Abstract
The histopathological effects of Zygocotyle lunata, Echinostoma trivolvis, and Ribeiroia ondatrae in naturally infected Helisoma trivolvis were studied in hematoxylin and eosin sections of infected digestive glands fixed in 10% neutral buffered formalin. The larval stages of all three trematodes damaged the snail digestive gland. Most notable histopathology included disrupted digestive gland tubules, lysed cells, compressed tubules, and edematous spaces between tubules. Considerable damage was done by rediae ingesting digestive cells. There was a detectable hemocytic response by H. trivolvis in response to the rediae and cercariae of Z. lunata. Histochemical studies on sectioned material stained with the Ayoub-Shklar method for keratin detected the presence of this protein in the rediae and cercariae of Z. lunata and R. ondatrae. The presence of keratin is probably related to its role in cercarial encystment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Various histopathological investigations have been made on digenean larvae in their molluscan hosts (James 1965; Wright 1966; Reader 1971; Huffman and Fried 1985). Previous studies have been concerned with larval trematode infections in various molluscan hosts (Hurst 1927; Pratt and Barton 1941; Cheng 1963a, b; Porter et al. 1967), but none has examined the relationship between Helisoma trivolvis (also known as Planorbella trivolvis) and its larval trematodes. H. trivolvis, a ubiquitous planorbid in North America, is infected with numerous species of larval trematodes (see review in Klockars et al. 2007). Of the larval trematodes reported in H. trivolvis, three occur in NJ and PA (Schmidt and Fried 1997; Klockars et al. 2007). These are Echinostoma trivolvis, Ribeiroia ondatrae, and Zygocotyle lunata. Some incidental information on the histopathology of these species was provided by Huffman and Fried (1990) for E. trivolvis, by Johnson et al. (2004) for R. ondatrae, and by Willey (1941) for Z. lunata; none of the aforementioned papers has detailed the histopathology and cellular reaction in the digestive gland of H. trivolvis in response to daughter rediae and cercariae of these larval trematodes. The purpose of our study is to provide information on the histopathological effects of these trematodes on the digestive gland of H. trivolvis. Incidental to the histopathology study, we made observations on the histochemical presence of keratin in the intramolluscan larval stages, and we report these observations herein. The role of keratin in the intramolluscan larval stages of trematodes is uncertain, although this protein may play a role in cercarial encystment.
Materials and methods
Specimens of H. trivolvis were collected from Delaware Pond, Warren County, NJ, USA (40° 55′ 19.1″ N; 75° 03′ 49.5″ W). The geographic coordinates of the collection site were determined by a global positioning system (Garmin eTrex Legend). Snails were collected by hand from the perimeter of the pond, no more than 1.5 m from the edge. The snails were isolated to determine infection with larval trematodes within 48 h of collection by placing them in individual wells in plastic multiwell trays (Costar Corporation, Cambridge, MA, USA) containing 3 ml of natural spring water in each well. Two 50-W bulbs were placed approximately 30 cm from the trays to maintain the snails at 28–29°C. The trays were examined up to 6 h after snail isolation for cercariae. Live cercariae were examined unstained or stained with 0.01% neutral red. Cercariae of E. trivolvis and Z. lunata were identified by one of us (BF), who has worked with these cercariae for many years. The gymnocephalus cercaria was identified as R. ondatrae by its unique morphological characteristics (Johnson et al. 2004) and by molecular analysis (Wilson et al. 2005). DNA was extracted from the cercariae using the MoBio Ultraclean Tissue DNA Isolation Kit (MoBio Laboratories Inc., Carlsbad, CA, USA). The intertranscribed spacer region 2 (ITS-2) of the ribosomal gene complex was targeted using the primer set 3 S (5′ GGT ACC GGT GGA TCA CGT GGC TAG TG 3′; Bowles et al. 1995) and ITS2.2 (5′ CCT GGT TAG TTT CTT TTC CTC CGC 3′ Hugall et al. 1999) as described in Wilson et al. (2005). Promega Master Mix (Promega Corporation, Madison WI, USA) was used for all polymerase chain reactions (PCR) at 25-µl reaction volume with template and primer concentrations based on manufacturer’s recommendations. PCR product was visualized using a 3% agarose gel for the presence of a 429 bp. The PCR product was sequenced using the Big Dye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Forest City, CA, USA) on the AB 3130 Genetic Analyzer (Applied Biosystems, Forest City, CA, USA), following the manufacturer’s protocol. The sequences were compared to GenBank sequences using the basic local alignment tool (BLAST; Altschul et al. 1990) based on the nucleotide–nucleotide BLAST search (blastn) with the default settings. BioEdit Sequence Alignment Editor was used to directly compare the project sequences to R. ondatrae ITS-2 sequences from GenBank (Hall 1999). The sequences were found to be identical to the ITS-2 region of R. ondatrae (Wilson et al. 2005).
Twenty snails were divided into four groups with five snails per group. Three groups contained snails infected with either E. trivolvis, Z. lunata, or R. ondatrae, and the fourth contained snails that were not infected with larval trematodes. All infected snails showed patent larval trematode infections and snails with prepatent infections were not used. During the course of this study, histopathological and histochemical observations were made on tissue sections from most snails in all four groups. Parasitized and nonparasitized snails were removed from their shells and fixed in 10% neutral buffered formalin. For the histopathological studies, tissues were dehydrated in an alcohol series, embedded in paraffin, sectioned at 6 μm, and stained with hematoxylin and eosin. To determine the histochemical presence of keratin, the tissues were dehydrated in an alcohol series, embedded in paraffin, sectioned at 6 μm, and stained with the Ayoub-Shklar method for keratin (Luna 1968).
Tissue damage was evaluated according to criteria established by Malek and Cheng (1974), including mechanical compression and lysis. Cell types were characterized using the criteria of Cheng (1983), granulocytes (variable in size from 12 to 25 μm, with a low nucleus to cytoplasm ratio and the presence of numerous large granules), and hyalinocytes (small, 6–13 um, cells with a high nucleus to cytoplasm ratio, and few granules).
Results
The uninfected digestive gland of H. trivolvis was brown in color and located in the posterior third of the snail. Just beyond the digestive gland was the gonad. However, the gonads of the infected snails were riddled with the intramolluscan stages of the three species of larval trematodes (parasitic castration), and gonadal tissue was seldom apparent. Hence, histopathological observations on the gonads were not made. The digestive gland of the uninfected snails contained numerous tubules surrounded by loose connective tissue containing the visceral hemocoelic space. The tubules were separated from the hemocoel by a thin layer of loose connective tissue and lined with glandular epithelium (Fig. 1). The epithelium was composed of two cell types, serous and mucous cells, and the serous cells were triangular. The cytoplasm at the base of each cell was basophilic, and the nucleus was round and situated close to the base. The cytoplasm toward the apex of the serous cells contained eosinophilic granules. The mucous cells were columnar with flattened nuclei which were crowded against the base of the cells. There was less basophilia at the base of these cells than in serous cells. The periphery of the digestive gland contained muscle. A membrane, comprised of a single squamous epithelium layer overlying a connective tissue layer, enclosed the digestive gland.
The digestive glands of H. trivolvis infected with the rediae of E. trivolvis, Z. lunata, and R. ondatrae were mottled with these intramolluscan stages, and the characteristic appearance of each species of redia was readily apparent in the digestive gland. Gross observations of the rediae of E. trivolvis were given in Huffman and Fried (1990) of the rediae of R. ondatrae in Johnson et al. (2004), and of Z. lunata rediae in Willey (1941).
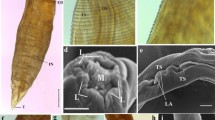
Z. lunata infection of H. trivolvis caused a disruption of the digestive glands; mechanical compression and rupture of the digestive cells were also evident (Fig. 2). A hemocytic infiltration of host tissues surrounding the larval parasites was present. There was no rupture of the tunica propria. The rediae of E. trivolvis were surrounded by clear zones devoid of cells (Fig. 3a). There was a detectable hemocytic response on the part of the molluscan host to the presence of the rediae. Lysis of host cells, mechanical compression, and displacement were seen in the digestive cells (Fig. 3b). The digestive glands were completely disrupted. No rupture of the tunica propria was seen. The rediae of R. ondatrae induced a hemocytic response. Mechanical compression and displacement of host tissues and lysis of cells were also observed (Fig. 4). No rupture of the tunica propria was seen.
Digestive gland cells in both infected and uninfected snails contained keratin. A heavy keratin response occurred in the rediae and cercariae of Z. lunata; a moderate response was present in R. ondatrae rediae and cercariae; no keratin was present in E. trivolvis rediae or cercariae.
Discussion
Despite numerous studies of the gastropod digestive gland, there is still uncertainty as to the terminology and classification of cell types. Pan (1958) described three cell types in the digestive gland of Biomphalaria glabrata: goblet, lime, and digestive. Porter et al. (1967) recognized two types in Oxytrema siliqua: liver and calcium cells. Reader (1971) recognized three types in Bithynia tentaculata: absorptive, mucous, and basophilic cells.
Mucous and secretory cells were present in H. trivolvis. The epithelial cells of the digestive gland in this gastropod have morphological characteristics typical of mucous and serous secreting columnar epithelium. These two morphologically distinct types are further differentiated on the basis of their cytoplasmic constituents.
Numerous studies have been made on the destruction of molluscan digestive glands by larval trematodes (Hurst 1927; Pratt and Barton 1941; Porter et al. 1967; Reader 1971; Huffman and Fried 1985). In the present study, we noted certain histopathological effects of the rediae and cercariae of Z. lunata, E. trivolvis, and R. ondatrae on the digestive gland of H. trivolvis.
One difficulty in studying naturally infected snails is that the age of the infection cannot be determined nor can the sequence of the pathology. The primary method of cell destruction in the digestive glands by the three species of trematodes we studied appeared to be through ingestion of host cells. Cheng (1963b) observed similar changes in H. trivolvis infected with Echinoparyphium sp. rediae. All species of rediae in our study exerted mechanical pressure on the digestive gland tubules as evidenced by constricted tubular lumina. It is also possible that the excretory products of the rediae had a lytic effect on host tissue, as evidenced by the clear zones surrounding the parasite. The clear zones were devoid of cells and were probably edematous.
Many workers have noted little or no cellular response to trematode larvae in molluscan hosts (Cheng 1963a; Cheng and Burton 1965; James 1965). Mechanisms by which larval trematodes inhibit the ability of the mollusk to recognize them as foreign are still poorly understood. In the present study, a hemocytic response was noted. Z. lunata elicited a distinct hemocytic response in H. trivolvis. Borges et al. (1998) reported the presence of amebocytes (hemocytes) in the ovotestis and tubular kidney of B. glabrata snails in response to Schistosoma mansoni. In contrast to the work of Borges et al. (1998), most other studies have reported little or no cellular response to larval trematodes in their molluscan hosts (Cheng 1963a; James 1965).
The primary function of keratin, a fibrous protein, is to protect epithelial cells from mechanical and nonmechanical stresses. Other functions include a stress response and apoptosis, as well as unique roles that are keratin specific and tissue specific (Coulombe and Omary 2002). Keratin was found within the rediae and cercariae of Z. lunata and R. ondatrae but not in E. trivolvis. This protein was reported by Dixon (1965) in the metacercarial cyst wall of Fasciola hepatica and in the rediae and cercariae of Philophthalmus megalurus by Huffman and Fried (1985). The latter two parasites encyst on vegetation or on the surfaces of other substrates. Z. lunata also encysts on vegetation and shell surfaces, and R. ondatrae encysts in the lateral line of fish or in the limb buds of ranid tadpoles; E. trivolvis encysts in the kidneys of snails and ranid tadpoles. The presence of keratin in the larval stages of Z. lunata and R. ondatrae may indicate that this protein is involved in cercarial encystment of these two digeneans.
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Borges CMC, de Souza CP, Andrade ZA (1998) Histopathologic features associated with susceptibility and resistance of Biomphalaria snails to infection with Schistosoma mansoni. Mem Inst Oswaldo Cruz 93:117–121
Bowles J, Blair D, McManus DP (1995) A molecular phylogeny of the human schistosomes. Mol Phylogenet Evol 4:103–109
Cheng TC (1963a) Histological and histochemical studies on the effects of parasitism of Musculum partumeium (Say) by the larvae of Gorgodera amplicava Loss. Proc Helminthol Soc Wash 30:101–107
Cheng TC (1963b) The effects of Echinoparyphium larvae on the structure and glycogen deposition in the hepatopancreas of Helisoma trivolvis and glycogenesis in the parasite larvae. Malacologia 1:291–303
Cheng TC (1983) Internal defense mechanisms of mollusks against invading microorganisms: personal reminiscences. Trans Am Microsc Soc 102:185–193
Cheng TC, Burton RW (1965) Relationships between Bucephalus sp. and Crassostrea virginica: histopathology and sites of infection. Chesapeake Sci 6:3–16
Coulombe PA, Omary MB (2002) ‘Hard’ and ‘soft’ principles defining the structure, function and regulation of keratin intermediate filaments. Curr Opin Cell Biol 14:110–122
Dixon KE (1965) The structure and histochemistry of the cyst wall of the metacercariae of Fasciola hepatica L. Parasitology 55:215–226
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Huffman JE, Fried B (1985) Histopathological and histochemical effects of larval trematodes in Goniobasis virginica (Gastropoda: Pleuroceridae). The Veliger 27:273–281
Huffman JE, Fried B (1990) Echinostoma and echinostomiasis. Adv Parasitol 29:215–269
Hugall A, Stanton J, Moritz C (1999) Reticulate evolution and the origins of ribosomal internal transcribed spacer diversity in apomictic Meloidogyne. Mol Biol Evol 16:157–164
Hurst CT (1927) Structural and functional changes produced in the gastropod mollusk, Physa occidentalis, in the case of parasitism by the larvae of Echinostoma revolutum. Univ Calif Pub Zool 29:321–404
James BL (1965) The effects of parasitism by larval Digenea on the digestive gland of the intertidal prosobranch, Littorina saxatilis (Olivi) subsp. tenebrosa (Montagu). Parasitology 55:93–115
Johnson PTJ, Sutherland DR, Kinsella JM, Lunde KB (2004) Review of the trematode genus Ribeiroia (Psilostomidae): ecology, life history, and pathogenesis with special emphasis on the amphibian malformation problem. Adv Parasitol 57:191–253
Klockars J, Huffman JE, Fried B (2007) Survey of seasonal trematode infections in Helisoma trivolvis (Gastropoda) from lentic ecosystems in New Jersey, U.S.A. Comp Parasitol 74:75–80
Luna LG (1968) Manual of histologic staining methods of the Armed Forces Institute of Pathology. McGraw-Hill, New York
Malek EA, Cheng TC (1974) Medical and economic malacology. Academic, NY, p 398
Pan CT (1958) The general histology and topographic microanatomy of Australorbis glabratus. Bull Mus Comp Zool 119:237–299
Porter CA, Pratt I, Owczarzak A (1967) Histopathological and histochemical effects of the trematode Nanophyetus salmincola (Chapin) on the hepatopancreas of its snail host, Oxytrema siliqua (Gould). Trans Am Microsc Soc 86:232–239
Pratt I, Barton CD (1941) The effects of four species of larval trematodes upon the liver and ovotestis of the snail, Stagnicola emarginata angulata (Sowerby). J Parasitol 27:284–288
Reader TAJ (1971) The pathological effects of sporocycts, rediae and metacercariae on the digestive gland of Bithynia tentaculata (Mollusca: Gastropoda). Parasitology 63:483–489
Schmidt KA, Fried B (1997) Prevalence of larval trematodes in Helisoma trivolvis (Gastropoda) from a farm pond in Northampton County, Pennsylvania with special emphasis on Echinostoma trivolvis (Trematoda) cercariae. J Helminthol Soc Wash 64:157–159
Willey CH (1941) The life history and bionomics of the trematode Zygocotyle lunata (Trematoda: Paramphistomatidae). Zoologica 26:65–88
Wilson WD, Johnson PTJ, Sutherland DR, Mone H, Loker ES (2005) A molecular phylogenetic study of the genus Ribeiroia (Digenea): Trematodes known to cause limb malformations in amphibians. J Parasitol 91:1040–1045
Wright CA (1966) The pathogenesis of helminthes in the Mollusca. Helminthol Abstr 35:207–224
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huffman, J.E., Klockars, J., Keeler, S.P. et al. Histopathological effects of the intramolluscan stages of Zygocotyle lunata, Echinostoma trivolvis, and Ribeiroia ondatrae on Helisoma trivolvis and observations on keratin in the trematode larvae. Parasitol Res 105, 1385–1389 (2009). https://doi.org/10.1007/s00436-009-1572-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-009-1572-0