Abstract
This paper is a comparative study of Diplostomum (Austrodiplostomum) compactum (Lutz, 1928) in Nile tilapia Oreochromis niloticus (Linneo) from two fish farms and two nearby coastal lagoons in Guerrero state, Mexico. The higher infections levels in cultured tilapia than wild tilapia is attributed to higher fish densities in the culture systems and higher abundance of the snail Biomphalaria cf. havanensis (Pteiffer), first intermediate host of this parasite in freshwater and brackish water systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parasitic diseases are sporadic or periodic limiting factors in aquaculture production. A wide variety of parasitic diseases are prevalent in extensive fish monocultures (Karvonen et al. 2005, 2006), and their control requires frequent use of drugs and chemicals.
One of the most important parasitic diseases in cultured freshwater fish is diplostomiasis, also known as diplostomatosis, parasite cataract, or eye-fluke disease (Chappell et al. 1994; Semenas 1998). Caused by Diplostomum and Tylodelphys genera helminth species, it is one of the most widespread diseases found in wild and cultured fish in temperate and tropical climates (Chappell et al. 1994). In cultured fish, it can cause massive mortalities at the breeding stage, especially in intensive conditions, but is not reported as deadly in natural fish populations (Paperna 1995; Overstreet and Curran 2004).
In Mexico, diplostomiasis is caused by the digenean Diplostomum (Austrodiplostomum) compactum (Pineda-López et al. 1985), a species recorded in 33 freshwater fish species from ten families (Salgado-Maldonado 2006), including the African tilapia Oreochromis mossambicus, O. niloticus, and O. urolepis hornorum (Pineda-López et al. 1985). In the state of Guerrero, D. (A.) compactum has been recorded in ten species of fish from the Coyuca and Tres Palos lagoons (Violante-González 2006; Violante-González and Aguirre-Macedo 2007; Violante-González et al. 2007).
Commercial and subsistence aquaculture in Guerrero have grown notably in recent years, meaning constant supervision of cultured populations is needed to prevent outbreaks of parasitic diseases such as diplostomiasis, and reduce high losses due to mortality or suboptimum growth performance. The aim of the present study was to make an initial comparative description of diplostomiasis in tilapia Oreochromis niloticus from two culture systems and two coastal lagoons in Guerrero, Mexico.
Materials and methods
From October 2007 to April 2008, samples were taken of Nile tilapia Oreochromis niloticus (Linneo) and the snail Biomphalaria cf. havanensis (Pteiffer) from two commercial farms and two natural lagoons. Aguas Blancas farm (17°02′N; 100°03′W) covers approximately 0.9 ha and utilizes 30 × 20 m2 earth ponds. Las Playas farm (16°49′N; 99°43′W) covers approximately 0.75 ha and utilizes 75 × 25 m2 earth ponds. Water temperature in the ponds at both farms ranged from 24 to 28°C (25.87 ± 1.73) during the study period. A total of 454 fish were collected at Aguas Blancas farm during a single sampling in December 2007, and 321 were collected at Las Playas farm in two samplings; one at the beginning of the culture period (n = 192, October 2007), and another 4 months later (n = 129, February 2008). A total of 569 snails B. cf. havanensis were collected from Aguas Blancas farm, and 552 from Las Playas farm. Samples were also taken of the snail Thiara (Melanoides) tuberculata (Müller, 1774) (n = 320) at Aguas Blancas farm, but only B. cf. havanensis was found to contain cercariae of D. (A.) compactum, and therefore only data for B. cf. havanensis are included here.
Collections of wild tilapia and B. cf. havanensis were made during the same period in two natural coastal lagoons near the farms. A total of 72 wild tilapia and 64 snails were collected from Coyuca Lagoon (16°57′ N; 100°02′ W), and 120 tilapia and 48 snails from Tres Palos Lagoon (16°48′ N; 99°47′ W).
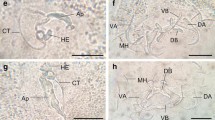
All fish and snail samples were transported for analysis to the laboratory of the Marine Ecology Academic Unit, Universidad Autonoma de Guerrero. Total length was measured for each fish, and the eyes dissected and then examined for metacercariae with a stereoscopic microscope. The metacercariae extracted from each eye were counted as separate lots, and placed in a Petri dish containing saline solution before fixing in 70% alcohol. Some metacercariae samples were fixed using slight ventral flattening in AFA (acetic acid–alcohol–formalin) fixer for 24 h, and then placed in small flasks containing 70% alcohol. Other samples were fixed directly in 70% alcohol, without flattening, to prevent errors in morphometric measurement. Parasite specimens were stained using carmine hydrochloric solution, and mounted in synthetic resin. Identification of larval stages and adult forms were based on the morphological and morphometrical descriptions of Ostrowski de Nuñez (1982) and tested with molecular analysis (data to be presented in separate article).
Shell diameter to the nearest millimeter was measured in snail specimens. These were dissected in a Petri dish containing a small quantity of pond or lagoon water to prevent cercariae from drying out and dying before being located. Cercariae samples were fixed in 70% alcohol for permanent preparation.
In an effort to collect data for the entire lifecycle of D. (A.) compactum, 15 cormorant Phalacrocorax olivaceus (Gmelin) specimens were collected. This piscivorous bird permanently inhabits the coastal lagoons of Guerrero and was observed feeding on tilapia fingerlings at the sampled farms during the study period. After killing, the birds were dissected, all helminth species extracted from their digestive system (esophagus, stomach, intestine and cloaca), and fixed in individual flasks containing 70% alcohol. Other piscivorous birds species observed at the collection sites included Ardea herodias (Linnaeus), Casmerodius albus (Linnaeus), Egretta thula (Molina), Egretta tricolor (Müller), and Nycticorax nycticorax (Linnaeus). Specimens of these species were also collected and examined, but only P. olivaceus harbored adults of D. (A.) compactum. Voucher specimens of D. (A.) compactum metacercariae and adults (6534, 6535, 6536, and 6537) were deposited in the National Helminth Collection, and voucher specimens B. cf. havanensis (3091) were deposited in the National Mollusks Collection, both in the Institute of Biology, National Autonomous University of Mexico, Mexico City.
Parasite infection levels in the different hosts were evaluated using the infection parameters of prevalence (percent infected host); mean intensity (number of parasites per infected host), expressed as the mean ± standard deviation; abundance (number of parasites per examined host); and range of intensity (Bush et al. 1997). Possible differences in infection prevalence between cultivated and wild fish were identified with a G test (Sokal and Rohlf 1998) and a Chi2-test was used to identify differences in mean intensity. The Spearman correlation coefficient (rs) was applied to fish size classes to determine if a linear relation existed between fish size and infection parameters. A Student test with n − 2 degrees of freedom and a p < 0.05 significance level was used to evaluate if the rs value was of sufficient magnitude to accurately indicate correlation between variables.
Results
In the sampled cultivated and wild tilapia Oreochromis niloticus, the metacercariae of Diplostomum (Austrodiplostomum) compactum was the cause of diplostomiasis. Metacercariae were found to be free inside the eyes of infected hosts, actively moving in the vitreous humor.
Infection prevalence was highest among fish sampled from Las Playas farm (G = 99.9, p < 0.05), although no differences in mean intensities were observed between cultivated and wild fish (Chi2 = 0.74, p > 0.05). Tilapia from Las Playas farm had the broadest range of intensity (1 to 11), followed by wild tilapia from Tres Palos Lagoon (1 to 8; Table 1). The cultured tilapia exhibited a higher percentage of infected fish, but the number of metacercariae per individual was low (one to two); indeed, only 20% of the cultured fish harbored more than six parasites (Fig. 1).
Analysis of infection levels considering fish size classes showed prevalence to be negatively correlated to total length, particularly in fish from the Las Playas (rs = −0.83, p < 0.05) and Aguas Blancas farms (rs = −0.79, p < 0.05; Fig. 2). However, no correlation was observed between total length and mean intensity at either farm (rs = −0.68, p = 0.09; rs = −0.60, p = 0.15, respectively). Further analysis considering the total number of examined hosts per farm (Las Playas, n = 321; Aguas Blancas, n = 454) showed total length to be negatively correlated to metacercariae abundance (number of parasites per infected fish) in the eyes (rs = −0.201, p < 0.01; rs = −0.233, p < 0.05, respectively). Prevalence was also negatively correlated to total length in the wild tilapia from Tres Palos Lagoon (rs = −0.85, p < 0.05). Due to small sample sizes and the low infection levels in infected wild specimens, no further analyses were done of the fish from Tres Palos or Coyuca lagoons.
Prevalence of cercariae in the population of B. cf. havanensis was 8.4% at Las Playas farm and 8.7% at Aguas Blancas farm. Shell diameter of snails from the farms ranged from 1.9 to 11 mm (5.01 ± 1.28), and only individuals with a shell diameter >4.1 mm were infected with D. (A.) compactum cercariae or redia. Prevalence increased with size, and was over 40% in the 9- to 11-mm size range (Fig. 3). Other unidentified cercariae were observed in snails from the farms, but co-occurrences with D. (A.) compactum cercariae were rare. Snails from Coyuca Lagoon had a mean size of 5.64 ± 1.10 mm and an infection prevalence of 12.5%, while those from Tres Palos Lagoon had a mean size of 6.97 ± 2.25 mm and a prevalence of 10.7%. As it occurred in snails from the farms, D. (A.) compactum almost never co-occurred with other cercariae in the snails from the lagoons.
Infection prevalence of D. (A.) compactum adults in the 15 examined cormorants (P. olivaceus) was 100%, with a mean abundance of 18.75 ± 16.45, and an intensity range of two to 54 helminths per infected host.
Discussion
Infection levels of the digenean Diplostomum (Austrodiplostomum) compactum were higher in the cultivated tilapia than in the wild tilapia. This is probably due to higher fish density in the aquaculture systems, and higher abundance of its primary host Biomphalaria cf. havanensis at both farms. At the farms, the highest overall infection levels and parasite counts in the eyes were registered in young fish (<5 cm fingerlings; Fig. 1).
Kennedy and Burrough (1977) commented that variations in parasite load in relation to host length are apparently rare in species of Diplostomum. More recent research, however, has shown positive correlations between standard length and D. (A.) compactum infection levels in fish from the Paraná River, Brazil (Machado et al. 2005), which suggest that the number of fish infected by D. (A.) compactum metacercariae increased proportionally to host length. Increased infection levels with host growth have also been reported for parasite species with longer life cycles, such as D. spathaceum (Muzzall et al. 1990).
In contrast, infection levels in the studied cultivated fish decreased as total host length (i.e., age) increased, probably due to behavioral differences between fingerlings and adults in the culture ponds. During early life stages, smaller (<5 cm) fingerlings take refuge among the aquatic vegetation (Desmanthus sp. and Panicum sp.) along the pond edges. Here, they ingest mainly aquatic insects (Noctonectidae Family), but are also in constant proximity to the snail B. cf. havanensis, greatly increasing their probability of becoming infected. In contrast, larger fish spend most of their time consuming the dry feed provided daily in the deeper areas of the ponds, which lack aquatic vegetation and therefore provide no habitat for B. cf. havanensis. This same behavior is exhibited by native and non-native cichlid fingerlings (e.g., Cichlasoma trimaculatum, O. niloticus, respectively) in the brackish water systems of Guerrero (unpublished data), suggesting that cichlid fingerlings in the two studied coastal lagoons may also have higher infection levels than adult stages.
Infection percentages were generally higher in the cultured fish than in the wild fish (Table 1). Studies of parasitic diseases in cultivated fish have suggested that extensive monoculture favors persistence of a wide variety of parasitic diseases, since high stocking densities favor increased parasite populations (Karvonen et al. 2005, 2006).
The prevalence and infection intensities observed for both the cultivated and wild fish (8.3 to 77.6%; 1.33 to 3.29), are relatively low when compared to the 100% (five at 20) reported for cultured Oncorhynchus mykiss in Argentina (Semenas 1998), the 71% to 100% (15.1 to 106.9) for wild Plagioscion squamossimus, and 20 to 90% (5.2 to 12.7) for wild Cichla ocellaris in Brazil (Sousa et al. 2002). The present results, however, are similar to the 55% prevalence and 3.9 metacercariae per infected fish reported by García et al. (1993) for the tilapias Oreochromis aureus and O. mosambicus from Amela Lagoon in Colima state, Mexico.
It is unlikely that the metacercariae counts in the eyes of either the cultivated or wild fish were sufficiently high to affect survival. Infection with cercariae such as D. (A.) compactum can produce severe damage in hosts, including death (Pineda-López et al. 1985). Indeed, massive fish kills due to D. (A.) compactum metacercariae infections in the eyes of cultivated tilapias have been documented in Malpaso and La Angostura reservoirs in the state of Chiapas, Mexico (Pineda-López et al. 1985). Total blindness can result when metacercariae counts exceed 40 individuals per eye, depending on fish size (Evans et al. 1976). Under these circumstances, the affected host can lose the capacity to feed, causing growth delays, weight loss and eventual direct mortality by starvation, or indirect mortality by increased vulnerability to predation (Kennedy 1974).
Infection patterns in the Biomphalaria cf. havanensis specimens collected from the farms showed larger (i.e., older) snails to have higher infection prevalence (>40%, Fig. 3), whereas smaller snails (<4 mm diameter) were not infected. Although smaller than the samples from the farms, the snail samples from the coastal lagoons clearly exhibited infection patterns similar to those in the farm samples. These results coincide with Karvonen et al. (2006), who reported that infection prevalence in the snail Lymnaea stagnalis, and the proportion of snails that released Diplostomum spathaceum cercariae, was strongly dependent on snail size. These authors observed that prevalence was low in snails <20 mm, that no infections occurred in snails <10 mm, and that those >40 mm were consistently infected. The above suggests that in snails the larger size classes are responsible for most cercariae production, and therefore that these classes are vital to consider when developing preventive measures against parasitic diseases in culture systems.
Infection prevalence in the B. cf. havanensis populations was 8% at the farms and 11 to 12% in the lagoons. The higher prevalence in the lagoons can be attributed to the smaller sample sizes and that the sampled snails were >5 mm in diameter, meaning that the percentage of infected individuals was higher. Nonetheless, an infection prevalence between 1% and 10% in snails is considered sufficient to generate 100% infection rates in fish (Stables and Chappell 1986).
Of the six piscivorous bird species examined, only the cormorant Phalacrocorax olivaceus harbored mature adult D. (A.) compactum. This suggests that P. olivaceus is the final preferential host of D. (A.) compactum in this region of Guerrero, which coincides with reports for other areas of Mexico (Pineda-López et al. 1985; Ramos 1995) and for other countries (Ostrowski de Núñez 1982; Rietschel and Werding 1978; Lunaschi et al. 2007).
Overall, the results indicate that even though the metacercariae counts in the eyes of either the studied cultivated or wild fish were not sufficiently high to affect survival, preventive measures such as vegetation control and removal of snail populations are needed to prevent any possible diplostomiasis outbreaks in the culture systems of the state of Guerrero.
References
Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83:575–583
Chappell LH, Hardie LJ, Secombes CJ (1994) Diplostomiasis: the disease and host-parasite interactions. In: Pike AW, Lewis JW (eds) Parasitic diseases of fish. Samara, pp 59-86
Evans RS, Heckmann RA, Palmieri J (1976) Diplostomiasis in Utah. Utah Acad Proc 53:20–25
García LJ, Osorio-Sarabia D, Constantino F (1993) Prevalencia de los parásitos y las alteraciones histológicas que producen a las tilapias de la laguna de Amela, Tecomán, Colima. Veter Méx 24:199–204
Karvonen A, Paukku S, Seppälä O, Valtonen ET (2005) Resistance against eye flukes: naïve versus previously infected fish. Parasitol Res 95:55–59
Karvonen A, Savolainen M, Seppälä O (2006) Dinamics of Diplostomum spathaceum infection in snail host. Parasitol Res 99:341–345
Kennedy CR (1974) The use of frequency distribution in an attempt to detect host mortality induced infections of diplostomatid metacercariae. Parasitol 89:209–220
Kennedy CR, Burrough R (1977) The population biology of 2 species of eye fluke Diplostomum spathaceum and Tylodelphys clavata in perch. J Fish Biol 11:619–633
Lunaschi LI, Cremonte F, Drago FB (2007) Checklist of digenean parasites of birds from Argentina. Zoot 1403:1–37
Machado PM, Takemoto RM, Pavanelli GC (2005) Diplostomum (Austrodiplostomum) compactum (Lutz, 1928) (Platyhelminthes, Digenea) metacercariae in fish from the floodplain of the Upper Paraná River, Brazil. Parasitol Res 97:436–444
Muzzall PM, Sweet RD, Milewski CL (1990) Occurrence of Diplostomum sp. (Trematoda, Diplostomatidae) in pond-reared walleyes from Michigan. Prog Fish Cult 52:53–56
Ostrowski de Núñez M (1982) Die Entwicklungszyklen von Diplostomum (Austrodiplostomum) compactum (Lutz 1928) Dubois 1970 und D. (A.) mordax (Szidat & Nani 1951) n. comb. in Szüdamerika. Zool Anz 208:393–404
Overstreet R, Curran S (2004) Defeating diplostomoid dangers in USA catfish aquaculture. Folia Parasitol 51:153–165
Paperna I (1995) Digenea (Phylum Platyhelminthes). In: Woo PT (ed) Fish diseases and disorders. Vol. I: protozoans and metazoans infections. CAB, pp 329-389
Pineda-López R, Andrade-Salas O, Páramo-Delgadillo S, Trejo PL, Almeida-Artigas J, Osorio-Sarabia D, Pérez-Ponce G (1985) Estudio del control sanitario de la piscifactoría Benito Juárez y en los Vasos de las Presas la Angostura y Malpaso. Dirección de Acuacultura, Secretaría de Pesca. México, 309 p
Ramos P (1995) Algunos tremátodos de vertebrados de la presa Miguel Alemán en Temascal, Oaxaca, México. Anales Inst Biol Univ Nac Autón Méx Ser Zool 66:241–246
Rietschel G, Werding B (1978) Trematodes of birds from Northern Columbia. Parasitenkunde 82:57–87
Salgado-Maldonado G (2006) Checklist of helminth parasites of freshwater fishes from Mexico. Zoot 1324:357
Semenas L (1998) Primer registro de diplostomiasis ocular en trucha arco iris cultivada en Patagonia (Argentina). Arch Med Vet 30:1–8
Sokal RR, Rohlf FJ (1998) Biometry, 2nd ed. Freeman, 859 p
Sousa R, Akira FD, Laterça M, Kazuyuki H, Garcia N (2002) Metacercárias de Diplostomum (Austrodiplostomum) compactum Lutz, 1928 (Digenea, Diplostomidae) em peixes do rio Paraná, Brasil. Prevalência, sazonalidade e intensidade de infecção. Maringá 24:475–480
Stables JN, Chappell LH (1986) The epidemiology of diplostomiasis in farmed rainbow trout from north-east Scotland. Parasitol 92:699–710
Violante-González J (2006) Comunidades de parásitos metazoarios de peces, en dos lagunas costeras del Estado de Guerrero, México. Tesis Doctorado, Cinvestav IPN, Mérida. México 155 p
Violante-González J, Aguirre-Macedo ML (2007) Metazoan parasites of fishes from Coyuca Lagoon, Guerrero, Mexico. Zoot 1531:39–48
Violante-González J, Aguirre-Macedo ML, Mendoza-Franco EF (2007) A checklist of metazoan parasites of fish from Tres Palos lagoon, Guerrero, Mexico. Parasitol Res 102:151–161
Acknowledgments
This research was financed by the PROMEP: Programa de Reincorporación de Exbecarios. The authors thank Drs. Edna Naranjo and Osamu Miura for snail species identification, Zacarias Blancarte Ahuatzin for aquatic plant species identification, and the students of the Marine Ecology Academic Unit (UAG) for their assistance in field and laboratory work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Violante-González, J., García-Varela, M., Rojas-Herrera, A. et al. Diplostomiasis in cultured and wild tilapia Oreochromis niloticus in Guerrero State, Mexico. Parasitol Res 105, 803–807 (2009). https://doi.org/10.1007/s00436-009-1458-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-009-1458-1