Abstract
The aim of this study was to investigate water samples collected in coastal areas of Southern Thailand in the years of 2005 and 2008 for their contamination by the protozoan parasites Cryptosporidium and Giardia. One hundred eighteen water samples of different origin were collected from six Tsunami affected southern provinces of Thailand in early 2005, and they have been analyzed using standardized methodology. Fifteen out of 118 samples (12.7%) were positive for Cryptosporidium spp. and nine (7.6%) positive for Giardia spp. Additional 42 samples from two same areas were examined 3 years later, in the early 2008. Five out of 42 (11.9%) samples were positive for Cryptosporidium spp., and three out of 42 (7.1%) were positive for Giardia spp.. Both protozoans were found in reservoir, river/canal, and pond waters. It appears no significant differences (p < 0.05) between Cryptosporidium and Giardia (oo)cysts levels during the two monitoring periods; however, the number of the investigated areas and samples in the second period was significantly less than in the first period. This is the first description on Cryptosporidium and Giardia (oo)cysts in water sources of Thailand, and it suggests the need for water quality control in the interest of public health safety.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cryptosporidium and Giardia are waterborne protozoa of human and animal fecal origin that causes widespread gastroenteritis in humans that is normally self-limiting but potentially life-threatening in the immune compromised persons. The transmissive stages, oocysts and cysts, are ubiquitous in aquatic environment, resistant to environmental stresses, and cannot be completely removed by conventional water treatment practices (Wolfe 1992; Graczyk et al. 1997; Karanis et al. 1996a, 1998, 2007). Cryptosporidium and Giardia (oo)cysts are transmitted by the fecal–oral route, not only interpersonally but also by food and water (Karanis et al. 2007). Drinking water and recreational waters particularly in water resources inhabited by domestic and wild animals can be significant sources of human exposure to these organisms (Bednarska et al. 1998; Cox et al. 2005; Heitman et al. 2002; Karanis et al. 1996b; Mead et al. 1999; Robertson and Gjerde 2001). Waterborne outbreaks of cryptosporidiosis and giardiasis have been reported worldwide associated with water from various sources including the contributions of farm animals, principally dairy cattle and sheep (Barwick et al. 2000; Craun et al. 1998; Karanis et al. 2007; Gray 1998; Lemmon et al. 1996; Ribeiro and Palmer 1986; Shield et al. 1990).
Although Cryptosporidium and Giardia have been reported as common enteric protozoa in many countries, details of their transmission to humans via drinking water remain unclear. In Southeast Asia, investigations on the distribution of Cryptosporidium and Giardia in surface waters and sources of water supply have not been reported. No previous data are available describing the occurrence of these organisms in water supplies in Thailand. After the tsunami in Asia on Sunday the 26th December 2004, the threat of waterborne disease was more significant than before. The heavily affected provinces in Southern Thailand are main tourism districts of Phang Nga, Ranong, Trang, Phuket, and Krabi where more than 5,000 people, including many foreigners, have been killed. More than 100,000 of the local population were displaced and accommodated in relocation camps having initially only with rudimentary sanitation facilities for up to a year or more. Contamination of drinking water sources by pathogenic bacteria, viruses, and protozoa represented a major human health hazard. Water supplies and sanitation facilities in affected areas were heavily damaged. For example, in Phang-nga, 1,000 households were without water or sewerage. Over 200 wells, 11 tap water supplies, eight surface water sources were unusable. Similar conditions occurred in the coastal portions of Krabi and somewhat less in similar area of the other provinces. Marine environments, including shallow coastal areas and river estuaries, were contaminated by untreated sewage introducing fecal contaminants. Coastal as well as inland surface waters were polluted by human and livestock fecal discharges including those from human corpses and animal remains following the disaster. These waters represent a potential risk for disease transmission via drinking and recreational water or through incidental contact while fishing or swimming.
The present study was conducted to determine the presence of the protozoan parasites Cryptosporidium and Giardia in environmental waters collected in areas of Southern Thailand and to provide information on (oo)cysts distribution in the investigated waters. Information on sources’ contamination of public water supplies would provide a basis for assessing their contribution to local public health risk.
Materials and methods
Locations and water sample collection
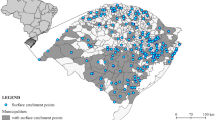
Sampling was conducted in tsunami-affected areas including the six southern provinces of Krabi, Phang-nga, Phuket, Ranong, Satun, and Trang (Fig. 1). Water samples were collected in two periods: the first set in the months immediately following the tsunami, between January 20th to May 10th 2005; the second just 3 years later, between February to April 2008. Samples were collected from a range of water sources including near-shore coastal, river and estuary, reservoir, ditch, and a functioning tap sites. Sites were selected to include potential sources of waterborne pathogen contamination. Overall in 2005, 118 samples were collected from various water sources included: 11 tap water, 42 well water, 12 estuary and river water, four reservoir water, six ditch water, 25 pond water, and 18 marine water. Additional of 42 samples have been collected from the same locations in Phuket and in Phang-Nga in the 2008 period. They included: two tap water, 10 well water, 12 estuaries and river water, four reservoir water, 12 pond water, and two marine water. The physical and chemical quality of each water sample has been examined used in situ using portable instruments. Water samples have been subsequently transported on ice to the laboratory in the Bangkok University facilities for further processing and examination.
Isolation and concentration of (oo)cysts from water
For both Cryptosporidium and Giardia (oo)cysts analysis, one of two protocols below was followed according to the turbidity levels of the water sample. For water samples with turbidity above of five NTU, a 10- to 20-l sample was filtered in the field through a Gelman Envirocheck filter (Pall Gelman Sciences, Inc; Ann Arbor, Mich) at 1 to 2 l/min according to manufacturer’s recommendation. Filters were transported to the laboratory on ice, and samples were eluted according to manufacturer’s recommendations within 36 h of sample collection. Eluted solids from the capsules were resuspended in 10 ml of laboratory grade water for each 0.5 ml of solids, stored at 4°C, then further processed for (oo)cysts isolation and concentration. For water samples with a turbidity less than five NTU, an each 20- to 30-l sample was collected in a clean container, and (oo)cysts were concentrated by filtration through a membrane filter (1.2 to 3 μm pore size, 47 mm diameter) using a vacuum pump. To keep moisture, the filters were replaced to a sterile plastic universal bottle containing 10 ml of sterile distilled water. Each bottle was then vortexed at low speed for 5 min to recover the (oo)cysts from the surface of the filters. Following centrifugation at 15,000×g for 10 min, the supernatant was carefully decanted and the pellet was resuspended in 1 ml of sterile distilled water and stored at 4°C until further processing. (Oo)cysts were further concentrated by sucrose flotation via density gradient centrifugation and, if the suspension after sucrose flotation contained debris, by immunomagnetic separation (IMS) with the Aureon CG kit (Aureon Biosystems GmbH, Vienna, Austria). Briefly, 100 μl of anti-Cryptosporidium and anti-Giardia beads were added to a 5-ml concentrate obtained after centrifugation or membrane filtration of the eluate. The sample was then processed according to the recommendations of the manufacturer (Dynal), using Dynal equipment for IMS processing. After dissociation from magnetic beads, (oo)cysts were transferred to a new microcentrifuge tube and treated with 5 μl of 1 N NaOH to neutralize the pH. The resultant suspension for screening was clean and approximately 50 μl total volume. This resulted final sample was divided into two portions, and one portion was examined by the immunofluorescent assay (IFA) to detect Cryptosporidium oocysts and Giardia cysts.
Detection and identification of Cryptosporidium and Giardia (oo)cysts by IFA and by DIC
The Cryptosporidium oocysts and Giardia cysts were stained with FITC conjugated anti-Cryptosporidium/anti-Giardia monoclonal antibodies (MERIFLUOR® C/G, UK), processed according to the recommendations of the manufacturer, and then were enumerated using fluorescence microscopy (OLYMPUS; Model BX 51/BH-2) and differential interference contrast (DIC) microscopy following the standard methodology as it is recommended by the Method 1623 (USEPA 2001). All samples which were found positive by IFA have been examined with DIC by ×100 magnification to confirm internal structures inside of the (oo)cysts.
Statistical data analysis
Statistical analyses were performed on logarithmically transformed data of Cryptosporidium oocysts and Giardia cysts numbers and then process using Pearson correlation analysis (SPSS for Windows software: version 11.00). The result were considered to be significant at α = 0.05.
Results
Among the 118 water samples of various quality and origin from the 2005 investigation period (11 tap water, 42 well water, 12 estuary and river water, four reservoir water, six ditch water, 25 pond water, and 18 marine water), 15 out of 118 samples (12.7%) were positive for Cryptosporidium spp., and nine (7.6%) were positive for Giardia spp. The highest detection rates were in samples from sources in Phang-Nga. Cryptosporidium spp. oocysts have been found in three of 42 (7.1%) well samples, two of 12 (16.7%) estuarine and river samples, two of four (50%) reservoir samples, two of six (33.3%) ditch samples, and six of 25 (24%) pond samples. Giardia spp. cysts have been detected only in estuarine and river water, reservoir, and pond water samples at 16.7%, 25%, and 24%, respectively (Tables 1 and 2). Whereas in the 2008 investigation period samples, five of 42 samples (11.9%) were positive for Cryptosporidium, and three of 42 (7.1%) were positive for Giardia (Tables 1 and 2).
For samples in which Cryptosporidium and/or Giardia (oo)cysts were detected, the geometric mean of Cryptosporidium oocysts was 5.16 oocysts per liter, ranging from 0.73 to 24.25 oocysts per liter (Table 3). For Giardia cysts, the geometric mean was 3.26 cysts per liter, ranging from 0.00 to 13.12 cysts per liter (Table 3). On the average, Cryptosporidium oocysts were presented 1.6 times more numerous than Giardia cysts (Fig. 2). Cryptosporidium occurrence was significantly correlated (r = 0.717, P < 0.01) to the Giardia occurrence. Cryptosporidium oocysts numbers were also significantly related to sample turbidity (r = 0.614, P < 0.05; Fig. 3) and Giardia cysts numbers similarly correlated to turbidity (r = 0.465, P > 0.05; Fig. 4), but neither organism numbers were significantly correlated to either total nor fecal coliform levels (data not shown here). No Cryptosporidium oocysts or Giardia cysts were found in any of the samples from Ranong, Trang or Satun (Table 1).
Dicussion
Although infections with Cryptosporidium spp. in HIV-infected patients, in cattle, and in food (green mussels) have been already reported from Thailand (Uga et al. 2000; Gatei et al. 2002; Saksirisampant et al. 2002; Tiangtip and Jongwutiwes 2002; Nuchjangreed et al. 2008; Srisuphanunt et al. 2009), no outbreaks of waterborne cryptosporidiosis or giardiasis have been reported in the country, and they could not be included in the systematic review covering all reports worldwide of waterborne protozoan outbreaks by Karanis et al. (2007). No data exist regarding protozoan parasite concentrations in wastewaters or in reuse and recycling of wastewaters for agriculture and industry and in backwash water from drinking water treatment plants in Thailand.
The present study aimed to provide information on the occurrence and distribution of Cryptosporidium and Giardia spp. (oo)cysts in environmental waters of Southern Thailand. The areas which have been affected by the tsunami at December 2004 have been investigated in the early and in late post-tsunami periods almost 3 years later in the year 2008. Water environments including fresh, estuarine, and marine areas were heavily affected during and after the tsunami period. Physical damage and inundation occurred in drinking water supplies, waste systems, estuaries and fisheries, and recreational areas. The microbiological quality of water and associated risks of diseases have been additionally affected from fecal contamination following these extreme events of the tsunami in which human and animal wastes were distributed indiscriminately with wastes from toilets, latrines, underground drain fields, sewage lagoons, and treatment facilities. Due to the study design, it will not be possible to give concrete comparative information on the (oo)cysts numbers before and/or after the tsunami events. The prevalence of the Cryptosporidium oocysts and Giardia cysts in both periods (early and late post-tsunami period) were similar (12.5% versus 11.9% for Cryptosporidium; 7.6% versus 7.1% for Giardia); however, the number of the investigated areas and samples differ remarkable (118 samples and six areas investigated in 2005 versus 42 samples and two areas investigated in 2008). These are reasons for indirect comparative figures of the degree of contamination of the investigated water supplies with the investigated protozoan. Furthermore, the different periods of investigations rely to the different contamination levels of the water sources, and this is valid also for periods independent of the tsunami or other physical phenomena. This will be difficult for direct comparisons in such studies because the concentration of both protozoans in water supplies is considerably affected by seasonal factors and other mechanisms of transportation and due to the differences in dry seasons or washed out in the rainy seasons. From the methodological point of view, the methods applied here for the detection leads to the underestimation of the parasites’ numbers. Nevertheless, it is obvious from the data presented that in both periods, the occurrence of Cryptosporidium and Giardia (oo)cysts in the samples correlated with the turbidity in the samples. In our investigations, we used membrane filtration with IFA as described by USEPA method 1623 that is widely used for the detection of Cryptosporidium and Giardia (oo)cysts in water. Recovery of Cryptosporidium and Giardia (oo)cysts using Method 1623 have been shown to vary significantly, depending on water quality (Ferguson et al. 2004; Lee et al. 2002; Quintero-Betancourt et al. 2003; Weintraub 2006). USEPA Method 1622/23 provides an estimation of the total oocyst count. Data produced by this method include essentially many species of Cryptosporidium and Giardia, having some ability to discriminate between live and dead organisms but virtually no ability to discriminate between genotypes. In assessing water quality in relation to public health risk, the first priority is the information on the presence or absence of a pathogen. This may be of overriding importance during or after natural disasters.
It has been suggested in the literature that some genotypes of both Cryptosporidium and Giardia are more strongly associated with human illness than others, and some genotypes are apparently related to specific symptomatology in human giardiasis and cryptosporidiosis cases, and methodologies have been described for the genetic differentiation of the pathogens (Ryan et al. 2005; Plutzer et al. 2008). Accordingly, more detailed information on pathogen occurrence including genotype, particularly for human-adapted Giardia species and Cryptosporidium species, would support to make risk estimation more precise in the future. However, it cannot be used directly for risk assessment, as it does not determine the fraction of infectious oocysts.
Thailand is a frequently visited country by tourists, and the number of travelers among the different districts is increasing every year. Hence, safe drinking water should be always a general concern to the Thai government. However, monitoring for Cryptosporidium and Giardia in water supplies in Thailand is not practiced, and epidemiological studies have been hindered by the lack of funding for systematic investigations. Systematic research studies are needed to assess the distribution of parasites in catchments areas of surface and groundwater resources (WHO 2005).
The present work is the first report on contamination with Giardia and Cryptosporidium spp. in natural and drinking water resources in Thailand, and it provides information of the contamination Cryptosporidium and Giardia spp. in investigated areas. In most cases, Cryptosporidium oocysts’ presence was at higher levels than that of Giardia cysts. In Southeast Asia, published reports on the occurrence and distribution of Cryptosporidium and Giardia in water supplies are sparse, and no data have previously described on detection of these organisms in the water environments. It is obvious that additional work to identify waterborne contaminants like Cryptosporidium and Giardia and in relation to the incidence of human illness in Asian countries towards the efforts for the protection of public health is needed.
References
Barwick RS, Craun LDA, GF BMJ, Calderon RL (2000) Surveillance for waterborne-disease outbreaks-United States, 1997–1998. MMWR 49:1–21
Bednarska M, Bajer A, Sinski E (1998) Calves as a potential reservoir of Cryptosporidium parvum and Giardia spp. Ann Agric Environ Med 5:135–138
Cox P, Griffith M, Angles M, Daniel D, Ferguson C (2005) Concentration of pathogens and indicators in animal faeces in the Sydney watershed. Appl Environ Microbiol 71:5929–5943
Craun GF, Hubbs SA, Frost F, Calderon RL, Via SH (1998) Waterborne outbreaks of cryptosporidiosis. J Am Wat Works Assoc 90:81–91
Ferguson C, Kaucner C, Krogh M, Deere D, Warnecke M (2004) Comparison of methods for the concentration of Cryptosporidium oocysts and Giardia cysts from raw waters. Can J Microbiol/Rev Can Microbiol 50:675–682
Gatei W, Suputtamongkol Y, Waywa D, Ashford RW, Bailey JW, Greensill J, Beeching NJ, Hart CA (2002) Zoonotic species of Cryptosporidium are as prevalent as the anthroponotic in HIV-infected patients in Thailand. Ann Trop Med Parasitol 96:797–802
Graczyk TK, Fayer R, Cranfield MR (1997) Zoonotic potential of Cryptosporidium parvum: implications for waterborne cryptosporidiosis. Parasitol Today 13:348–351
Gray MJ (1998) Assessment of water supply and associated matters in relation to the incidence of cryptosporidiosis in West Hertfordshire and North London in February and March 1997, Appendix, vol 1. Department of the Environment, Transport and the Regions, Welsh Office, Cardiff, pp 17–26
Heitman TL, Frederick LM, Viste JR, Guselle NJ, Morgan UM, Thompson RCA, Olson ME (2002) Prevalence of Giardia and Cryptosporidium and characterization of Cryptosporidium spp. isolated from wildlife, human, and agricultural sources in the North Saskatchewan river basin in Alberta, Canada. Can J Microbiol 48:530–541
Karanis P, Schoenen D, Seitz HM (1996a) Giardia and Cryptosporidium in backwash water from rapid sand filters used for drinking water production. Zent Bl Bakteriol 284:107–114
Karanis P, Opiela K, Al-Arousi M, Seitz HM (1996b) A comparison of phase contrast microscopy and an immunofluorescence test for the detection of Giardia spp. in faecal specimens from cattle and wild rodents. Trans R Soc Trop Med Hyg 90:250–251
Karanis P, Schoenen D, Seitz HM (1998) Distribution and removal of Giardia and Cryptosporidium in water supplies in Germany. Wat Sci Technol 37:9–18
Karanis P, Kourenti C, Smith H (2007) Waterborne transmission of protozoan parasites: a review of world-wide outbreaks and lessons learned. J Wat Health 5:1–38
Lee SH, Levy DA, Craun GF, Beach MJ, Calderon RL (2002) Surveillance for waterborne - disease outbreaks- United States, 1999–2000. MMWR 51:1–47
Lemmon JM, McAnulty JM, Bawden–Smith J (1996) Outbreak of cryptosporidiosis linked to an indoor swimming pool. Med J Austr 165:613
Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, Griffin PM, Tauxe RV (1999) Food-related illness and death in the United States. Emerg Infect Dis 5:607–625
Nuchjangreed C, Boonrod K, Ongerth J, Karanis P (2008) Prevalence and molecular characterization of human and bovine Cryptosporidium isolates in Thailand. Parasitol Res 103:1347–1353
Plutzer J, Karanis P, Domokos K, Törökné A, Márialigeti K (2008) Detection and characterisation of Giardia and Cryptosporidium in Hungarian raw, surface and sewage water samples by IFT, PCR and sequence analysis of the SSUrRNA and GDH genes. Int J Hyg Environ Health 211:524–533
Quintero-Betancourt W, Gennacaro AL, Scott TM, Rose JB (2003) Assessment of methods for the detection of infectious Cryptosporidium oocysts and Giardia cysts in reclaimed effluents. Appl Environ Microbiol 69:5380–5388
Ribeiro CD, Palmer SR (1986) Family outbreak of cryptosporidiosis. Brit Med J 292:377
Robertson LJ, Gjerde B (2001) Factors affecting recovery efficiency in isolation of Cryptosporidium oocysts and Giardia cysts from vegetables for standard method development. J Food Prot 64:1799–1805
Ryan U, Read C, Hawkins P, Warnecke M, Swanson P, Griffith M, Deere D, Cunningham M, Cox P (2005) Genotypes of Cryptosporidium from Sydney water catchment areas. J Appl Microbiol 98:1221–1229
Saksirisampant W, Eampokalap B, Rattanasrithong M, Likanonsakul S, Wiwanitkit V, Nasingkarn A, Denmasae N (2002) A prevalence of Cryptosporidium infections among Thai HIV-infected patients. Parasitol 85:424–428
Shield J, Baumer JH, Dawson JA, Wilkinson PJ (1990) Cryptosporidiosis - an educational experience. J Infect 21:297–301
Srisuphanunt M, Saksirisampant W, Karanis P (2009) Detection of Cryptosporidium oocysts in green mussels (Perna viridis) from shell-fish markets of Thailand. Parasite 16:235–239
Tiangtip R, Jongwutiwes S (2002) Molecular analysis of Cryptosporidium species isolated from HIV-infected patients in Thailand. Trop Med Int Health 7:357–364
Uga S, Kawamura T, Hotta H, Endo T, Masuda K, Yamamoto A, Shiba Kumar R, Tsuji H, Ono K (2000) Prevalence of Cryptosporidium parvum infection and pattern of oocyst shedding in calves in Japan. Appl Environ Microbiol 67:3832–3836
USEPA (2001) Cryptosporidium and Giardia in water by filtration/IMS/FA, USEPA Method 1623. USEPA, Washington, DC, pp 1–58
Weintraub JM (2006) Improving Cryptosporidium testing methods: a public health perspective. J Wat Health 4:23–26
WHO (2005) Document for WHO conference on the Health aspects of the Tsunami Disaster in Asia, Phuket, Thailand, 4–6 May, 2005
Wolfe MS (1992) Giardiasis. Clin Microbiol Rev 5:93–100
Acknowledgements
We would like to thank the Public Utilities Board, Singapore for supplying some materials for parasite testing. We are also indebted to Mr. Sumetha Wichienpet, Director of Emergency Response and Remediation Division, Pollution Control Department, Ministry of Natural Resources and Environment, Thailand; Dr. Raywadee Roachankanan, Faculty of Environment and Resources, Mahidol University for their assistance with field water sampling; and to Dr. Kittipong Harncharoen and Dr. Dusit Sujirarat, Department of Epidemiology, Faculty of Public Health, Mahidol University for their assistance with the statistical analyses. This work was partially funded by the DAAD, Germany, by institutional funds of the Anatomy Center in Cologne University, by the Thai government on the health aspect of the Tsunami disaster in Thailand and partially supported for publication by the China Medical Board, Faculty of Public Health, Mahidol University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Srisuphanunt, M., Karanis, P., Charoenca, N. et al. Cryptosporidium and Giardia detection in environmental waters of southwest coastal areas of Thailand. Parasitol Res 106, 1299–1306 (2010). https://doi.org/10.1007/s00436-010-1795-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-010-1795-0