Abstract
The aim of the present work was to evaluate the in vitro efficacy of the flubendazole (FLBZ) and ivermectin (IVM) combination against Echinococcus granulosus protoscoleces and metacestodes. Protoscoleces and groups of ten peritoneal cysts obtained from BALB/c mice were incubated with the two drugs, either separately or in combination, at the following final concentrations: 10 µg/mL FLBZ, 1 µg/mL FLBZ, 1 µg/mL IVM, 10 µg/mL FLBZ + 1 µg/mL IVM, and 1 µg/mL FLBZ + 1 µg/mL IVM. The maximum protoscolicidal effect was found with the combination 10 µg/mL FLBZ + 1 µg/mL IMV. After 1 day of incubation, the presence of numerous blebs in the tegument of protoscoleces was observed. Ultrastructural studies revealed that the primary site of damage was the tegument of the parasite. The effect of the two drugs on hydatid cysts obtained from mice was more rapidly detected in cysts treated with the combination of FLBZ + IVM than when drugs were used separately. Ultrastructural studies revealed that the germinal layer of treated cysts lost the multicellular structure feature and underwent considerable degenerative changes after in vitro treatment. The outcomes obtained demonstrated the favorable effect of the combination of FLBZ and IVM against E. granulosus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cystic echinococcosis (CE) caused by the larval stage of the cestode Echinococcus granulosus is a life-threatening disease of serious public health and economic concern of global proportion (Eckert and Deplazes 2004). Traditionally, the treatment of echinococcosis relies primarily on surgery and/or chemotherapy, and the treatment strategy depends largely on different factors, such as metacestode size and location, viability status, the interaction between the expanding parasite and the adjacent host tissue, complicating bacterial or fungal infection, and potential complications related to cyst rupture and spillage of protoscoleces (Kern 2003, 2006).
Mebendazole (MBZ) and albendazole (ABZ) were initially tested in animal models and were first used in humans in the late 1970s (Bekhti et al. 1977; Schantz et al. 1982; Morris et al. 1983). Approximately 20–40% of cases do not respond favorably to such chemotherapy. Praziquantel (PZQ), a heterocyclic pyrazinoisoquinoline derivative, has been proposed for use together with benzimidazoles in CE patients (Cobo et al. 1998).
MBZ, ABZ, its metabolite albendazole sulfoxide (ABZSO), and flubendazole (FLBZ) were shown to be active against protoscoleces and metacestodes of E. granulosus under in vitro and in vivo conditions (Chinnery and Morris 1986; Morris et al. 1987; Pérez Serrano et al. 1994, 1997; Casado et al. 1996; Elissondo et al. 2006, 2007; Ceballos et al. 2006). On the other hand, PZQ exhibits a high efficacy against protoscoleces and metacestodes in animal experiments (Urrea-Paris et al. 1999, 2000, 2002) and it was suggested that PZQ exerts a substantial effect against metacestodes and protoscoleces when applied in combination with ABZ (Urrea-Paris et al. 1999).
Ivermectin (IVM), a macrocyclic lactone endectocide, is used to control nematode and arthropod parasites that cause diseases such as lymphatic filariasis, strongyloidosis, and scabies in humans and parasitic gastroenteritis in farm animals and different ectoparasites as well (McKellar and Benchaoui 1996). Campbell et al. (1983) suggested the total lack of efficacy of IVM on cestodes. However, Casado et al. (1989) found a marked protoscolicidal effect in in vitro incubations. These contradictory results may be explained by the different methodologies used. Thus, Martínez et al. (1999) observed an alteration in heat shock proteins after the in vitro incubation of E. granulosus protoscoleces with IVM, and Pérez-Serrano et al. (2001) later found a tegumental depolarization on the protoscoleces incubated in vitro with IVM that could be fundamental for the survival of the parasite.
Casado et al. (2002) studied the combination of IVM with ABZ and they demonstrated an improvement of IVM efficacy when it is combined with ABZ. This treatment also killed protoscoleces and metacestodes in less time than ABZ alone.
In previous works, we reported the in vitro effect of FLBZ on E. granulosus protoscoleces and metacestodes (Elissondo et al. 2006, 2007). The aim of the present work was to evaluate the in vitro efficacy of the FLBZ and IVM combination against E. granulosus protoscoleces and metacestodes.
Materials and methods
Protoscoleces collection and culture
Hydatid cysts from the liver and lungs of naturally infected cattle were obtained from an abattoir located in the southeast of Buenos Aires Province, Argentina. Protoscoleces were collected aseptically and washed several times with phosphate-buffered saline (pH 7.2). Viability was assessed by the methylene blue exclusion test (Elissondo et al. 2004). Moreover, other criteria, such as muscular movements, morphological perfectness of the whole body, and motility of flame cells, were taken into account to confirm the viability.
Mouse infection and collection of cysts
BALB/c mice (8 weeks old at the start of the experiments) were infected by intraperitoneal infection of 1,500 protoscoleces in 0.5 mL of medium 199 to produce experimental secondary hydatid disease. The animals were housed in a temperature-controlled (22 ± 1°C), light-cycled (12-h light/dark cycle) room.
At 8 months post-infection, mice with experimental secondary CE were euthanized, and necropsy was carried out immediately thereafter. At necropsy, the peritoneal cavity was opened and the hydatid cysts were carefully removed.
Drugs and in vitro incubation procedures
FLBZ (kindly provided by Janssen Animal Health, Beerse, Belgium) and IVM (Sigma, USA) were dissolved in dimethyl sulfoxide (DMSO) at a drug concentration of 1 and 0.1 mg/mL. The two drugs were added to the medium either separately or in combination at the following final concentrations: 10 µg/mL FLBZ (31.9 µM), 1 µg/mL FLBZ (3.19 µM), 1 µg/mL IVM (1.14 µM), 10 µg/mL FLBZ + 1 µg/mL IVM, and 1 µg/mL FLBZ + 1 µg/mL IVM.
Protoscoleces (1,500 per Leighton tube) were cultured in medium 199 (Gibco), supplemented with 100 IU penicillin, 100 µg/mL streptomycin, and 4 mg/mL glucose. Cultures were performed in 10 mL of incubation medium at 37°C without changes of medium (Elissondo et al. 2006). Protoscoleces incubated with culture medium containing 20 µl DMSO served as controls. Each experiment was repeated three times.
Groups of ten peritoneal cysts obtained from BALB/c mice were also placed in Leighton tubes containing 10 mL of culture medium. The tubes were maintained at 37°C without changes of medium during the entire drug incubation period (Elissondo et al. 2007). The cysts incubated with the culture medium containing DMSO were used as controls. Each experiment was repeated three times.
Evaluation by light and electron microscopy
Culture tubes with protoscoleces were followed up microscopically everyday to determine the appearance of morphological alterations. Samples of protoscoleces (approximately 70–90 protoscoleces in 0.5 mL of incubation medium) from each of the dosing groups and the controls were taken every 5 to 6 days for viability assessment using the methylene blue exclusion test. Additionally, ultrastructure studies with scanning and transmission electron microscope (SEM and TEM, respectively) were performed. For ultrastructure studies, protoscoleces cultures were processed as described by Elissondo et al. (2006).
Culture tubes with cysts were followed macroscopically and microscopically everyday. Samples of cysts from each of the dosing groups and the control were taken every 6 days for up to 12 days and then fixed for electron microscopy. The criteria for cysts vitality assessment included loss of turgidity, collapse of cysts, and ultrastructural observation of the germinal layer as described by Elissondo et al. (2007).
Results
Effects of FLBZ + IVM on E. granulosus protoscoleces
The survival of E. granulosus protoscoleces after exposure to FLBZ, IVM, and FLBZ + IVM combination is shown in Fig. 1. Control protoscoleces viability was 96.1 ± 0.8% after 24 days of incubation (Fig. 1). Viability decreased slowly, reaching 77.7 ± 7.5% at day 60. The maximum protoscolicidal effect was found with the combination 10 µg/mL FLBZ + 1 µg/mL IMV (Fig. 1); viability was reduced to 4.4 ± 0.3% after 24 days of incubation compared with 14.5 ± 4.7% with 1 µg/mL FLBZ + 1 µg/mL IMV, 18.2 ± 4.8% with 1 µg/mL IVM, 22.2 ± 0.8% with 10 µg/mL FLBZ, and 26 ± 1.1% with 1 µg/mL FLBZ.
The results of the viability tests coincide with the tissue damage observed at the structural level. Protoscoleces incubated with DMSO (control group) revealed no changes in structure throughout the experimental period (Fig. 2a). After 1 day of incubation, the presence of numerous blebs in the tegument of protoscoleces treated with 10 µg/mL FLBZ + 1 µg/mL IVM was observed by inverted microscope (Fig. 2b). Other alterations such as rostellar disorganization, loss of hooks, and contraction of posterior region could be seen after 2 days of incubation. With the other concentrations and combinations of drugs, the same alterations could be observed later, between 3 and 6 days post-incubation (p.i.).
These results were confirmed on the ultrastructural level by SEM and TEM. Control cultures exhibited no ultrastructural alterations in parasite tissue during the whole incubation period (Figs. 3a and 4a). In contrast, morphological and ultrastructural damages were detected in treated protoscoleces. The primary site of damage was the tegument of the parasite.
SEM of E. granulosus protoscoleces incubated in vitro with FLBZ and IVM. a Evaginated control protoscolece. b Protoscolece incubated with IVM alone (1 µg/mL) during 12 days. Note tegumental alterations of the soma region. c Altered protoscoleces after 12 days p.i. with the combination 1 µg/mL FLBZ + 1 µg/mL IVM. d–f Protoscoleces incubated with 10 µg/mL FLBZ + 1 µg/mL IVM (12 days p.i.). Note the loss of morphology, rostellar disorganization, loss of hooks, shedding of microtriches, and formation of tegumental vesicles
TEM of E. granulosus protoscoleces incubated in vitro with FLBZ + IVM. a Soma region of a control protoscolece (12 days p.i.; g glycocalix, dc distal cytoplasm; ×6,700). b Treated protoscolece (12 days p.i., 1 µg/mL IVM). Note the presence of large vacuoles (v) in the distal cytoplasm. c–d Protoscolece incubated with 10 µg/mL FLBZ + 1 µg/mL IVM (12 days p.i.). Internal tissue was severely affected. c Note the presence of vacuoles (v) and lipid droplets (l; ×6,700). d Residual lamellar bodies (b; ×10,000)
At 12 days p.i., observations by SEM and TEM of protoscoleces incubated with IVM alone revealed the presence of tegumental alterations, contraction of the soma region (Fig. 3b), and the presence of large vacuoles in the distal cytoplasm (Fig. 4b). On the other hand, microtriches remained unaltered. The ultrastructural effects observed after FLBZ + IVM incubation were greater than those caused by the drugs incubated separately, although vitality percentages at that time were rather similar (Fig. 1). Moreover, the combination 1 µg/mL FLBZ + 1 µg/mL IVM produced less marked alterations than 10 µg/mL FLBZ + 1 µg/mL IVM (Fig. 3c–f). The ultrastructural changes included tegumental alterations (Fig. 3c), rostellar disorganization, loss of hooks, and shedding of microtriches of the scolex region (Fig. 3d). Formation of tegumental vesicles was observed in several protoscoleces (Fig. 3e). In some protoscoleces, loss of morphology was evident (Fig. 3f). Ultrastructural studies by TEM revealed the severely affected internal tissue, resulting in an increase of vacuoles and lamellar residual bodies (Fig. 4c, d).
Effects of FLBZ + IVM on E. granulosus cysts
The in vitro results of the incubation of hydatid cysts obtained from mice with the different drugs are shown in Table 1. The effect was more rapidly detected in cysts treated with the combination of FLBZ + IVM than when drugs were used separately.
Control cultures exhibit no ultrastructural alterations in parasite tissue during the whole incubation period (Figs. 5a and 6a). Studies by SEM revealed that the germinal layer of treated cysts lost the multicellular structure feature (Fig. 5b–d). There was a correlation between the intensity of damage and the drug and/or combination assayed. This fact coincides with the macroscopic changes presented in Table 1. As was mentioned for protoscoleces, the combination 10 µg/mL FLBZ + 1 µg/mL IVM produced greater ultrastructural effects than when the drugs were incubated separately (Fig. 5b).
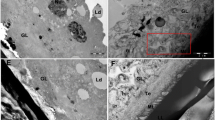
SEM of E. granulosus metacestodes incubated in vitro with FLBZ + IVM. a Control murine cyst with an intact germinal layer (12 days p.i.; gl germinal layer, ll laminar layer). b Murine cyst incubated in vitro with 10 µg/mL FLBZ + 1 µg/mL IVM (12 days p.i.). Note the extensive damage of the germinal layer. Only cellular debris could be observed. c Murine cyst (1 µg/mL FLBZ + 1 µg/mL IMV, 12 days p.i.). The germinal layer is altered. d Murine cyst (1 µg/mL IVM, 12 days p.i.). Germinal layer showing disintegrated areas
TEM of E. granulosus metacestodes incubated in vitro with FLBZ + IVM. a Control cyst (ll laminar layer, mt microtriches, gl germinal layer; ×7,500). b Treated murine cyst (12 days p.i., 1 µg/mL FLBZ + 1 µg/mL IVM). The internal tissue is altered with the presence of lipid droplets (l; ×8,000). c Murine cyst incubated during 12 days with 1 µg/mL IVM. Note the presence of numerous vacuoles (v)
These results were confirmed on the ultrastructural level by TEM (Fig. 6). After 12 days p.i., treated metacestodes underwent considerable degenerative changes after in vitro treatment. The presence of numerous vacuoles and lipid droplets was observed (Fig. 6b, c).
Discussion
In previous studies (Elissondo et al. 2006, 2007), the in vitro and in vivo effect of FLBZ on E. granulosus protoscoleces and metacestodes has been established. The present work is the first report of the effect of FLBZ and IVM combination against E. granulosus protoscoleces and metacestodes.
There are very few studies on the effects of IVM on cestodes. Casado et al. (1989) demonstrated a rapid protoscolicidal activity of IVM at 100 and 10 µg/mL. Moreover, Pérez-Serrano et al. (2001) recorded a depolarization of the protoscolex tegument which precedes the cestodicidal effect. Later on, Casado et al. (2002) reported that the effects of the in vitro IVM and IVM + ABZ combined treatment against protoscoleces and metacestodes were different. They studied the combination of ABZ + IVM in order to check for possible synergic effects. A higher susceptibility of the parasite was observed when it was incubated with the drug combination.
FLBZ was shown to be more effective and killed protoscoleces and metacestodes in less time than ABZ (Elissondo et al. 2006, 2007). In this work, the combination FLBZ + IVM not only exerted a marked effect on the larval stages of the parasite, but this effect was also observed early compared with the ABZ + IVM combination.
Protoscoleces cultured with FLBZ + IVM were killed considerably faster than protoscoleces cultured with ABZ + IVM. After 18 days of exposure to FLBZ + IVM, viability was approximately 20 ± 1.4% and it was reduced to 4.4 ± 0.3% after 24 days of incubation. Casado et al. (2002) reported a vitality of 35% after 18 days of culture with ABZ + IVM, but they did not describe what happened after 18 days of incubation.
Metacestodes incubated with FLBZ + IVM underwent considerable degenerative changes in less time than when the drugs were used separately. Moreover, the observed changes were identical to those reported by Casado et al. (2002). In contrast, they observed loss of turgidity after 3 days in cysts incubated with ABZ + IVM. In this work, these changes were observed during the first day of the experiment.
As evidenced in our experiments, the incubation of drugs separately, as well as in combination, produced ultrastructural alterations with the tegument of the parasite as the primary site of damage. However, treatment efficacy was considerably improved by applying the FLBZ + IVM combination. Ultrastructural changes observed in protoscoleces included tegumental alterations, rostellar disorganization, loss of hooks, shedding of microtriches of the scolex region, and the internal tissue being severely affected.
On the other hand, the ultrastructural effects on cysts were observed on the germinal layer. An increased vacuolization of the distal cytoplasm and the presence of lipid droplets were observed. The intensity of the damage depends on the drug and/or combination assayed. The combination 10 µg/mL FLBZ + 1 µg/mL IVM produced profound morphological and degenerative changes, killing almost all cells of the germinal layer.
Our results at the ultrastructural level are consistent with those reported by Casado et al. (2002). In this work, they suggested that the treatment with IVM produced a depolarization on the surface of the tegument in the initial stage of incubation (3 h), before the ultrastructural changes occur. This depolarization, together with the ultrastructural effects, is, however, not enough to produce the death of the parasite. But, since the tegument is the contact surface of the parasite with the drug, its depolarization, together with the effect of benzimidazole carbamate on the protoscoleces tubulin, could cause more damage to the parasite tissue leading to death.
The outcomes obtained demonstrated the favorable effect of the combination of FLBZ and IVM against E. granulosus. However, the IVM concentration (1 µg/mL) used in the present experimental work was much higher than that observed in the plasma of IVM-treated humans in which, after a dose of 15 mg/kg, an IVM peak plasma concentration of 0.045 µg/mL was reported (Fink and Porras 1989). Further thorough studies of FLBZ + IVM chemotherapeutic effect in vitro at a concentration lower than 1 µg/mL should be undertaken.
Additionally, after the in vivo coadministration of both FLBZ and IVM, potential pharmacokinetic interactions between components may occur and need to be addressed. In fact, pharmacokinetic interaction between ABZ and IVM has been recently reported in sheep (Alvarez et al. 2008). Although it was unclear at which level ABZ/metabolites and IVM interact, two possible explanations were as follows: (1) IVM-induced inhibition of ABZ metabolism or (2) drug-to-drug interaction via drug efflux transporters-mediated mechanisms. Moreover, in vivo studies will be required to evaluate the potential of the combination as a useful option for the treatment of human CE.
References
Alvarez L, Lifschitz A, Entrocasso C, Manazza J, Mottier L, Borda B, Virkel G, Lanusse C (2008) Understanding the pharmacokinetic interaction between ivermectin and albendazole following their combined use in lambs. J Vet Pharmacol Ther 31:230–239
Bekhti A, Schaaps JP, Capron M, Dessaint JP, Santoro F, Capron A (1977) Treatment of hepatic hydatid disease with mebedazole: preliminary results in four cases. Br Med J 2:1047–1051
Campbell WC, Fisher MH, Stapley EO, Albers-Schonberg G, Jacob TA (1983) Ivermectin: a potent new antiparasitic agent. Science 221:823–828
Casado N, Rodríguez-Caabeiro F, Jiménez A, Criado A, De Armas C (1989) In vitro effects of levamisol and ivermectin against Echinococcus granulosus protoscoleces. Int J Parasitol 19:945–947
Casado N, Pérez Serrano J, Denegri G, Rodríguez-Caabeiro F (1996) Development of a chemotherapeutic model for in vitro drug screening in Echinococcus granulosus cysts: assessment of the effects of albendazole and albendazole sulphoxide combination-therapy. Int J Parasitol 26:59–65
Casado N, Moreno MJ, Urrea-París MA, Rodríguez-Caabeiro F (2002) Could ivermectin have a synergic effect with albendazole in hydatidosis therapy? 1. In vitro studies. Parasitol Res 88:153–159
Ceballos L, Álvarez L, Sánchez Bruni S, Elissondo C, Dopchiz M, Denegri G, Torrado J, Lanusse C (2006) Development of a cyclodextrin-based flubendazole formulation to control secondary echinococcosis: pharmacokinetics, hydatid cyst morphology and efficacy in mice. J Vet Pharmacol Ther 29:85–86
Chinnery JB, Morris DL (1986) Effect of albendazole sulphoxide on viability of hydatid protoscoleces in vitro. Trans R Soc Trop Med Hyg 80:815–817
Cobo F, Yarnoz C, Sesma B, Fraile P, Aizcorbe M, Trujillo R (1998) Albendazole plus praziquantel versus albendazole alone as a pre-operative treatment in intra-abdominal hydatidosis caused by Echinococcus granulosus. Trop Med Int Health 3:462–466
Eckert J, Deplazes P (2004) Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev 17:107–135
Elissondo MC, Dopchiz MC, Brasesco M, Denegri G (2004) Echinococcus granulosus: first report of microcysts formation from protoscoleces of cattle origin using the in vitro vesicular culture technique. Parasite 11:415–418
Elissondo MC, Dopchiz MC, Ceballos L, Alvarez L, Sánchez Bruni S, Lanusse C, Denegri G (2006) In vitro effects of flubendazole on Echinococcus granulosus protoscoleces. Parasitol Res 98:317–323
Elissondo MC, Ceballos L, Dopchiz MC, Andresiuk MV, Alvarez L, Sánchez Bruni S, Lanusse C, Denegri G (2007) In vitro and in vivo effects of flubendazole on Echinococcus granulosus metacestodes. Parasitol Res 100:1003–1009
Fink E, Porras A (1989) Pharmacokinetics of ivermectin in animal and humans. In: Campbell W (ed) Ivermectin and abamectin. Springer, New York, pp 113–130
Kern P (2003) Echinococcus granulosus infection: clinical presentation, medical treatment and outcome. Langenbecks Arch Surg 388:413–420
Kern P (2006) Medical treatment of echinococcosis under the guidance of Good Clinical Practice (GCP/ICH). Parasitol Int 55:273–282
Martínez J, Pérez-Serrano J, Bernardina WE, Rodríguez-Caabeiro F (1999) Echinococcus granulosus: in vitro effects of ivermectin and praziquantel on HSP60 and HSP70 levels. Exp Parasitol 93:171–180
McKellar Q, Benchaoui H (1996) Avermectins and milbemycins. J Vet Pharmacol Ther 19:331–351
Morris DL, Dykes PW, Dickson B, Marriner SE, Bogan JA, Burrows FG (1983) Albendazole in hydatid disease. Br Med J 286:103–104
Morris DL, Chinnery JB, Ubhi C (1987) A comparison of the effects al albendazole, its sulphone metabolite, and mebendazole on the viability of protoscoleces of Echinococcus granulosus in an in vitro culture system. Trans R Soc Trop Med Hyg 81:804–806
Pérez Serrano J, Casado N, Denegri G, Rodríguez-Caabeiro F (1994) The effects of albendazole and albendazole sulphoxide combination- therapy on Echinococcus granulosus in vitro. Int J Parasitol 24:219–224
Pérez-Serrano J, Denegri G, Casado N, Rodríguez-Caabeiro F (1997) Treatment of experimental echinococcosis with albendazole and albendazole sulphoxide. Int J Parasitol 27:1341–1345
Pérez-Serrano J, Grossman C, Urrea-París MA, Denegri G, Casado N, Rodríguez-Caabeiro F (2001) Depolarization of the tegument precedes morphological alterations in Echinococcus granulosus protoscoleces incubated with ivermectin. Parasitol Res 87:804–807
Schantz PM, van den Bossche H, Eckert J (1982) Chemotherapy for larval 235 echinococcosis in animals and humans: report of a workshop. Z Parasitenkd 67:5–26
Urrea-Paris MA, Moreno MJ, Casado N, Rodríguez-Caabeiro F (1999) Echinococcus granulosus: praziquantel treatment against the metacestode stage. Parasitol Res 85:999–1006
Urrea-Paris MA, Moreno MJ, Casado N, Rodríguez-Caabeiro F (2000) In vitro effect of praziquantel and albendazole combination therapy of the larval stage of Echinococcus granulosus. Parasitol Res 86:957–964
Urrea-Paris MA, Moreno MJ, Casado N, Rodríguez-Caabeiro F (2002) Relationship between the efficacy of praziquantel treatment and the cystic differentiation in vivo of Echinococcus granulosus metacestode. Parasitol Res 88:26–31
Acknowledgements
The authors acknowledge Dr. Leo Van Leemput and Dr. Kathleen Vlaminck (Janssen Animal Health, Beerse, Belgium) and Dr. Gustavo Viana (Janssen, Buenos Aires, Argentina) for providing the FLBZ used in the present experimental work and for their critical review of this manuscript. The help of Dr. González and Sr. Chasma is gratefully appreciated. This work was supported by the PICT 02 No. 01-12535, BID 1201/OC-AR.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elissondo, M.C., Ceballos, L., Alvarez, L. et al. Flubendazole and ivermectin in vitro combination therapy produces a marked effect on Echinococcus granulosus protoscoleces and metacestodes. Parasitol Res 105, 835–842 (2009). https://doi.org/10.1007/s00436-009-1469-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-009-1469-y