Abstract
Studies were conducted to determine the role of sibling species of Anopheles funestus complex in malaria transmission in three agro-ecosystems in central Kenya. Mosquitoes were sampled indoors and outdoors, and rDNA PCR was successfully used to identify 340 specimens. Anopheles parensis (91.8%), A. funestus (6.8%), and Anopheles leesoni (1.5%) were the three sibling species identified. A. parensis was the dominant species at all study sites, while 22 of 23 A. funestus were collected in the non-irrigated study site. None of the 362 specimens tested was positive for Plasmodium falciparum circumsporozoite proteins by enzyme-linked immunosorbent assay. The most common blood-meal sources (mixed blood meals included) for A. parensis were goat (54.0%), human (47.6%), and bovine (39.7%), while the few A. funestus s.s. samples had fed mostly on humans. The human blood index (HBI) for A. parensis (mixed blood meals included) in the non-irrigated agro-ecosystem was 0.93 and significantly higher than 0.33 in planned rice agro-ecosystem. The few samples of A. funestus s.s. and A. funestus s.l. also showed a trend of higher HBI in the non-irrigated agro-ecosystem. We conclude that agricultural practices have significant influence on distribution and blood feeding behavior of A. funestus complex. Although none of the species was implicated with malaria transmission, these results may partly explain why non-irrigated agro-ecosystems are associated with higher risk of malaria transmission by this species compared to irrigated agro-ecosystems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anopheles funestus Giles is one of the major vectors of malaria in sub-Saharan Africa. This species is an important bridge vector for malaria transmission during the dry season because their larvae develop in permanent swampy habitats that continue to be productive when Anopheles gambiae Giles habitats shrink (Gillies and De Meillon 1968). At taxonomic level, A. funestus comprises at least nine sibling species that are morphologically indistinguishable as adults and barely as eggs and larvae, yet they differ in their behavioral and vectorial attributes (Gillies and Coetzee 1987). A. funestus sensu stricto (henceforth referred to as A. funestus) is highly anthropophilic and a major vector of malaria, while the other species, Anopheles vaneedeni Gillies and Coetzee, Anopheles parensis Gillies, Anopheles aruni Sobti, Anopheles confusus Evans and Leesoni, Anopheles rivulorum Leesoni, Anopheles fuscivenosus Leesoni, Anopheles leesoni Evans, and Anopheles brucei Service are mainly zoophilic and non-vectors. However, A. rivulorum is known to be a minor vector in Tanzania (Wilkes et al. 1996) and A. vaneedeni has shown experimental susceptibility to Plasmodium falciparum in the laboratory (De Meillon et al. 1977) but no evidence of malaria transmission in nature. Analysis of rDNA sequences has revealed the occurrence of a new taxon provisionally referred to as A. rivulorum-like because of its relatedness to A. rivulorum (Cohuet et al. 2003).
Precise identification of members of the A. funestus complex is essential for vector control programs because misidentification of non-vector species as A. funestus can lead to massive wastage of time and resources through misdirected vector control efforts. The choice of vector control tactic to be used is also dependent on behavior of the target vector species. For instance, indoor residual spraying relies upon mosquitoes resting on the sprayed surface and picking up lethal doses of insecticide. This method is therefore ineffective against exophilic and exophagic members of the funestus complex.
Despite the presence of larval habitats that appear conducive for A. funestus in irrigated rice agro-ecosystems in Africa, this species is less frequently encountered compared to A. gambiae s.l. Ahero rice irrigation scheme; Kenya and the highlands of Madagascar are the only two places where this species occurs abundantly in relation to rice cultivation (Marrama et al. 1995; Githeko et al. 1996). It is unclear why A. funestus does not do well in African rice agro-ecosystems but it could be due to some unknown aspects of its biology as well as vector control activities. Interestingly, this species plays a significant role in malaria transmission in these areas (Ijumba et al. 1990; Ijumba et al. 2002; Dolo et al. 2004; Muturi et al. 2008), yet its species composition, and the role of different members of the complex in malaria transmission, has been least studied (Kamau et al. 2003a).
In a recent study conducted in the Mwea Rice Irrigation Scheme Central Kenya, the numbers of A. funestus were 11-fold lower than those of Anopheles arabiensis but the human blood index (HBI) for A. funestus was 3-fold higher than for A. arabiensis (Muriu et al. 2008). Further studies revealed that adult mosquito densities, HBI, and the risk of malaria transmission by A. funestus complex were significantly lower within the scheme than in neighboring non-irrigated villages (Muriu et al. 2008; Muturi et al. 2008). Earlier studies based on indoor collected samples within the rice scheme had shown that A. parensis (99.8%) and A. leesoni (one specimen) were the two species of A. funestus complex occurring in the area, and none was positive for malaria parasites (Kamau et al. 2003a). Apart from these studies, little is known about the species composition of A. funestus complex and their role in malaria transmission in Mwea Rice Irrigation Scheme and adjacent areas. This information is critical for development of effective evidence-based vector control programs specifically tailored to malaria vectors within this species complex.
The objective of this study was to determine how different agricultural practices influence the distribution and blood feeding pattern of sibling species of A. funestus complex and the role of different species within the complex in malaria transmission. Because the HBI for A. funestus s.l. increases with increasing distance from the rice scheme (Muturi et al. 2008), it was our hypothesis that species composition shifts from A. parensis or other zoophilic members of the complex within the scheme to anthropophilic A. funestus outside the scheme.
Materials and methods
Study sites
The study was conducted in five villages, Mbuinjeru, Karima, Rurumi, Kiamachiri, and Murinduko located in Mwea division, Kirinyaga District, 100 km North East of Nairobi. The study sites have been described in details previously (Muturi et al. 2006; Muturi et al. 2007). The study area has two annual rainfall seasons, the long rains in April/May and the short rains in October/November. The annual average rainfall is 950 mm. The average maximum temperatures are in the range of 16–26.5°C and the average relative humidity varies from 52% to 67%. A. arabiensis and A. funestus s.l. are the main drivers of malaria transmission in the area (Muturi et al. 2008). The prevalence of malaria in Mbuinjeru, Kiamachiri, and Murinduko among children 9 years and younger is 0%, 17%, and 54%, respectively (Mutero et al. 2004).
Mbuinjeru, Karima, and Rurumi are within the Mwea Rice Irrigation Scheme, and about 75% of land is under rice cultivation. Farmers in these villages follow a defined rice cropping cycle as determined by the National Irrigation Board (planned rice cultivation). Kiamachiri is ≈5 km away from the rice scheme, and about 20% of the village land is used for rice cultivation. Individual farmers decide their own cropping cycle depending on water availability. Murinduko is ≈15–20 km from the scheme and is generally a non-rice growing village mainly because of its hilly terrain that renders much of the area (about 90%) unsuitable for rice cultivation. Limited rice growing activity (<5% of the total area) is done along one major river valley that runs along the edge of the village.
Mosquito sampling and handling
Anopheline mosquitoes were sampled twice per month between April 2005 and March 2007 by two sampling methods: pyrethrum spray catch (PSC) technique (World Health Organization 1975) for indoor population and Centers for Disease Control and Prevention (CDC) light traps (J.W. Hock Ltd, Gainesville, FL, USA) for outdoor population. PSC was conducted twice per month in 20 randomly selected houses, and six CDC light traps were operated the night before and the night after PSC. The samples were transported to the laboratory and identified to species using morphologic characteristics (Gillies and Coetzee 1987). All A. funestus samples were scored as unfed, blood-fed, semi-gravid, or gravid by visual examination of abdomen under a dissecting microscope and later preserved dry in silica gel. A detailed description of the sampling strategy can be found elsewhere (Muturi et al. 2006).
Sample processing and analysis
The heads and thoraces of all A. funestus were tested for P. falciparum circumsporozoite proteins by enzyme-linked immunosorbent assay (ELISA; Wirtz et al. 1987). The hind guts of blood-fed samples were tested for human, cattle, goat, chicken, dog, and cat as possible hosts by ELISA (Beier et al. 1988). The legs and wings of each specimen were used for A. funestus sibling species identification by rDNA polymerase chain reaction (Koekemoer et al. 2002). Chi-square test was used to determine whether the human blood index for A. parensis differed significantly among the three study sites.
Results
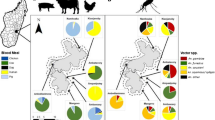
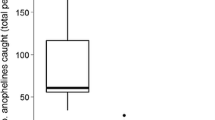
In total, 340 out of 362 specimens were successfully characterized into respective species of A. funestus complex by PCR (Table 1). A. parensis (91.8%), A. funestus (6.8%), and A. leesoni (1.5%) were the three sibling species identified in the samples. A. parensis was the dominant species at all study sites, while 22 of the 23 samples of A. funestus identified in this study were from the non-irrigated study site. Of the 66 A. funestus s.l. samples collected in Murinduko, 20 (all unfed) were from light trap collections outdoors. These comprised 10 A. parensis, six A. funestus, and four A. leesoni. The remaining samples as well as those from the other study sites were collected indoors. None of the 342 specimens examined for P. falciparum circumsporozoite proteins by ELISA tested positive. Because of the rarity in which members of A. funestus complex occur in the area, only 94 blood-fed specimens were collected during the study period and tested for host blood meal (Table 2). Including specimens that had mixed blood meals, goat (54.0%), human (47.6%), and bovine (39.7%) were the most common blood-meal sources for A. parensis, while the few A. funestus s.s. (100.0%) and A. funestus s.l. (78.6%) samples had fed mostly on humans. A few specimens were found to have fed on dogs, cats, and chicken. The HBI for A. parensis (including specimens with mixed blood meals) in the non-irrigated agro-ecosystem was 0.93 and significantly higher than 0.33 in planned rice agro-ecosystem (χ 2 = 16.08, df = 1, P < 0.01). Samples of A. parensis in unplanned rice agro-ecosystem were too few to allow logical comparison of HBI (0.67) with other study sites. Similarly, there were too few samples of A. funestus s.s. and A. funestus s.l. to facilitate reasonable comparisons of blood-meal hosts in the three study sites. However, A. funestus s.l. showed a trend of high HBI in the non-irrigated agro-ecosystem (0.86) than in planned rice agro-ecosystem (0.57). The three blood-fed A. funestus were collected in the non-irrigated agro-ecosystem and had human blood meals. Mixed blood feeding was also a common phenomenon (Table 2).
Discussion
A. parensis, A. funestus, and A. leesoni were the three sibling species of A. funestus complex identified in the study area. A. parensis was the dominant species in the three study sites, whereas 96% of all A. funestus occurred in the non-irrigated site. Previous studies in the same area had shown that A. parensis and A. leesoni are the two species of funestus complex occurring within the rice scheme, the former constituting 99.8% of the samples (Kamau et al. 2003a; Kamau et al. 2003b). Approximately 6% of the specimens morphologically recorded as A. funestus s.l. by two independent experts could not be identified by PCR. Possible explanations for lack of amplification in PCR may include misidentification of some specimens, DNA degradation due to problems during storage of samples, or DNA extraction process as well as presence of other members of A. funestus complex that could not be identified in this study (Kamau et al. 2003a; Temu et al. 2007). The PCR method used in this study identifies five of the nine members of the funestus complex: A. funestus s.s, A. parensis, A. rivulorum, A. leesoni, and A. vaneedeni.
Sympatric occurrence of members of A. funestus complex in Africa is not uncommon. Studies across 10 isolated study sites in Kenya (Kamau et al. 2003b) and in coastal Tanzania (Temu et al. 2007) demonstrated that up to four sibling species of A. funestus complex, A. funestus, A. parensis, A. rivulorum, and A. leesoni occur in sympatry. In the latter study, A. funestus was the dominant species, whereas in the former, species distribution and abundance varied across the study sites. For instance, A. parensis was the dominant species in Mwea and Baringo, Kenya, while A. funestus was dominant in western Kenya (Kamau et al. 2003b). Such observations highlight the importance of correct identification of members of funestus complex to malaria control programs.
Very few samples of blood-fed A. funestus s.l. were caught in the current study, significantly reducing the statistical power of our results. Because this species occurs in relatively low numbers than the main malaria vector, A. arabiensis (Muturi et al. 2006), we believe the current data, though insufficient for detailed conclusions, provide a general picture of blood-meal hosts for A. funestus group. A. parensis fed mostly on goat (54.0%), human (47.6%), and bovine (39.7%) while the three blood-fed A. funestus samples had human blood meals. The highly anthropophilic nature of A. parensis in the study area contradicts previous findings that this species is zoophilic (Kamau et al. 2003a). Nothing much can be said about A. funestus because of the small sample size, but detection of human blood meals in the three A. funestus samples suggests that this species is anthropophilic. The indoor presence of zoophilic A. parensis is indicative of exophagic and endophilic behavior.
In our previous studies, we reported that anthropophily by A. funestus s.l. was significantly higher in adjacent non-irrigated areas than within the rice scheme (Muriu et al. 2008; Muturi et al. 2008). The HBI for A. parensis and A. funestus s.l. in the current study followed this pattern, confirming that land use has significant impact on blood feeding behavior of members of A. funestus complex. Several reasons are believed to account for reduced anthropophily of malaria vectors in irrigated rice agro-ecosystems. These include wide-scale use of bednets as a result of higher mosquito densities (Mutero et al. 2004) especially A. gambiae s.l. and Culex quinquefasciatus, large numbers of cattle that cause a shift from human to animal feeding, as well as economic empowerment created by rice cultivation, enabling communities to invest on mosquito-bite protection measures (Ijumba and Lindsay 2001).
The role of the three members of funestus group in malaria transmission in the study area remains questionable. All A. parensis and A. funestus samples that were tested for P. falciparum cirmsporozoite proteins by ELISA were negative. Previous studies that did not distinguish between the different members of this species found A. funestus s.l. to be an important malaria vector in the area (Ijumba et al. 1990). Further studies indicated that this species was more abundant and an important malaria vector in neighboring non-irrigated villages than within the scheme (Muturi et al. 2008). Because A. parensis was found to be zoophilic and non-vector within the scheme (Kamau et al. 2003a), it was expected that A. funestus would be more common in surrounding non-irrigated villages than within the scheme. Unfortunately, very few samples were identified as A. funestus to permit evaluation of this prediction. The trend was however clear because 96% of A. funestus were from the non-irrigated. A recent study using PCR found the three species identified in this study to be positive for P. falciparum in coastal Tanzania (Temu et al. 2007). These findings together with reports of sporozoite positive A. funestus s.l. in the study area (Mukiama and Mwangi 1989; Ijumba et al. 1990; Muturi et al. 2008) suggest that the three species could be playing an unknown role in malaria transmission in similar areas. Further studies are needed to establish this role as well as ecology of the three sibling species.
One limitation of the current study is that samples were collected in three study sites in planned rice agro-ecosystem compared to one study site in other agro-ecosystems. The study sites within the planned rice agro-ecosystem did not show any disparity in mosquito species composition, feeding behavior, and sporozoite rates. We assumed that sites outside the scheme would have similar characteristics and effects on these parameters and would provide a broad perspective on the influence of land use on malaria transmission by A. funestus complex.
In conclusion, A. parensis, A. funestus, and A. leesoni were the three sibling species occurring in the study area. The results revealed that agricultural practices have significant effect on distribution and blood feeding patterns of A. funestus complex. We were unable to link any of the three species with malaria transmission in the study area. However, the increasing proportion of A. funestus and the high frequency of human–vector contact in the non-irrigated agro-ecosystem may partly explain why these areas are associated with greater risk of malaria transmission by A. funestus complex compared to irrigated agro-ecosystems.
References
Beier JC, Perkins PV, Wirtz RA, Koros J, Diggs D, Gargan TP, Koech DK (1988) Blood meal identification by direct enzyme-linked immunosorbent assay (ELISA), tested on Anopheles (Diptera: Culicidae) in Kenya. J Med Entomol 25:9–16
Cohuet A, Simard F, Toto JC, Kengne P, Coetzee M, Fontenille D (2003) Species identification within the Anopheles funestus group of malaria vectors in Cameroon and evidence for a new species. Am J Trop Med Hyg 69:200–205
De Meillon B, Van Eeden GJ, Coetzee L, Coetzee M, Meiswinkel R, Du Toit CLN, Hansford CF (1977) Observations on a species of the Anopheles funestus subgroup, a suspected exophilic vector of malaria parasites in north east Transvaal, South Africa. Mosq News 37:657–661
Dolo G, Briet OJT, Dao A, Traore SF, Bouare M, Sogoba N, Niare O, Bagayogo M, Sangare D, Teuscher T, Toure YT (2004) Malaria transmission in relation to rice cultivation in the irrigated Sahel of Mali. Acta Trop 89:147–159
Gillies MT, De Meillon B (1968) The anophelinae of Africa south of the Sahara. S Afri Inst Med Res, Johannesberg
Gillies MT, Coetzee M (1987) A supplement to the Anophelinae of Africa south of the Sahara (Afro-tropical region). Johannesberg. Pub South Afr Inst Med Res 55:1–143
Githeko A, Service M, Mbogo C, Atieli F (1996) Resting behaviour, ecology and genetics of malaria vectors in large scale agricultural areas of Western Kenya. Parassitologia 38:481–489
Ijumba J, Lindsay S (2001) Impact of irrigation on malaria in Africa: paddies paradox. Med Vet Entomol 15:1–11
Ijumba J, Mwangi R, JC B (1990) Malaria transmission potential of Anopheles mosquitoes in the Mwea-Tebere irrigation scheme, Kenya. Med Vet Entomol 4:425–432
Ijumba J, Mosha F, Lindsay S (2002) Malaria transmission risk variations derived from different agricultural practices in an irrigated area of northern Tanzania. Med Vet Entomol 16:28–38
Kamau L, Koekemoer LL, Hunt RH, Coetzee M (2003a) Anopheles parensis: the main member of the Anopheles funestus (Diptera: Culicidae) species group found resting inside human dwellings in Mwea area of central Kenya. J Am Mosq Control Assoc 19:130–133
Kamau L, Munyekenye GO, Koekemoer LL, Hunt RH, Coetzee M (2003b) A survey of the Anopheles funestus (Diptera: Culicidae) group of mosquitoes from 10 sites in Kenya with special emphasis on population genetic structure based on chromosomal inversion karyotypes. J Med Entomol 40:664–671
Koekemoer L, Kamau L, Hunt R, Coetzee M (2002) A cocktail polymerase chain reaction (PCR) assay to identify the Anopheles funestus (Diptera: Culicidae) group. Amer J Trop Med Hyg 66:804–811
Marrama L, Rajaonarivelo E, Laventure S, Rabarison P (1995) Anopheles funestus and rice culture on the Plateau of Madagascar. Cahiers d'Etudes et de Recherches Francophones Sante´ 5:415–419
Mukiama T, Mwangi R (1989) Seasonal population changes and malaria transmission potential of Anopheles pharoensis and minor anophelines in Mwea Irrigation Scheme, Kenya. Acta Trop 46:181–189
Muriu SM, Muturi EJ, Shililu JI, Mbogo CM, Mwangangi JM, Jacob BG, Irungu LW, Mukabana RW, Githure JI, Novak RJ (2008) Host choice and multiple blood feeding behaviour of malaria vectors and other anophelines in Mwea rice scheme. Kenya. Malar J 7:43
Mutero C, Kabutha C, Kimani V, Kabuage L, Gitau G, Ssennyonga J, Githure J, Muthami L, Kaida A, Musyoka L, Kiarie E, Oganda M (2004) A transdisciplinary perspective on the links between malaria and agroecosystems in Kenya. Acta Trop 89:171–186
Muturi J, Shililu J, Jacob B, Githure J, Gu W, Novak R (2006) Mosquito species diversity and abundance in relation to land use in a riceland agroecosystem in Mwea, Kenya. J Vector Ecol 31:129–137
Muturi E, Shililu J, Gu W, Jacob B, Githure J, Novak R (2007) Larval habitat dynamics and diversity of Culex mosquitoes in rice agro-ecosystem in Mwea, Kenya. Am J Trop Med Hyg 76:95–102
Muturi J, Muriu S, Shililu J, Mwangangi J, Jacob B, Mbogo C, Githure J, Novak R (2008) Effect of rice cultivation on malaria transmission in central Kenya. Am J Trop Med Hyg 78:270–275
Temu EA, Minjas JN, Tuno N, Kawada H, Takagi M (2007) Identification of four members of the Anopheles funestus (Diptera: Culicidae) group and their role in Plasmodium falciparum transmission in Bagamoyo coastal Tanzania. Acta Trop 102:119–125
Wilkes RA, Matola YG, Charlwood JD (1996) Anopheles rivulorum, a vector of human malaria in Africa. Med Vet Entomol 10:108–110
Wirtz R, Zavala F, Graves P, Andre G (1987) Field evaluation of ELISA for P. falciparum and P. vivax sporozoites in mosquitoes (Diptera: Culicidae) from Papua New Guinea. J Med Entomol 24:433–437
World Health Organization (1975) Manual on practical entomology in malaria, part II. Methods and techniques. World Health Organization, Geneva
Acknowledgments
We are grateful to Prof. Christian Borgemeister, Director General, International Centre of Insect Physiology and Ecology, for his strong support in this project. We acknowledge the technical support provided by Lucy Wachira and Geoffrey Gikandi of Kenya Medical Research Institute and by International Centre of Insect Physiology and Ecology Mwea field staff especially James Wauna, Peter Barasa, and Enock Mpanga. This research was supported by NIH/NIAID grant no. U01A1054889 (Robert Novak).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muturi, E.J., Kamau, L., Jacob, B.G. et al. Spatial distribution, blood feeding pattern, and role of Anopheles funestus complex in malaria transmission in central Kenya. Parasitol Res 105, 1041–1046 (2009). https://doi.org/10.1007/s00436-009-1543-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-009-1543-5