Abstract
The aim of the study was to report tick infestations on wild birds in a region of the eastern Brazilian Amazon and evaluate the rickettsial infection of these ticks. Wild birds captured by mist nets were examined for the presence of ticks, which were collected and identified to species by morphology or molecular methods. In addition, part of these ticks was individually tested by polymerase chain reaction targeting portions of the rickettsial genes gltA and ompA. Among 331 captured birds, representing 56 species, 133 individuals (40.2%) from 34 species were found infested by 443 ticks, being Amblyomma longirostre (Koch) the most common (103 larvae, 12 nymphs), followed by Amblyomma humerale Koch (15 larvae, 3 nymphs), Amblyomma geayi Neumann (seven larvae, one nymph), Amblyomma calcaratum Neumann (one larva, four nymphs), Amblyomma coelebs Neumann (two larvae), and Haemaphysalis juxtakochi Cooley (one larva, two nymphs). Other 285 larvae and 7 nymphs collected from birds could not be identified to species and were morphologically identified as Amblyomma spp. The species A. humerale and A. geayi are recorded for first time parasitizing birds in the Neotropical region. Among 67 A. longirostre and 7 A. geayi, 38 (56.7%) and 4 (57.1%), respectively, were found infected by Rickettsia amblyommii. In spite of R. amblyommii being not currently recognized as human or animal pathogen, there has been serological evidence for human and canine infection by this agent in the USA and in the Brazilian western Amazon.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wild birds are essential host for various ectoparasites including ticks, arthropods vectors of various pathogens responsible for causing diseases in humans and animals (Hoogstraal 1961). Among 61 tick species reported in Brazil (Dantas-Torres et al. 2009), 18 species from five genera have been reported parasitizing wild birds in different biomes other than the Amazon rainforest (Aragão 1911, 1936; Arzua et al. 2003, 2005; Arzua and Barros-Battesti 1999; Barros-Battesti et al. 2003; Evans et al. 2000; Labruna et al. 2007a; Ogrzewalska et al. 2008, 2009; Szabó et al. 2008; Terrassini et al. 2006). The Amazon biome is the largest rainforest of the world, with most of its extension (4,900,000 km2) in northern Brazil. Approximately 1,000 bird species (≈1/3 of South American bird species) are reported occurring in the Amazon (Sick 1997). Despite the high diversity of birds, the ixodid tick fauna of Brazilian Amazon is poorly known. Consequently, there has been no report of tick species infesting wild birds in the Brazilian Amazon.
The vast majority of bird-infesting tick reports in Brazil refer to immature stages (larvae and nymphs) of the genus Amblyomma. However, many of these reports are limited to genus level (i.e., Amblyomma sp.) because most of the ≈30 Amblyomma species currently known to occur in Brazil have their immature stages still not described (Guglielmone et al. 2003); consequently, there is no sufficient literature for a proper identification of Brazilian Amblyomma immature ticks. Immature specimens of most Amblyomma species established in Brazil can be identified with certainty to the species level by only two procedures: (1) rearing the subadult specimen until the adult stage and then identifying the species of the adult tick; (2) use of molecular tools (Labruna et al. 2007a).
Bacteria of the genus Rickettsia are obligate intracellular organisms that infect invertebrate hosts worldwide. Some of the known Rickettsia species are capable of causing diseases (i.e., rickettsiosis) in humans, to whom they are transmitted by hematophagous vectors, mostly ticks. An increasing number of Rickettsia species previously regarded as nonpathogenic or of unknown pathogenicity, because they were known to occur only in ticks, are currently considered agents of emerging human rickettsioses throughout the world (Parola et al. 2005, 2009). Thus, several of the currently recognized pathogenic Rickettsia species were first reported in ticks, and some years to decades later, they were shown to cause human disease.
The present study evaluated tick infestations on wild birds in a region of the eastern Brazilian Amazon. In addition, we tested part of these ticks for rickettsial infection.
Materials and methods
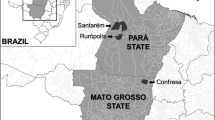
Bird capture was performed in Jutaituba farm, located among Portel, Baião, Bagre and Oeiras Municipalities in the state of Pará, Northern Brazil, between the Tocantins and Pacajá Rivers (central point of the farm: 02°57′18′′S, 50°01′54′′ W). The region has suffered relatively low deforestation, although it is localized in the proximity of so called “deforestation arc” on the southern and eastern part of the Amazon forest region. The most prevalent types of vegetation within the farm are the dense broadleaf forest, the open broadleaf forest with lianas with selective logging, and the open broadleaf flood forest.
Between 16 and 27 of November 2008, wild birds were caught using 20 mist nets (12 m long × 2 m wide, 36 mm mesh) displayed along human and animal trails within five forest areas (2 work-days per area). In each area, mist nets were left open from 6 a.m. to 5 p.m. in the first day and from 6 a.m. and 12 p.m. on the second day, totalizing 1,700 net-hours for the whole study. Mist nets were checked every 30 min; captured birds were identified to species according to Sick (1997), Ridgely and Tudor (1989, 1994), Sigrist and Quirino (2007), and Souza (2002). Captured birds were examined for the presence of ticks by checking carefully their whole body. All ticks found attached to birds were removed with forceps. Before releasing captured birds, they were marked by cutting head feathers in order to verify any recapture, which was not considered in the counting. For the present study, we followed the protocol that agrees with Ethical Principles in Animal Research adopted by the Brazilian College of Animal Experimentation, which was approved by the Faculdade de Medicina Veterinária e Zootecnia /USP-Ethical Committee for Animal Research. Permits and approvals are on file in the office of M.B.L.
Fully engorged ticks (larvae and nymphs) removed from birds were placed in plastic vials containing several grass leaves and covered by a cork containing several minute holes to keep ticks alive until arriving at the laboratory. In the laboratory, engorged ticks were placed in an incubator at 25°C and RH 90% to allow them to molt to nymphs or adults. Each adult specimen obtained from an engorged nymph was used for taxonomic identification of the species of the former corresponding nymph collected on bird. In the case of Amblyomma longirostre Koch, species identification was performed by examining the nymphal stage obtained directly from birds or from engorged larvae that molted to nymphs in the laboratory. The A. longirostre nymph can be easily identified by its pointed hypostome and elongate scutum (Keirans and Durden 1998).
Either nonengorged or partially engorged ticks collected from birds were immediately preserved in absolute isopropanol. In this case, for identification of tick species, we used molecular tools as described elsewhere (Ogrzewalska et al. 2009). Briefly, each immature specimen was submitted to DNA extraction and submitted to polymerase chain reaction (PCR) using primers that amplify a ≈460 bp of the tick mitochondrial rDNA gene (Mangold et al. 1998). Amplified products were purified and DNA sequenced as previously described (Labruna et al. 2004a), and compared with NCBI Nucleotide BLAST searches (Altschul et al. 1990) and with mitochondrial 16S rDNA gene partial sequences obtained in our laboratory during the present study, by processing adult ticks of the following species (locality of origin in parentheses): Amblyomma humerale Koch (a male collected from a Geochelone sp. in Monte Negro, State of Rondônia; 10°15′35′′S, 63°18′06′′W); and Amblyomma goeldii Neumann (a male collected from a Tamandua tetradactyla (L.) in Manaus, State of Amazonas, 03°06′48′′S, 60°01′31′′W).
Prevalence of ticks on birds and mean intensity of the tick infestations on each bird species were calculated following Bush et al. (1997); prevalence is the number of infested birds/number of examined birds × 100 within each bird species, and mean intensity is total number of ticks/number of infested birds within each bird species.
Ticks identified to the species level were individually tested for the presence of Rickettsia by PCR using primers CS-78 and CS-323, which target a 401-bp fragment of the gltA gene that occurs in all Rickettsia species (Labruna et al. 2004a), and primers Rr190.70F and Rr190.602R targeting a fragment of 532 bp of the ompA gene present only in Rickettsia species belonging to the spotted fever group (SFG) (Regnery et al. 1991). PCR products were DNA sequenced and submitted to BLAST analysis to determine similarities to other Rickettsia species (Altschul et al. 1990).
Results
A total of 331 birds were captured, representing 56 species from five orders. Passeriformes constituted the most numerous bird order, with 48 species encompassing 298 (90%) individual birds. A total of 133 (40.2%) birds from 34 species (orders Passeriformes and Galbuliformes) were infested with 414 larvae and 29 nymphs from the genera Amblyomma and Haemaphysalis (Table 1). Among engorged ticks, 16 nymphs (15 Amblyomma and 1 Haemaphysalis) collected from birds were reared to the adult stage in the laboratory, where they molted to A. longirostre (12 nymphs), Amblyomma calcaratum Neumann (three nymphs), and Haemaphysalis juxtakochi Cooley (one nymph). A total of 27 Amblyomma engorged larvae molted to 27 nymphs of A. longirostre. Ticks identified to species by molecular analysis were A. longirostre (76 larvae), A. humerale (15 larvae, 3 nymphs), Amblyomma geayi Neumann (seven larvae, one nymph), H. juxtakochi (one larva, one nymph), A. calcaratum (one larva, one nymph), and Amblyomma coelebs Neumann (two larvae). A total of 285 larvae and 7 nymphs died before reaching the adult stage or did not generate high-quality DNA sequence; therefore, they were identified morphologically as Amblyomma spp. For molecular identification of larvae or nymphs, mean individual fragment lengths of 419 ± 30 bp (range 249–446 bp) were 95.8–100% identical to the corresponding 16S rDNA sequence of the Amblyomma species-adult stage or GenBank available sequences (Table 2).
All bird species parasitized by ticks are presented in Table 1. Individual infestations usually consisted of few ticks, with mean intensity values lower than 4.0 ticks/bird at most of the times. No ticks were found on the following bird species (number of individuals in parentheses): Columbiformes, Columbidae: Geotrygon montana L. (4); Strigiformes, Strigidae: Glaucidium hardyi Vielliard (1); Apodiformes, Trochilidae: Phaethornis ruber (L.) (2), Phaethornis superciliosus (L.) (14), Florisuga mellivora (L.) (1), Thalurania furcata (Gmelin) (6); Passeriformes, Thamnophilidae: Myrmotherula leucophthalma (Pelzeln) (7), Dichrozona cincta (Pelzen) (1), Cercomacra nigrescens (Cabanis and Heine) (1), Hypocnemis cantator (Boddaert) (1), Formicariidae: Formicarius colma Boddaert (3), Scleruridae: Sclerurus mexicanus Sclater (2), Sclerurus rufigularis Pelzeln (1), Furnariidae: Philydor erythrocercus (Pelzeln) (2), Xenops minutus (Sparrman) (2), Tyrannidae: Terenotriccus erythrurus (Cabanis) (4), Attila cinnamomeus (Gmelin) (1), Cotingidae: Lipaugus vociferans (Wied) (2), Vireonidae: Hylophilus ochraceiceps Sclater (1), Coerebidae: Coereba flaveola L. (1), Emberizidae: Arremon taciturnus (Hermann) (1), Cardinalidae: Cyanocompsa cyanoides (Lafresnaye) (2).
Among 65 larvae and 2 nymphs of A. longirostre tested by PCR targeting rickettsial genes, 38 larvae (56.7%) yielded positive results by both gltA and ompA PCR assays, as well as four out of seven A. geayi larvae (57.1%). The gltA product from these ticks were sequenced and were shown to be identical to each other, and 100% (343/343 bp) identical to the corresponding sequence of Rickettsia amblyommii (GenBank accession number AY375163) previously detected in ticks Amblyomma cajennense (Fabricius) and A. coelebs from the state of Rondônia, western Brazilian Amazon (Labruna et al. 2004b), and with R. amblyommii strain An13 (DQ517290) previously detected in Amblyomma neumanni Ribaga from Argentina (Labruna et al. 2007b). The ompA product of these ticks showed to be 99.8% (487/488) identical to the corresponding sequence of R. amblyommii previously detected Amblyomma americanum (L.) in the United States (EF450696, EF689733, AF453408), and 98.9% (483/488) identical to both R. amblyommii strain AL (EU274656) isolated from A. longirostre in the state of São Paulo, Brazil (Ogrzewalska et al. 2008), and R. amblyommii strain Aranha (AY360213) detected in A. longirostre from the state of Rondônia, Brazil (Labruna et al. 2004c). No A. humerale (14 larvae, 1 nymph) or A. calcaratum (two nymphs) yielded positive results by PCR.
Voucher tick specimens collected during this study have been deposited in the “Coleção Nacional de Carrapatos” of the Faculty of Veterinary Medicine, University of São Paulo, SP, Brazil (accession numbers: 1371–1387). GenBank nucleotide sequence accession numbers for the partial mitochondrial 16S rDNA sequences obtained in the present study are GQ891947 (A. calcaratum larva), GQ891948 (A. coelebs larva), GQ891949 (A. geayi larva), GQ891950 (A. goeldii adult), GQ891951 (A. longirostre larva), GQ891952 (A. humerale larva), GQ891953 (H. juxtakochi larva), and GQ891954 and GQ891955 for partial sequences of R. amblyommii (gltA and ompA genes, respectively).
Discussion
The present study reports immature stages of five Amblyomma species and H. juxtakochi parasitizing wild birds in a region of Amazon rainforest in the State of Pará. The predominant tick species-infesting birds was A. longirostre. This tick is widely distributed in the Neotropical region (Guglielmone et al. 2003; Jones et al. 1972). Adults of A. longirostre feed primarily on porcupines (Coendou spp.) whereas subadult stages feed primarily on birds, mostly Passeriformes (Aragão 1936; Jones et al. 1972; Labruna et al. 2007a; Ogrzewalska et al. 2008). Except for Turdus albicollis Vieillot, which has been previously recorded parasitized by A. longirostre nymphs in Brazil (Labruna et al. 2007a) and Uruguay (Venzal et al. 2005), all bird species records for A. longirostre of the present study (Table 1) are being reported for the first time.
Two tick species, A. humerale and A. geayi, are recorded for first time parasitizing birds in the Neotropical region. Although there is a published record of an A. humerale nymph (identified by molecular methods) on a migratory bird [Catharus minimus (Lafresnaye)] in Canada (Morshed et al. 2005), the DNA sequence generated from this nymph refers, in fact, to Amblyomma sabanerae Stoll (L. Beati, personal communication). A. humerale seems to be a species restricted to the North-Central parts of South America including the Amazon forest (Aragão 1936; Guglielmone et al. 2003; Labruna et al. 2002). The adult stage of A. humerale is known to parasitize primarily the land tortoises Geochelone denticulata (L.) and Geochelone carbonaria (Spix) (Aragão 1936; Labruna et al. 2002). Nymphs have been reported on the lizards Plica plica (L.), Plica umbra (L.), Kentropyx calcarata Spix, on the marsupial Didelphis marsupialis L., and on the edentate Cyclopes didactylus L. (Labruna et al. 2002). Our present records of A. humerale on eight bird species suggest that small Passeriformes birds with terrestrial habits play a role in the life history of A. humerale. We provided the first host record for the larval stage of A. humerale.
The tick A. geayi is a relatively rare species occurring in Brazil, Colombia, Guyana, French Guiana, Panama, Peru, and Surinam (Guglielmone et al. 2003). Adults parasite animals from the order Xenartha, generally sloths, while there has been no host record for immature stages (Guglielmone et al. 2003). In the present study, we found six bird species parasitized by seven larvae and one nymph of A. geayi which are the first records of A. geayi immature stages in nature. A. geayi is morphologically and genetically close-related to A. longirostre and Amblyomma parkeri Fonseca and Aragão (Labruna et al. 2009). Our results suggest that A. geayi has also ecological similarities with A. longirostre and A. parkeri; i.e., immature stages might feed primarily on Passeriformes.
A. calcaratum, A. coelebs, and H. juxtakochi have been reported on wild birds in other South American Biomes (Beldomenico et al. 2003; Labruna et al. 2007a; Ogrzewalska et al. 2009). We found these three tick species sporadically on a few bird species, which were never reported to be infested by any of them. A. calcaratum has been reported in several countries of Central and South America (Guglielmone et al. 2003). The adult stage parasitizes chiefly anteaters, Tamandua spp. and Myrmecophaga tridactyla L., while subadults seem to feed primarily on birds (Jones et al. 1972; Labruna et al. 2007a). H. juxtakochi seems to be a typical Neotropical deer tick (Szabó et al. 2006), although previous records of immature stages on Passeriformes in southern Brazil, Uruguay, and Argentina suggest that birds may also play a role in the life-history of H. juxtakochi (Arzua et al. 2005; Beldomenico et al. 2003; Venzal et al. 2005). Adults of A. coelebs feed primarily on tapirs (Tapirus spp.) (Labruna et al. 2005a), whereas nymphs have been recorded in Brazil on jaguars [Panthera onca L. and Puma concolor (L.)], opossums [Didelphis albiventris (Lund)], peccary [Tayassu tajacu L.], humans (Labruna et al. 2005a, 2005b), and on a few birds with terrestrial habits (Ogrzewalska et al. 2009).
Overall, 56.7% and 57.1% of the A. longirostre and A. geayi ticks were found to be infected by the SFG agent R. amblyommii. Different strains of this Rickettsia have been reported infecting at least five Amblyomma species from the USA to Argentina, including A. longirostre from the state of Rondônia, western Brazilian Amazon, and from the state of São Paulo, southeastern Brazil (Bermúdez et al. 2009; Labruna et al. 2004c, 2007b; Ogrzewalska et al. 2008; Parola et al. 2007; Weller et al. 1998). While R. amblyommii is not currently recognized as human or animal pathogen, there has been serological evidence for human and canine infection by this agent in the USA and in the Brazilian western Amazon, respectively (Apperson et al. 2008; Labruna et al. 2007c). In fact, it has been proposed that some of the rickettsiosis cases reported as Rocky Mountain spotted fever (presumably caused by Rickettsia rickettsii) in the USA may have been caused by R. amblyommii (Apperson et al. 2008).
Ticks are known to carry pathogens of importance for human and animal health. In this context, birds are particularly important because they can transport ticks to continental distances, with great importance for tick-borne diseases. Herein, we provide a series of new host records for Neotropical Amblyomma species, suggesting that birds are much more important than currently recognized for the life histories of these ticks in the Amazonian biome.
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Apperson CS, Engber B, Nicholson WL, Mead DG, Engel J, Yabsley MJ, Dail K, Johnson J, Watson DW (2008) Tick-borne diseases in North Carolina: is “Rickettsia amblyommii rdquo; a possible cause of rickettsiosis reported as Rocky Mountain spotted fever? Vector Borne Zoonotic Dis 8:1–10
Aragão HB (1911) Notas sobre ixodidas brazileiros. Mem Inst Oswaldo Cruz 3:145–195
Aragão HB (1936) Ixodidas brasileiros e de alguns paizes limitrophes. Mem Inst Oswaldo Cruz 31:759–843
Arzua M, Barros-Battesti DM (1999) Parasitism of Ixodes (Multidentatus) auritulus Neumann (Acari: Ixodidae) on birds from the city of Curitiba, State of Paraná, Southern Brazil. Mem Inst Oswaldo Cruz 94:597–603
Arzua M, Silva N, Famadas KM, Beati L, Barros-Battesti DM (2003) Amblyomma aureolatum and Ixodes auritulus (Acari: Ixodidae) on birds in southern Brazil, with notes on their ecology. Exp Appl Acarol 31:238–296
Arzua M, Onofrio VC, Barros-Battesti DM (2005) Catalogue of the tick collection (Acari, Ixodida) of the Museu de História Natural Capão da Imbuia, Curitiba, Paraná. Brazil Rev Bras Zol 22:623–632
Barros-Battesti DM, Arzua M, Pichorim M, Keirans JK (2003) Ixodes (Multidentatus) paranaensis nsp (Acari: Ixodidae) a parasite of Streptoprocne biscutata (Sclater 1865) (Apodiformes: Apodidae) birds in Brazil. Mem Inst Oswaldo Cruz 98:93–102
Beldomenico PM, Baldi CJ, Antoniazzi LR, Orduna GM, Mastropaolo M, Macedo AC, Ruiz MF, Orcellet VM, Peralta JL, Venzal JM, Mangold AJ, Guglielmone AA (2003) Ixodid ticks (Acari: Ixodidae) present at Parque Nacional El Rey, Argentina. Neotrop Entomol 32:273–277
Bermúdez SE, Eremeeva ME, Karpathy SE, Samudio F, Zambrano ML, Zaldivar Y, Motta JA, Dasch GA (2009) Detection and identification of rickettsial agents in ticks from domestic mammals in Eastern Panama. J Med Entomol 46:856–861
Bush AO, Lafferty KD, Lotz JM, Shostaka AW (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83:575–583
Dantas-Torres F, Onofrio VC, Barros-Battesti DM (2009) The ticks (Acari: Ixodida: Argasidae, Ixodidae) of Brazil. Syst Appl Acarol 14:30–46
Evans DE, Martins JR, Guglielmone AA (2000) A review of ticks (Acari: Ixodida) of Brazil, their hosts and geographic distribution—1 The state of Rio Grande do Sul, southern Brazil. Mem Inst Oswaldo Cruz 95:453–470
Guglielmone AA, Estrada-Peña A, Keirans JE, Robbins RG (2003) Ticks (Acari: Ixodida) of the Neotropical Zoogeographic Region. Atlanta, Houten, The Netherlands
Hoogstraal H (1961) Migrating birds and their ectoparasites in relation to disease. East Afr Med J 38:221–226
Jones EK, Clifford CM, Keirans JE, Kohls GM (1972) The ticks of Venezuela (Acarina: Ixodoidea) with a key to the species of Amblyomma in the Western hemisphere. Brigham Young Univ Sci Bull Biol Ser 17:1–40
Keirans JE, Durden LA (1998) Illustrated key to nymphs of the tick genus Amblyomma (Acari: Ixodidae) found in the United States. J Med Entomol 35:489–495
Labruna MB, Camargo LMA, Terrassini FA, Schumaker TTS, Camargo EP (2002) Notes on Parasitism by Amblyomma humerale (Acari: Ixodidae) in the State of Rondônia, Western Amazon. J Med Entomol 39:814–817
Labruna MB, Whitworth T, Horta MC, Bouyer DH, McBride JW, Pinter A, Popov V, Gennari SM, Walker DH (2004a) Rickettsia species infecting Amblyomma cooperi ticks from an area in the state of Sao Paulo, Brazil, where Brazilian spotted fever is endemic. J Clin Microbiol 42:90–98
Labruna MB, Whitworth T, Bouyer DH, McBride J, Camargo CLMA, EP PV, Walker DH (2004b) Rickettsia bellii and Rickettsia amblyommii in Amblyomma ticks from the State of Rondônia, Western Amazon. J Med Entomol 41:1073–1081
Labruna MB, McBride JW, Bouyer DH, Camargo LMA, Camargo EP, Walker DH (2004c) Molecular evidence for a spotted fever group Rickettsia species in the tick Amblyomma longirostre in Brazil. J Med Entomol 41:533–537
Labruna MB, Camargo LM, Terrassini FA, Ferreira F, Schumaker TS, Camargo EP (2005a) Ticks (Acari: Ixodidae) from the state of Rondônia, western Amazon, Brazil. Syst Appl Acarol 10:17–32
Labruna MB, Jorge R, Sana D, Jácomo A, Kashivakura C, Furtado M, Ferro C, Perez S, Silveira L, Santos JRT, Marques S, Morato R, Nava A, Adania C, Teixeira R, Gomes A, Conforti V, Azevedo F, Prada C, Silva J, Batista A, Marvulo M, Morato R, Alho C, Pinter A, Ferreira P, Ferreira F, Barros-Battesti D (2005b) Ticks (Acari: Ixodida) on wild carnivores in Brazil. Exp Appl Acarol 36:151–165
Labruna MB, Sanfilippo LF, Demetrio C, Menezes AC, Pinter A, Guglielmone AA, Silveira LF (2007a) Ticks collected form birds in the state of São Paulo, Brazil. Exp Appl Acarol 43:147–160
Labruna MB, Pacheco RC, Nava S, Richtzenhain LJ, Guglielmone AA (2007b) Infection by Rickettsia bellii and Candidatus ‘Rickettsia amblyommii’ in Amblyomma neumanni ticks from Argentina. Microb Ecol 54:126–133
Labruna MB, Horta MC, Aguirar DM, Cavalcante GT, Pinter A, Gennari SM, Camargo LMA (2007c) Prevalence of Rickettsia infection in dogs from the urban and rural areas of Monte Negro Municipality, Western Amazon, Brazil. Vector Borne Zoonotic Dis 7:249–256
Labruna M, Onofrio V, Beati L, Arzua M, Bertola P, Ribeiro A, Barros-Battesti D (2009) Redescription of the female, description of the male, and several new records of Amblyomma parkeri (Acari: Ixodidae), a South American tick species. Exp Appl Acarol 49:243–260
Mangold AJ, Bargues MD, Mas-Coma S (1998) Mitochondrial 16S rDNA sequences and phylogenetic relationships of species of Rhipicephalus and other tick genera among Metastriata (Acari: Ixodidae). Parasitol Res 84:478–484
Morshed MG, Scott JD, Fernando K, Beati L, Mazerolle DF, Geddes G, Durden LA (2005) Migratory songbirds disperse ticks across Canada, and first isolation of the Lyme Disease spirochete, Borrelia burgdorferi, from the avian tick, Ixodes auritulus. J Parasitol 91:780–790
Ogrzewalska M, Pacheco R, Uezu A, Ferreira F, Labruna MB (2008) Ticks (Acari: Ixodidae) infesting wild birds in an Atlantic Forest area in the state of São Paulo, Brazil, with isolation of Rickettsia from the tick Amblyomma longirostre. J Med Entomol 45:770–774
Ogrzewalska M, Pacheco R, Uezu A, Richtzenhain LJ, Ferreira F, Labruna MB (2009) Ticks (Acari: Ixodidae) infesting birds in an Atlantic rain forest region of Brazil. J Med Entomol 46:1225–1229
Parola P, Paddock CD, Raoult D (2005) Tick-borne rickettsioses around the world: emerging diseases challenging old concepts. Clin Microbiol Rev 18:719–756
Parola P, Matsumoto K, Socolovschi C (2007) A tick-borne rickettsia of the spotted-fever group, similar to Rickettsia amblyommii, in French Guyana. Ann Trop Med Parasitol 101:185–188
Parola P, Rovery C, Rolain JM, Brouqui P, Davoust B, Raoult D (2009) Rickettsia slovaca and R. raoultiio in tick-borne rickettsioses. Emerg Infect Dis 15:1105–1108
Regnery RL, Spruill CL, Plikaytis BD (1991) Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J Bacteriol 173:1576–1589
Ridgely RS, Tudor G (1989) The birds of South America, Volume 1, the oscine passerines. Oxford University Press, London
Ridgely RS, Tudor G (1994) The birds of South America, Volume 2, the suboscine passerines. Oxford University Press, London
Sick H (1997) Ornitologia brasileira. Nova Fronteira, Rio de Janeiro
Sigrist T, Quirino MT (2007) Aves do Brasil Oriental—birds of Eastern Brazil. Avis Brasilis, São Paulo
Souza D (2002) All the birds of Brazil: an identification guide. Dall, Feira de Santana
Szabó MPJ, Labruna MB, Vogliotti A, Duarte JMB (2006) Ticks (Acari: Ixodidae) on Small Red Brocket Deer (Mazama bororo Duarte) along deer trails in the Atlantic Rain Forest of Southeastern Brazil. Syst Appl Acarol 11:41–45
Szabó MPJ, Pascoli GVT, Júnior OM, Franchin AG, Torga K (2008) Brown dog tick Rhipicephalus sanguineus parasitizing the bird Coereba flaveola in the Brazilian cerrado. Ciência 38:543–545
Terrassini FA, Venzal JM, Estrada-Peña AA, Camargo LMA, Labruna MB (2006) Argas (Persicargas) sp (Acari: Argasidae) em um urubu na Vila Princesa, Município de Porto Velho, Rondônia. I Simpósio Brasileiro de Acarologia Diversidade, taxonomia e manejo de ácaros neotropicais. Anais do simpósio 63-72, Viçosa MG, Brasil
Weller SJ, Baldridge GD, Munderloh UG, Noda H, Simser J, Kurtti TJ (1998) Phylogenetic placement of Rickettsiae from the ticks Amblyomma americanum and Ixodes scapularis. J Clin Microbiol 36:1305–1317
Venzal JM, Félix ML, Olmos A, Mangold AJ, Guglielmone AA (2005) A collection of ticks (Ixodidae) from wild birds in Uruguay. Exp Appl Acarol 36:325–331
Acknowledgments
This work was supported by FAPESP (grant to M.B.L. and scholarship to M.O. and A.U.), CNPq (Academic Career Scholarship to M.B.L.), and IPÊ—Institute for Ecological Research. We are deeply grateful to the farm owner, Group Martins, who turned our field work possible.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ogrzewalska, M., Uezu, A. & Labruna, M.B. Ticks (Acari: Ixodidae) infesting wild birds in the eastern Amazon, northern Brazil, with notes on rickettsial infection in ticks. Parasitol Res 106, 809–816 (2010). https://doi.org/10.1007/s00436-010-1733-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-010-1733-1