Abstract
Eimeria bovis infections commonly have clinical impact only on young animals, as homologous reinfections generally are under immunological control. So far, the nature of the immune responses delivering protection to calves has not been investigated. In this study we therefore analysed local and peripheral proliferative T cell activities of primary- and challenge-infected calves and investigated the occurrence of T cell phenotypes in the peripheral blood and in mucosal gut segments isolated either by bioptic means or by necropsies. We show that lymphocytes of E. bovis-infected calves exhibit effective, transient antigen-specific proliferative responses in the course of prepatency of primary infection but fail to react after homologous reinfection suggesting early abrogation of parasite development. Whilst in primary infection an expansion of peripheral CD4+ T cells was observed, reinfection had no effect on the proportions of CD4+, CD8+ subsets or γδTCR+ T cells. In contrast, both E. bovis primary and challenge infections had an impact on local tissue T cell distribution. Primary infection was characterised by a CD4+ T cell infiltration early in prepatency in ileum and later in colon mucosa, whereas CD8+ T cells were only found accumulating in the latter gut segment. Challenge infection led to infiltration of both CD4+ and CD8+ T cells in small intestine and large intestine segments indicating protective functions of both cell types. In contrast, infiltration of ileum and colon mucosa with γδTCR+ T cells was restricted to primary infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eimeria bovis infections cause important coccidian diseases of cattle severely affecting animal health and profitability of cattle industry (Daugschies et al. 1998; Daugschies and Najdrowski 2005; Fitzgerald 1980). Clinical symptoms related with bovine coccidiosis are usually restricted to primary-infected calves as they develop protective immunity against subsequent homologous infections.
In general, the termination of Eimeria spp. primary infections as well as the control of homologous reinfections rely on cellular adaptive immune reactions of the host (Wakelin and Rose 1990; Zahner et al. 1994). However, immunity against Eimeria spp. generally is species specific (Rose 1973; Rose 1987) or even strain specific (Fitz-Coy 1992; Martin et al. 1997; Norton and Hein 1976; Shirley and Bellatti 1988; Smith et al. 2002) and, in consequence, protective cross immunity is rare. So far, cellular immune responses against Eimeria spp. affecting cattle have scarcely been investigated. Considering the few studies available the first meront stage in E. bovis-infected calves—in contrast to primary E. zuernii infections (Stockdale 1977)—seem hardly associated with lymphocyte infiltration of the mucosa (Friend and Stockdale 1980). However, lymphocytes of E. bovis infected animals displayed enhanced antigen-specific proliferative activities (Hermosilla et al. 1999; Hughes et al. 1988; Hughes et al. 1989), whilst non-specific reactions towards a mitogen seemed not influenced by infection (Hermosilla et al. 1999). Data generated in rodent models or avian eimeriosis showed gut infiltration with both αβTCR+ T cells (Lillehoj 1994; Rothwell et al. 1995; Vervelde et al. 1996) and γδTCR+ T cells (Findly et al. 1993; Rothwell et al. 1995). Studies with αβTCR T cell deficient animals reveal these T cells as key actors (Roberts et al. 1996; Smith and Hayday 2000b), whilst γδTCR+ T cells seem of minor importance in the development of immunity (Roberts et al. 1996; Rose et al. 1996). It appears generally accepted, that primary Eimeria spp. infections are predominantly controlled by Th1-associated reactions of CD4+ T with whilst cytotoxic CD8+ T cells seem to be the major effector cell type against challenge infections (Findly et al. 1993; Ovington et al. 1995; Rose et al. 1992a; Shi et al. 2001; Smith and Hayday 2000b). However, data appear somewhat conflicting as depletion or defects of CD4+ T cells did not influence primary avian E. acervulina or murine E. papillata infections (Schito et al. 1998a; Trout and Lillehoj 1996) but seemed essential for protective immunity after challenge in murine E. vermiformis and E. papillata infections (Schito et al. 1998a; Smith and Hayday 1998). Both CD4+ and CD8+ T cell subsets were demonstrated to expand during primary E. bovis infection (Hermosilla et al. 1999) and recent data showing enhanced antigen-specific IFN-γ production in prepatent E. bovis infections in calves suggest Th1-dominated immune responses within this period of infection (Taubert et al. 2008).
In this work, we investigate T cell-mediated immune reactions of E. bovis primary-infected and challenged calves with respect to antigen-specific proliferative activities and infiltration of T cell subsets in the parasite affected mucosa. We show that T cells proliferate effectively during a restricted time span during prepatency of primary infection but fail to do so after challenge suggesting early abrogation of reinfection. In accordance, we demonstrate expansion of peripheral CD4+ T cells during primary infection whilst after challenge neither the proportions of CD4+ or CD8+ T cell subsets nor those of γδTCR+ T cells were influenced. Overall, analyses of T cell infiltration into parasitized gut mucosa suggest a major involvement of CD4+ T cells in the termination of primary infection and a role of both CD4+ and CD8+ T cells in the control of reinfections.
Materials and methods
Animals
Calves were purchased from a local farmer at the age of 2 weeks, treated with Baycox® (Bayer) and Halocur® (Intervet) in the second week after birth, tested for parasitic infections, and when deemed parasite free, maintained under parasite-free conditions in autoclaved stainless steel cages (Woetho) until experimental E. bovis infection. They were fed with milk substitute (Hemo Mischfutterwerke) and commercial concentrates (Raiffeisen). Water and sterilized hay were given ad libitum.
Parasite maintenance
The E. bovis strain H was maintained by passages in calves. For the production of oocysts, calves were infected at the age of ten weeks with 5 × 104 sporulated oocysts each. Excreted oocysts were isolated from the faeces beginning 19 days p.i. according to the method of Jackson (1964). Sporulation was achieved by incubation in a 2 % (w/v) potassium dichromate (Sigma) solution at room temperature. Sporulated oocysts were stored in this solution at 4°C until further use.
Sporozoites were excysted from sporulated oocysts as previously described (Hermosilla et al. 2002). For in vitro infections, bovine umbilical vein endothelial cells (BUVEC) isolated and grown to confluence in endothelial cell growth medium (Promocell) as described elsewhere (Taubert et al. 2006) were infected with freshly isolated E. bovis sporozoites (106 sporozoites/75 cm2 culture flask). Culture medium was changed 24 h p. i. and thereafter every second day. From day 18 p. i. onwards, E. bovis merozoites I were harvested from culture as previously described (Hermosilla et al. 2002).
Infections of animals, biopsies and necropsies
Four groups (A–D) of three calves each were used, aged 8–12 weeks. Groups A and B were orally infected on day 0 with 5 × 104 sporulated oocysts and challenged on day 48 with 3 × 104 sporulated oocysts. Group C experienced only the primary infection. Group D served as non-infected control. Shedding of oocysts was determined from day 18 p.i. onwards by daily faecal examination (McMaster technique).
Biopsies were performed on days 8 and 40 after primary and 8 days after challenge infection. Animals were sedated with xylazine (0.1 mg/kg, i. m., Rompun®, Bayer) and fixed in left lateral position. The incision site at the right abdominal wall (approximately one hand proximally to the tuber coxae) was infiltrated with procaine (2%, Procasel®, Selectavet). Calves were then anaesthetised with ketamine (3 mg/kg, i.v., Ursotamin®, Serumwerk Bernburg). Laparotomy followed standard surgery procedures. The Plica ileocaecalis of the ileum and the Ansa spiralis coli were advanced for withdrawal of the L. ileocaecales and L. colici, respectively. Lymph nodes were fixed with a clamp, removed and submitted to sterile medium (RPMI, 1% penicilline/streptomycine) for subsequent lymphocyte isolation. Draining vessels were ligated (Serafit®, Serag-Wiessner) and mesentery defects were closed. Gut segments (ileum on days 8 after primary and challenge infection, colon on day 40 after infection) were advanced and the content in the respective area was removed by massage. The gut was then fixed with a clamp and mucosal samples were taken using a biopsy punch (ø 8 mm, Stiefel Laboratorium GmbH) and submitted to sterile medium. The mucosal defect was closed according to standard surgical methods (Serafit®, Serag-Wiessner) and checked for closeness. After flushing (0.9% NaCl, 37°C), the gut was relocated and the wound was sutured. Calves were treated with flunixin-meglumin (2.2 mg/kg, i.v., Finadyne®, Essex) and procaine-penicillin G (0.1 ml/kg, s.c. for 5 days, Animedica) and monitored.
Calves of group A and B were necropsied on days 60 and 74, respectively, i.e., 12 and 26 days after challenge infection, those of group C 26 days p.i. and group D at a corresponding age to group B. Gut mucosal tissue samples (jejunum, ileum, caecum, colon) were withdrawn for immunohistochemical analyses. Lymph nodes (L. jejunales, L. ileocaecales and L. colici) were excised for immediate lymphocyte isolation.
Preparation and cryoconservation of gut wall samples
Intestinal biopsies and half of the gut samples isolated at necropsies were cryopreserved. The mucosal site of the sample was applied to an equally sized cube of bovine liver, covered with a drop of OCT reagent (Tissue-Tec®, Sankura Finetec Inc.) and cooled in 2-methylbutane (15 s in liquid nitrogen). The samples were wrapped in aluminum foil and stored at -80°C until further use.
The second half of the gut samples obtained at necropsies was fixed in 4% formaldehyde (Merck) in phosphate-buffered saline for 24 h, dehydrated and embedded in paraffin according to standard procedures.
E. bovis merozoite I antigen
E. bovis merozoites I collected from culture were homogenized by repeated freezing followed by sonication (20 kHz, 5 × 15 s pulses) on ice. After centrifugation (11,000 × g, 4°C, 20 min) the supernatants were passed through 0.2 µm sterile filters (Renner). Protein concentration was determined using the Bradford method (Bradford 1976). The E. bovis merozoite I antigen (EbAg) was stored at −80°C.
Isolation of peripheral blood mononuclear cells and lymphocytes
Calves were bled by puncture of the jugular vein on days 0, 4, 6, 8, 12, 15, 19, 26, 48, 49, 52, 54, 56 and 60 p.i. Blood was collected in 50 ml plastic tubes (Nunc) containing 0.1 ml heparin (Sigma). For peripheral blood mononuclear cells (PBMC) isolation, 20 ml of heparinized blood were mixed with equal parts of 0.9 % NaCl. Four ml of the mixture were applied on top of 3 ml Ficoll-Paque (density = 1.077 g/l, Biochrom) in glass tubes with an inner diameter of 12 mm. After centrifugation [45 min, 400 × g, room temperature (RT)] the PBMC layer was collected and the cells were washed three times (10 min, 400 × g, 4°C) in medium RPMI 1640. Viable cells (trypan blue, Sigma, exclusion test) were counted in a Neubauer chamber.
For preparation of lymph node cells, lymph nodes were cut into pieces and gently teased through sterile nylon sieves (meshes of 180 µm; Reichelt Chemietechnik) flotating in RPMI. After three washings (10 min, 400 × g, 4°C), the cells were suspended in RPMI, supplemented with 1% penicillin (Sigma), 5 mM glutamine (Gibco BRL), 10% foetal calf serum (FCS, Biochrom KG) and 1.7 µl/500 ml 2-mercaptoethanol (Serva). Viable cells (trypan blue exclusion test) were counted in a Neubauer chamber.
Cells were either subsequently used in lymphocyte proliferation assays or suspended in dimethylsulphoxide (1% final concentration, Merck) in RPMI supplemented with 10% FCS (Biochrom), pre-cooled (1 h, 4°C) and cryopreserved in liquid nitrogen until required for flow cytometry analyses.
In vitro stimulation of lymphocytes with EbAg and lymphocyte proliferation assays
Freshly isolated PBMC or lymph node cells were resuspended in culture medium (CM), composed of RPMI, 2 mM l-glutamine (Sigma), 0.22% NaCO3 (Merck), 1 mM 2-mercaptoethanol (Sigma), 200 UI/ml penicilline (Sigma), 50 µg/ml streptomycin (Sigma) and 10% FCS. Cells (2 × 105 lymphocytes/well, 96-well microtiter plates, Nunc) were stimulated either with EbAg (10 µg/ml, 96 h), Con A (5 µg/ml, Biochrom, 48 h) or plain RPMI. Cultures were incubated at 37°C, 5% CO2 atmosphere and thereafter pulsed for the final 16 h with 50 µl [3H] thymidine (0.5 µCi/ml, Amersham). Subsequently, cells were harvested on glass-fibre filters using a 96-well cell harvester (Skatron). After drying (60°C, 1 h), filters were saturated with scintillation fluid (Roth) and radioactivity was measured in a β-liquid scintillation counter (Tri-Carb 2700 TR, Packard Instruments).
Phenotypical characterization of T cells by fluorescence antibody cell sorting
Frozen PBMC and lymph node cells were rapidly thawn, suspended in V-shape bottomed 96-well microtiter plates (Nunc) at a density of 1 × 105 cells/well and washed three times with RPMI (10 min, 150 × g, 4°C). The cell pellet was suspended in 60 µl of monoclonal antibody solutions (IL-A 11, directed against bovine CD4; IL-A 105, directed against bovine CD8 or D86, directed against the bovine γδ+TCR; all antibodies were kindly donated by C. Menge, Giessen) and incubated for 30 min on ice. Thereafter, cells were washed in 150 µl PBS and incubated in 50 µl FITC-conjugated goat anti-mouse antibodies (1:200 in PBS, 20 min on ice; Dianova) supplemented with 1 µl propidiumiodide solution (2 µg/ml), washed twice in PBS and transferred to plastic test tubes (Renner) previously filled with 300 µl PBS. Immunofluorescence staining was measured using a Coulter Epics Elite-FACS (Coulter Electronic). Tests were performed in triplicates.
Immunohistology
For the detection of CD4+, CD8+ and WC1+ (=γδTCR+) T cells 4 µm cryo section were dried over night at room temperature on Superfrost plus object slides (Menzelgläser). Samples were fixed in ice cold acetone (10 min) and dried. Endogenous peroxidase was inactivated in 0.5 % H2O2 (30 min, RT, Roth). After five washings in TBS (5 min), samples were probed with primary monoclonal antibodies (anti-bovine CD4, CC30: 1:5; anti-bovine CD8, CC63: 1:200; anti-bovine WC1, CC15: 1: 100; all Serotec) for 1 h (37°C, humidity chamber). After rinsing the samples thrice in PBS, they were incubated in sheep anti-mouse antibodies conjugated with peroxidase (1:50, Amersham). Following three further washings in TBS (5 min), binding was visualised by adding substrate (0.048 g DAB, Fluka, and 800 µl 3% H2O2 in 80 ml imidazole buffer, 3–5 min). After rinsing three times in TBS (5 min) and once in aqua dest. (5 min), the tissue probes were counterstained for 15 s in Papanicolaou solution (1:10, Merck), washed in tap water (5 min), dehydrated according to standard histological procedures and mounted in Aquatex® (Merck).
Immunostained T cells were counted in ten randomly chosen vision fields (200 × magnification) placing the vision field in a way that one half comprised the tip and the other half the basis of a villus.
Statistical analysis
Statistical analyses used the programme package BMDP for XP, Release 8.1 (Dixon, 1993). For the description of the data arithmetical means were calculated. To describe the variability of the data standard deviations were used. As some statistical distributions of the original data were skewed to the right, if necessary, arc-sine or logarithmic transformation were performed to obtain an approximately normal distribution of the values. In accordance to the design of the experiments, data were compared by two or three-factorial analysis of variance with repeated measures (BMDP2V). Differences were regarded as significant at a level of p ≤ 0.05.
Results
E. bovis challenge-infected calves are immune
All E. bovis primary-infected calves shed oocysts beginning on 19 days p.i. (Fig. 1). There was a rapid increase in oocyst shedding from 20 days p.i. onwards with highest amounts found 21–24 days p.i. Thereafter, oocyst shedding rapidly decreased and ceased with 29 days p.i. Primary-infected calves were immune to challenge infection and hardly shed any oocysts (difference to primary infection: p < 0.0001). If at all, very few oocysts were found in the faeces of challenged animals from days 20 to 24 p.i. (Fig. 1).
Oocyst shedding of E. bovis primary- and challenge-infected calves. Calves (n = 3) were infected orally (5 × 104 sporulated oocysts of E. bovis/animal) and challenged after 48 days (3 × 104 sporulated oocysts of E. bovis/animal). Oocyst counts (oocysts per gram faeces, OpG) were determined by MacMaster technique
E. bovis primary-infected calves exhibit antigen-specific proliferative immune responses
PBMC of E. bovis primary-infected calves reacted to EbAg only during prepatency (Fig. 2) and exhibited a short-timed proliferative response on days 8–15 p.i. with maximum reactions on day 8 days p.i. Non-infected control animals as well as challenge calves failed to react to EbAg (difference to primary infection: p < 0.02; Fig. 2).
Antigen-specific proliferative responses of peripheral blood mononuclear cells isolated from E. bovis primary- and challenge-infected calves. Peripheral blood mononuclear cells, isolated from E. bovis-infected calves (n = 3, black squares) and non-infected control animals (n = 3, grey triangles), were stimulated in vitro with E. bovis merozoite I-antigen (10 µg/ml, 96 h). Proliferative T cell activity was measured by 3H thymidine incorporation. Arithmetical means and standard deviations. SI stimulation index
Enhanced proliferative activity of T lymphocytes in the peripheral blood corresponded to reactions found in draining lymph nodes. Thus, lymphocytes isolated from L. ileocaecales of primary-infected calves on day 8 p.i. showed signficantly enhanced antigen-specific T cell proliferation, but failed to do so 12 days p.i. or 8 days after challenge infection (Fig. 3). Lymphocytes isolated from L. colici 40 days after primary and 26 days after challenge infection also lacked antigen-specific proliferation (Fig. 3). Cells of non-infected controls never reacted to EbAg (differences between infected animals 8 days after primary infection and all other data: p < 0.0001 −0.001; Fig. 3).
Antigen-specific proliferative responses of lymphocytes isolated from L. ileocaecales and L. colici of E. bovis primary- and challenge-infected calves. Lymphocytes isolated from L. ileaocaecales and L. colici of E. bovis primary- and challenge-infected calves (n = 3, black columns) and non-infected control animals (n = 3, grey columns), were stimulated in vitro with E. bovis merozoite I-antigen (10 µg/ml, 96 h). Proliferative T cell activity was measured by 3H thymidine incorporation. Arithmetical means and standard deviations. SI = stimulation index
E. bovis infection does not modulate non-specific proliferative T cell reactions
PBMC or lymph node cells responded to stimulation with the mitogen Con A with significantly enhanced proliferative activity (p < 0.001). Responses varied irregularly in all groups regardless of E. bovis infections (data not shown).
E. bovis primary infection leads to expansion of peripheral CD4+ T cells whilst challenge infection fails to influence the composition of peripheral T cell phenotypes
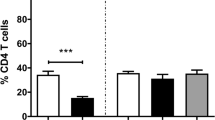
Phenotyping peripheral blood lymphocytes after primary infection revealed expansion of CD4+ T cells beginning 15 days p. i. (p < 0.0001), leading to a plateau-like time course from 20 days p.i. onwards (Fig. 4). Challenge infection did not influence the level of CD4+ T cells and the proportions of CD4+ T cells in the blood of reinfected animals remained stable (p < 0.02, Fig. 4).
T cell subpopulations in the peripheral blood of E. bovis primary- and challenge-infected calves. Peripheral blood mononuclear cells, isolated from E. bovis primary-infected and challenged calves (n = 3, black triangles) and non-infected control animals (n = 3, grey quarders), were probed with antibodies directed against bovine CD4, CD8 and WC1 (=γδTCR-specific) and analysed by flow cytometry. The time point of challenge infection (48 d p. i.) is indicated by an arrow. Arithmetical means and standard deviations
The proportions of CD8+ T cells did not expand after primary or challenge infection and, however, seemed to be reduced during prepatency of the primary infection when compared to non-infected control animals (p < 0.02, Fig. 4). Challenge infection did not influence the level of CD8+ T cells (Fig. 4).
The proportions of γδTCR+ T cells, as measured by the detection of WC1 on lymphocytes, appeared decreased throughout primary and challenge infection when compared to non-infected control animals. Thus, γδTCR+ T cells declined immediately after primary infection and remained on a constantly decreased level until challenge infection (Fig. 4). After challenge, the numbers of γδTCR+ T cells increased, however, equal changes were detected in the non-infected group (Fig. 4).
E. bovis primary and challenge infections induce local cellular immune responses
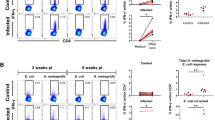
Biopsies were taken to analyze local tissue responses (infiltration of CD4+, CD8+ and γδTCR+ T cells) to meront I formation in the ileum at 8 days after primary and challenge infection and subsequent to oocyst excretion 40 days after primary infection in the colon. The data suggest slightly increased numbers of these cell types in the ileum at 8 days after primary and, more pronounced, after challenge infection (Fig. 5) although differences generally remained below a significant level. Only the numbers of CD4+ T cells in challenged animals differed significantly (p < 0.02) from those of non-infected controls. More distinct reactions were observed 40 days after primary infection in the colon (Fig. 6). CD4+ T cells (p < 0.02) and γδTCR+ T cells (p < 0.004) accumulated significantly when compared to non-infected controls. The numbers of CD8+ T cells seemed enhanced in infected animals, too, but appeared rather variable overall.
T cell subtypes in the ileum mucosa of non-infected, E. bovis primary- and challenge-infected calves. Ileum samples of non-infected, E. bovis primary-infected and challenged (each group: n = 3) calves were obtained by bioptic means at different time points p.i., fixed and subjected to immunohistological analyses using antibodies directed against bovine CD4, CD8 and WC1 (=γδ-TCR-specific). Arithmetical means and standard deviations
T cell subtypes in biopsies of colon mucosa of E. bovis primary and non-infected calves. Colon samples of non-infected and E. bovis primary-infected (each group: n = 3) calves were obtained by bioptic means at 40 days p.i., fixed and subjected to immunohistological analyses using antibodies directed against bovine CD4, CD8 and WC1 (=γδ-TCR-specific). Arithmetical means and standard deviations
Owing to technical reasons, analyses of tissues obtained by necropsies had to be restricted to samples isolated 26 days after primary and 12 and 26 days after challenge infection (Fig. 7). Overall, mucosal samples of challenged calves showed significant more CD4+ T cells in the various gut segments (p < 0.005–0.05) than those of primary-infected animals. There were, however, no significant differences between animals necropsied 12 and 26 days after challenge infection. Data depicted in Fig. 7 suggest a similar tendency for CD8+ T cells, but significant differences between primary- and challenge-infected calves were restricted to the jejunum (p < 0.05). γδTCR+ T cell contents did not differ significantly between the groups and gut sections (Fig. 7).
T cell subtypes in intestine mucosa of E. bovis primary- and challenge-infected calves. Different groups (n = 3, each) of E. bovis primary-infected or challenged calves were killed at different days p.i. Different gut mucosa samples (black column, jejunum; dark grey column, ileum; bright grey column, caecum; white column, colon) were fixed and subjected to immunohistological analyses using antibodies directed against bovine CD4, CD8 and WC1 (γδ-TCR-specific). Arithmetical means and standard deviations
Discussion
Infections of calves with suitable doses of E. bovis oocysts resulted in rapidly increasing, approximately one week lasting oocyst excretion as observed previously (Hermosilla et al. 1999). Infected animals developed effective protective immunity to homologous reinfection in accordance to Fiege et al. (1992). We furthermore show that primary infection is associated with a transient antigen-specific proliferation of PBMC during prepatency, which is in agreement with previous reports of Hermosilla et al. (1999). Transient antigen-specific proliferative activities of T cells were also reported for E. vermiformis-infected BALB/c mice (Wakelin et al. 1993) and E. tenella-infected chickens (Breed et al. 1996) in prepatency and patency, respectively. The proliferative activity was limited to the prepatency starting after day 6 p.i. and leading to peak activity on day 8 p.i., which means that the onset of these reactions coincides with the beginning of parasite proliferation. Interestingly, this time frame also overlaps with the first-time appearance of parasite-specific antigens on the surface of infected host cells (Badawy et al. 2010). Given that the host cells of E. bovis during first merogony, endothelial cells, are, in principle, capable of antigen presentation (Behling-Kelly and Czuprynski 2007; Bosse et al. 1993; Knolle 2006; Wagner et al. 1984) and can activate T cells in an antigen-dependent manner (Epperson and Pober 1994; Pober and Cotran 1991; Rodig et al. 2003), these data may suggest early meront I-induced T cell reactivity.
Peripheral antigen-specific proliferative activities during prepatency coincided with respective responses of lymphocytes isolated from the draining lymph node (L. ileocaecales) by bioptic means and with enhanced antigen-specific IFN-γ production (Taubert et al. 2008) suggesting an overall pattern of Th1 activity in this early phase of infection.
The rapid decline of peripheral and local T cell reactivity following 8 days p.i. may form part of mechanisms allowing long term development of macromeronts in the endothelial host cell. Interestingly, in animals infected with other Eimeria spp. that replicate much faster and do not develop macromeronts, e. g. in E. intestinalis-infected rabbits, antigen-specific proliferative responses of lymphocytes indeed also coincided with the onset of meront formation, but, in contrast, were even enhanced with ongoing infection (Renaux et al. 2003).
The failure of lymphocytes to proliferate in response to antigenic stimulation after challenge infection appears surprising at a first view, although it is in concert with other studies describing an impaired antigen-specific lymphocyte proliferative activity after E. papillata (Schito et al. 1998b) and E. vermiformis (Wakelin et al. 1993) challenge infections in mice. It may be explained, however, by the fact that sporozoites or early intracellular stages represent the targets of protective immune effects in Eimeria spp. infections (Rose et al. 1992b; Shi et al. 2001) resulting in a lack of T cell-stimulating early meronts.
T cell subsets in primary infection were dominated by CD4+ T cells which expanded beginning during late prepatency/early patency and remained on an elevated level throughout the observation period. According to these findings and in agreement with reports in other Eimeria spp. infections (Rothwell et al. 1995; Shi et al. 2001; Vervelde et al. 1996), immunohistological analyses of parasitized gut mucosa revealed infiltration of CD4+ T cells early after infection (8 days p. i.) in the ileum and later after infection (40 days p.i.) in the colon. The weak expansion of CD8+ T cells observed in the peripheral blood of E. bovis-infected calves by Hermosilla et al. (1999) could not be verified in the present study. Proportions of CD8+ T cells even decreased during prepatency in primary-infected calves and returned to the level of non-infected controls during patency. Accordingly, counts of CD8+ T cells were not significantly elevated in the ileum mucosa at day 8 p.i. However, the late CD8+ T cell infiltration of the colon mucosa after all suggests an expansion of this T cell subset.
Although challenge infection was neither associated with antigen-specific T cell proliferation nor with significant CD4+ and CD8+ subset expansion in peripheral blood, both subpopulations were found enhanced in the mucosa of the small and large intestine of challenge infected animals when compared with the situation after primary infection. Again, infiltration with CD4+ T cells was detected earlier after reinfection on day 8 post-challenge than accumulation of the CD8+ subset, which was firstly found at 12 days after challenge. Whilst CD8+ T cells have often been assumed to represent the key cell type for control of challenge infections (Rose et al. 1992a; Shi et al. 2001; Trout and Lillehoj 1995; Trout and Lillehoj 1996), only some reports point at a potential role of CD4+ T cells in protective immune effects against challenge infections with Eimeria spp. (Schito et al. 1998a; Smith and Hayday 1998). However, both subsets are known to exhibit effective cytotoxicity against intracellular apicomplexa (Denkers et al. 1993; Kasper et al. 1992; Khan and Kasper 1996; Staska et al. 2003) and may therefore both be considered as important cell types for the control of E. bovis challenge.
Immunhistological analyses also revealed an infiltration of γδTCR+ T cells during primary E. bovis infection in the ileum and colon mucosa, while they did not accumulate in the mucosal tissue after challenge infection. Gut infiltration by γδTCR+ T cells was also observed in primary avian and murine Eimeria infections (Findly et al. 1993; Rothwell et al. 1995). Considering that these cells contribute to 40% or more of PBMC in young calves (Wilson et al. 1996) this cell type may be of particular importance in cattle. γδTCR+ T cells have a wide range of functions, such as immunomodulation, cytokine production, cytotoxicicty and the regulation of inflammatory processes (reviewed by Pollock and Welsh 2002). Due to their general accumulation in the gut mucosa, a particular sentinel function is attributed to this cell type (De Libero 1997). However, the precise role of γδTCR+ T cells in Eimeria infections is still uncertain. On the one hand, Rose et al. (1996) and Roberts et al. (1996) did not find a protective role of γδTCR+ T cells in Eimeria vermiformis infections of mice, on the other hand Smith and Hayday (2000a) report on a higher susceptibility of E. vermiformis-infected αβTCR−/γδTCR− mice to homologous challenge infection compared to αβTCR- controls. Independent from this question, however, the anti-inflammatory efficacy of γδTCR+ T cells seems to play a role at least in murine coccidiosis as γδTCR-deficient mice showed strongly escalated intestinal damage after primary E. vermiformis infection when compared with wildtype controls (Roberts et al. 1996).
In conclusion, our data show distinct antigen-specific proliferative T cell activities with exclusive reactions occurring during primary E. bovis infection and a failure after reinfection indicating early abrogation of parasite development after challenge. Primary infection is characterized by an early CD4+ T cell infiltration into the intestine, whilst both CD4+ and CD8+ T cells accumulate in intestine mucosa of challenged animals. In contrast, gut infiltration with γδTCR+ T cells was restricted to primary infection. Overall, these data promote a better understanding of peripheral and local adaptive cellular immune responses of E. bovis infections and call for functional analyses of T cell subsets in ruminant Eimeria reinfections.
References
Badawy AI, Lutz K, Taubert A, Zahner H, Hermosilla C (2010) Eimeria bovis meront I-carrying host cells express parasite-specific antigens on their surface membrane. Vet Res Commun, doi:10.1007/s11259-009-9336-y
Behling-Kelly E, Czuprynski CJ (2007) Endothelial cells as active participants in veterinary infections and inflammatory disorders. Anim Health Res Rev 8:47–58
Bosse D, George V, Candal FJ, Lawley TJ, Ades EW (1993) Antigen presentation by a continuous human microvascular endothelial cell line, HMEC-1, to human T cells. Pathobiology 61:236–238
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Breed DG, Dorrestein J, Vermeulen AN (1996) Immunity to Eimeria tenella in chickens: phenotypical and functional changes in peripheral blood T-cell subsets. Avian Dis 40:37–48
Daugschies A, Najdrowski M (2005) Eimeriosis in cattle: current understanding. J Vet Med B Infect Dis Vet Public Health 52:417–427
Daugschies A, Bürger HJ, Akimaru M (1998) Apparent digestibility of nutrients and nitrogen balance during experimental infection of calves with Eimeria bovis. Vet Parasitol 77:93–102
De Libero G (1997) Sentinel function of broadly reactive human gamma delta T cells. Immunol Today 18:22–26
Denkers EY, Sher A, Gazzinelli RT (1993) CD8+ T-cell interactions with Toxoplasma gondii: implications for processing of antigen for class-I-restricted recognition. Res Immunol 144:51–57
Epperson DE, Pober JS (1994) Antigen-presenting function of human endothelial cells. Direct activation of resting CD8 T cells. J Immunol 153:5402–5412
Fiege N, Klatte D, Kollmann D, Zahner H, Bürger HJ (1992) Eimeria bovis in cattle: colostral transfer of antibodies and immune response to experimental infections. Parasitol Res 78:32–38
Findly RC, Roberts SJ, Hayday AC (1993) Dynamic response of murine gut intraepithelial T cells after infection by the coccidian parasite Eimeria. Eur J Immunol 23:2557–2564
Fitz-Coy SH (1992) Antigenic variation among strains of Eimeria maxima and E. tenella of the chicken. Avian Dis 36:40–43
Fitzgerald PR (1980) The economic impact of coccidiosis in domestic animals. Adv Vet Sci Comp Med 24:121–143
Friend SC, Stockdale PH (1980) Experimental Eimeria bovis infection in calves: a histopathological study. Can J Comp Med 44:129–140
Hermosilla C, Bürger HJ, Zahner H (1999) T cell responses in calves to a primary Eimeria bovis infection: phenotypical and functional changes. Vet Parasitol 84:49–64
Hermosilla C, Barbisch B, Heise A, Kowalik S, Zahner H (2002) Development of Eimeria bovis in vitro: suitability of several bovine, human and porcine endothelial cell lines, bovine fetal gastrointestinal, Madin-Darby bovine kidney (MDBK) and African green monkey kidney (VERO) cells. Parasitol Res 88:301–307
Hughes HP, Thomas KR, Speer CA (1988) Antigen-specific lymphocyte transformation induced by oocyst antigens of Eimeria bovis. Infect Immun 56:1518–1525
Hughes HP, Whitmire WM, Speer CA (1989) Immunity patterns during acute infection by Eimeria bovis. J Parasitol 75:86–91
Jackson AR (1964) The isolation of variable coccidial sporozoites. Parasitology 54:87–93
Kasper LH, Khan IA, Ely KH, Buelow R, Boothroyd JC (1992) Antigen-specific (p30) mouse CD8+ T cells are cytotoxic against Toxoplasma gondii-infected peritoneal macrophages. J Immunol 148:1493–1498
Khan IA, Kasper LH (1996) IL-15 augments CD8+ T cell-mediated immunity against Toxoplasma gondii infection in mice. J Immunol 157:2103–2108
Knolle PA (2006) Cognate interaction between endothelial cells and T cells. Results Probl Cell Differ 43:151–173
Lillehoj HS (1994) Analysis of Eimeria acervulina-induced changes in the intestinal T lymphocyte subpopulations in two chicken strains showing different levels of susceptibility to coccidiosis. Res Vet Sci 56:1–7
Martin AG, Danforth HD, Barta JR, Fernando MA (1997) Analysis of immunological cross-protection and sensitivities to anticoccidial drugs among five geographical and temporal strains of Eimeria maxima. Int J Parasitol 27:527–533
Norton CC, Hein HE (1976) Eimeria maxima: a comparison of two laboratory strains with a fresh isolate. Parasitology 72:345–354
Ovington KS, Alleva LM, Kerr EA (1995) Cytokines and immunological control of Eimeria spp. Int J Parasitol 25:1331–1351
Pober JS, Cotran RS (1991) Immunologic interactions of T lymphocytes with vascular endothelium. Adv Immunol 50:261–302
Pollock JM, Welsh MD (2002) The WC1(+) gammadelta T-cell population in cattle: a possible role in resistance to intracellular infection. Vet Immunol Immunopathol 89:105–114
Renaux S, Quere P, Buzoni-Gatel D, Sewald B, Le VY, Coudert P, Drouet-Viard F (2003) Dynamics and responsiveness of T-lymphocytes in secondary lymphoid organs of rabbits developing immunity to Eimeria intestinalis. Vet Parasitol 110:181–195
Roberts SJ, Smith AL, West AB, Wen L, Findly RC, Owen MJ, Hayday AC (1996) T-cell alpha beta + and gamma delta + deficient mice display abnormal but distinct phenotypes toward a natural, widespread infection of the intestinal epithelium. Proc Natl Acad Sci U S A 93:11774–11779
Rodig N, Ryan T, Allen JA, Pang H, Grabie N, Chernova T, Greenfield EA, Liang SC, Sharpe AH, Lichtman AH, Freeman GJ (2003) Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur J Immunol 33:3117–3126
Rose ME (1973) Immunity. In: Hammond DM, Long PL (eds) The coccidia. University Park Press and Butterworth, Baltimore, pp 295–341
Rose ME (1987) Immunity to Eimeria infections. Vet Immunol Immunopathol 17:333–343
Rose ME, Hesketh P, Wakelin D (1992a) Immune control of murine coccidiosis: CD4+ and CD8+ T lymphocytes contribute differentially in resistance to primary and secondary infections. Parasitology 105(Pt 3):349–354
Rose ME, Millard BJ, Hesketh P (1992b) Intestinal changes associated with expression of immunity to challenge with Eimeria vermiformis. Infect Immun 60:5283–5290
Rose ME, Hesketh P, Rothwell L, Gramzinski RA (1996) T-cell receptor gamma–delta lymphocytes and Eimeria vermiformis infection. Infect Immun 64:4854–4858
Rothwell L, Gramzinski RA, Rose ME, Kaiser P (1995) Avian coccidiosis: changes in intestinal lymphocyte populations associated with the development of immunity to Eimeria maxima. Parasite Immunol 17:525–533
Schito ML, Chobotar B, Barta JR (1998a) Major histocompatibility complex class I- and II-deficient knock-out mice are resistant to primary but susceptible to secondary Eimeria papillata infections. Parasitol Res 84:394–398
Schito ML, Chobotar B, Barta JR (1998b) Cellular dynamics and cytokine responses in BALB/c mice infected with Eimeria papillata during primary and secondary infections. J Parasitol 84:328–337
Shi M, Huther S, Burkhardt E, Zahner H (2001) Lymphocyte subpopulations in the caecum mucosa of rats after infections with Eimeria separata: early responses in naive and immune animals to primary and challenge infections. Int J Parasitol 31:49–55
Shirley MW, Bellatti MA (1988) Live attenuated coccidiosis vaccine: selection of a second precocious line of Eimeria maxima. Res Vet Sci 44:25–28
Smith AL, Hayday AC (1998) Genetic analysis of the essential components of the immunoprotective response to infection with Eimeria vermiformis. Int J Parasitol 28:1061–1069
Smith AL, Hayday AC (2000a) An alphabeta T-cell-independent immunoprotective response towards gut coccidia is supported by gammadelta cells. Immunology 101:325–332
Smith AL, Hayday AC (2000b) Genetic dissection of primary and secondary responses to a widespread natural pathogen of the gut, Eimeria vermiformis. Infect Immun 68:6273–6280
Smith AL, Hesketh P, Archer A, Shirley MW (2002) Antigenic diversity in Eimeria maxima and the influence of host genetics and immunization schedule on cross-protective immunity. Infect Immun 70:2472–2479
Staska LM, McGuire TC, Davies CJ, Lewin HA, Baszler TV (2003) Neospora caninum-infected cattle develop parasite-specific CD4+ cytotoxic T lymphocytes. Infect Immun 71:3272–3279
Stockdale PH (1977) The pathogenesis of the lesions produced by Eimeria zuernii in calves. Can J Comp Med 41:338–344
Taubert A, Zahner H, Hermosilla C (2006) Dynamics of transcription of immunomodulatory genes in endothelial cells infected with different coccidian parasites. Vet Parasitol 142:214–222
Taubert A, Hermosilla C, Sühwold A, Zahner H (2008) Antigen-induced cytokine production in lymphocytes of Eimeria bovis primary and challenge infected calves. Vet Immunol Immunopathol 126:309–320
Trout JM, Lillehoj HS (1995) Eimeria acervulina infection: evidence for the involvement of CD8+ T lymphocytes in sporozoite transport and host protection. Poult Sci 74:1117–1125
Trout JM, Lillehoj HS (1996) T lymphocyte roles during Eimeria acervulina and Eimeria tenella infections. Vet Immunol Immunopathol 53:163–172
Vervelde L, Vermeulen AN, Jeurissen SH (1996) In situ characterization of leucocyte subpopulations after infection with Eimeria tenella in chickens. Parasite Immunol 18:247–256
Wagner CR, Vetto RM, Burger DR (1984) The mechanism of antigen presentation by endothelial cells. Immunobiology 168:453–469
Wakelin D, Rose ME (1990) Immunity to coccidiosis. In: Long PL (ed) Coccidiosis of man and domestic animals. CRC Press, Florida, pp 281–306
Wakelin D, Rose ME, Hesketh P, Else KJ, Grencis RK (1993) Immunity to coccidiosis: genetic influences on lymphocyte and cytokine responses to infection with Eimeria vermiformis in inbred mice. Parasite Immunol 15:11–19
Wilson RA, Zolnai A, Rudas P, Frenyo LV (1996) T-cell subsets in blood and lymphoid tissues obtained from fetal calves, maturing calves, and adult bovine. Vet Immunol Immunopathol 53:49–60
Zahner H, Homrighausen-Riester C, Bürger HJ (1994) Eimeriosen. In: Röllinghoff M, Rommel M (eds) Immunologische und molekulare Parasitologie. Gustav Fischer Verlag, Jena, Stuttgard, pp 67–82
Acknowledgements
We acknowledge B. Hofmann and C. Scheld for their excellent technical assistance in cell culture. We also thank K. Failing (Giessen) for support in statistical analyses of the data. This work was supported by the German Research Foundation (DFG; project Za 67/6-1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sühwold, A., Hermosilla, C., Seeger, T. et al. T cell reactions of Eimeria bovis primary- and challenge-infected calves. Parasitol Res 106, 595–605 (2010). https://doi.org/10.1007/s00436-009-1705-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-009-1705-5