Abstract
Within the entomological monitoring program of the German federal ministry of food, agriculture, and user protection (BMELV), at 12 cattle farms in Rhineland-Palatinate and two in Saarland, ultraviolet lamp traps were used to monitor the distribution and seasonal appearance of potential vectors of the bluetongue virus, with special consideration of species of Culicoides. Using the traps during the first seven nights of each month from April 2007 to May 2008, 5,000–120,000 ceratopogonids were caught at different locations, in total about 500,000 and mainly females. Ninety-four percent belonged to the genus Culicoides, and of these, 90% were Culicoides obsoletus s.l., 6% were Culicoides pulicaris s.l., and 4% were other species of this genus. In all traps, the first ceratopogonids were caught in April 2007, the total number peaking in August 2007. After a reduction in September, a lower peak occurred in October. During the whole winter, some ceratopogonids were active. At nearly all locations, the total numbers of C. obsoletus s.l., C. pulicaris s.l., and of other ceratopogonids were significantly correlated with the temperatures, and higher population densities of C. obsoletus s.l. seemed to occur at altitudes of about 300 m above sea level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Species of the family Ceratopogonidae are small Diptera, usually with a length of 0.5–3 mm. They are distributed worldwide, and in some regions, the hematophagous species are vectors of human and animal diseases. In Central Europe, members of the genus Culicoides are important ectoparasites, often preferring specific mammals or birds (Mehlhorn 2008). The larvae of ceratopogonids develop in very different, but always wet, species-specific habitats, ranging from bogs over dung to salt water marshes (Kettle and Lawson 1952). Adults are mainly found close to the larval habitat but can actively fly about 3 km or drift by wind over much greater distances (summarized by Mellor et al. 2000). The lifespan of adult Culicoides spp. is strongly temperature-dependent, e.g., in Culicoides sonorensis ranging from 6 days to 3 months (Lysyk and Danyk 2007). The duration of development also differs between species and is strongly affected by ambient temperature varying between some days and several weeks. In addition, Palearctic species undergo diapause as larvae (Becker 1961). In North Germany, uni- and multivoltine species are described (Olbrich 1987), but only little is known about the biology and distribution of hematophagous species of Culicoides in other regions of Germany.
Culicoides are vectors of the bluetongue virus (BTV). Transmission is proved for Culicoides actoni, Culicoides fulvus, Culicoides imicola, Culicoides nubeculosus, Culicoides variipennis, and Culicoides wadai, but further species are suspected to be regional potential vectors (Mellor 1990; Mehlhorn et al. 2007). The bluetongue disease is widespread on the African continent moving northwards up to the Mediterranean region of Europe in the second half of the 20th century. In Central Europe, it was first reported in August 2006. Until December 2006, more than 2,000 farms were affected (Mehlhorn et al. 2007). As a reaction to the epidemic in Central Europe, the European community initiated an entomological monitoring which was funded from March 2007 to May 2008 by the German federal ministry of food, agriculture, and user protection (BMELV) at 90 locations in Germany to gain further information on the distribution and seasonal appearance of potential vectors of BTV, with special consideration of species of Culicoides.
Monitoring was performed by our group in Rhineland-Palatinate and Saarland. According to the information obtained from the farmers, one of the ten cattle farmers in Rhineland-Palatinate had an infected cow just before the beginning of the monitoring, but none of the two cattle farmers in the Saarland had an infected cow. On most farms, first infections occurred between July and September 2007, and until the end of the monitoring, 11 of the 12 farms were affected. Usually, only single animals were infected, but at one farm in Rhineland-Palatinate, 75% of the cattle was seropositive.
Materials and methods
The cattle farmers were selected by the veterinary administration of the respective district at ten locations in Rhineland-Palatinate and two in Saarland and possessed 50–250 cows of Black-and-White breed cattle maintained in open stables with open fronts or on a pasture nearby. Only one farm in Rhineland-Palatinate bred Galloway cattle kept only on the pasture (Fig. 1 and Table 1).
Insects were trapped in ultraviolet light lamp traps (“BG-Sentinel” BioGents, Regensburg) using a battery. The distance of the traps to the cattle was always less than 20 m. The traps were covered by a plastic plate as protection to rain (distance to the intake funnel 0.5 m) and stripped to the ground to avoid movement by wind. Insects were attracted by UV light and drawn into the trap by an air draft created by a ventilator at the bottom of the trap. To avoid the catch of Lepidoptera etc., the intake funnel was covered by a net of 5 mm mesh size. Insects in the trap were drawn by the airstream into a plastic beaker filled with 70% ethanol to kill and preserve the insects. Traps were run in the first seven nights of each month from April 2007 to May 2008. If difficulties with the traps occurred, the period was slightly shifted. The lamps were switched on and off automatically at sunset and sunrise, respectively, by a photosensoric device. The alcohol containing the insects was replaced at different time intervals according to the size of the catch and transferred to the laboratory.
There, the whole batch or—if the batch contained >500 Culicoides—an aliquot was examined under a stereomicroscope (magnification 20—40×) to separate Ceratopogonidae and the other insects, then non-Culicoides and Culicoides, the latter identified by the occurrence of spotted wings (Campbell and Pelham-Clinton 1960; Goffredo and Meiswinkel 2004). In the Culicoides spp., the pigmentation patterns on the wings were used to determine up to the complexes Culicoides obsoletus s.l., Culicoides pulicaris s.l., and other Culicoides spp. (Campbell and Pelham-Clinton 1960; Goffredo and Meiswinkel 2004). However, the classification of some species into these complexes is discussed controversially (summarized by Meiswinkel et al. 2004). After separation of males according to the plumose antennae of males, females of C. obsoletus s.l. and C. pulicaris s.l. were further classified according to the feeding stage into engorged and unengorged specimens, the latter into groups of gravid (presence of eggs) or parous (burgundy-red spots on the abdomen) or nulliparous females (Dyce 1969).
The identification of the Culicoides species (all males and about 10% of females) was based on morphological characteristics like wing pattern, the localization of setae and spines of the antennae, and the structure of male and female genitalia (Campbell and Pelham-Clinton 1960; Boorman 1993; Rawlings 1996). The other females were sent to the national reference laboratory at the Friedrich-Loeffler Institute (Riems, Germany) for the identification of infections with BTV.
Temperature, absolute humidity, and relative humidity were recorded in 4-h intervals by a data logger (HOBO Pro RH/Temp, Typ H08-032-IS; ProSorb, Gottenheim). At each location, the logger was positioned close to the trap on the same level. For correlations of the number of ceratopogonids with mean, minimum and maximum temperatures, and relative humidities, only data from the collection periods were considered. Statistics were done by Statistica software version 8 (StatSoft, Hamburg).
Results
About one million insects were caught in 91 nights, slightly more non-ceratopogonids (51%) and of these often bark beetles. The percentage of the ceratopogonids in the catches varied between 1% and 71%. Of these, only about 6% belonged to the genus Forcipomyia and not to species of the genus Culicoides. Within the latter, about 90% were determined as C. obsoletus s.l., about 6% as C. pulicaris s.l., and 4% as other species of Culicoides.
According to the detailed determination, 22 different species occurred in Rhineland-Palatinate but only 12 in Saarland (Table 2). In a comparison of the individual locations, the highest and lowest numbers of species were present in Ahrweiler (AW; 18) and Germersheim (GE; 7) in the northwest and southeast of Rhineland-Palatinate, respectively. In Saarland, both locations showed a relatively low number of eight and nine species. The diversity of Culicoides spp. changed in 2007. In September and October, the highest number of species was caught, 17 and 15 species, respectively. At the beginning of 2008, only one species occurred (Fig. 2).
In all traps, the first ceratopogonids were caught in April 2007. Their total number increased from April to June 2007 followed by a slight reduction in July and a peak in August (Fig. 3). After a reduction by 84% in September, a second peak in total numbers occurred in October with about 58% of the total numbers of August. The number of peaks of abundance varied between the locations. At five locations, three peaks were evident, at four locations, two peaks. At three locations the peak occurred in August. From December 2007 to April 2008, only 305 ceratopogonids were caught. In December 2007, at six of the 12 locations, 177 ceratopogonids were trapped in total, 83% of them at one location. In January 2008, 25 were caught at two farms; in February, 36 at three farms; in March, 6 at one farm; and in April, 61 culicoids at six farms. In May 2008, the next strong increase of the populations started at all locations with more than 45,000 specimens in total. In nine of the 12 farms, the number was higher than in May of the previous year.
In all months, C. obsoletus s.l. was collected and dominated at each location during all months (Figs. 3 and 4). Low numbers of C. pulicaris s.l. were found in nearly all months, except January and March 2008, relatively high numbers only in May 2008. The highest numbers in the group of other species were collected in June and August 2007.
Considering each location separately, the total number of ceratopogonids varied between 5,000 in Trier-Saarburg (TR) and 120,000 in Daun (DA; Fig. 4). At the latter, 24% of all ceratopogonids were caught, 19% in AW and 14% in Bad Dürkheim (DÜ); about 9% in Merzig-Wadern (MW) and Kusel (KU); 5–8% in the Rhein-Hunsrück-Kreis (RH), Mayen-Koblenz (MY), and Alzey-Worms (AZ); and 1–2% of all ceratopogonids in Altenkirchen (AK), GE, TR, and St. Wendel (WE), respectively. Highest numbers of C. obsoletus s.l. were found in DA with 110,000, AW and DÜ with more than 60,000 specimens, and lowest numbers in TR and AK with less than 5,000 individuals. The numbers of C. pulicaris s.l. and individuals of other species varied stronger between the different locations. For both complexes, the highest numbers were found in AW (Fig. 4), C. pulicaris s.l. displaying about 15% of individuals. Similar levels were reached in GE, TR, and AK with 18%, 17%, and 14%, respectively. In TR, the individuals of other genera possessed the highest percentages with 38%, followed by AK and AZ with 26% and 17% of all ceratopogonids (Fig. 5).
Focussing on the gender, only a small number of males (5%) were collected in the UV light traps. For C. obsoletus s.l., the highest percentage of males was caught in April 2008, for C. pulicaris s.l. in November 2007 (Figs. 6 and 7). In both complexes, the percentages of males increased slowly until June 2007 and were reduced in July and August. Those of engorged females increased from June until the end of the year. In May 2008, the percentage of engorged females was much higher than one year before.
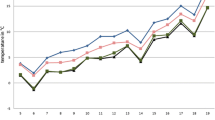
Mean temperatures increased in the collection period from about 8°C in April to 17°C in June 2007 (Fig. 8). After a reduction by about 2°C in July, again, 17°C was reached in August. Afterwards, temperatures dropped until January 2008 to 1°C and increased to 12°C in May 2008. Between the different locations, the mean temperatures differed by 2–4°C. At all locations, the mean temperatures in the sampling period of May 2008 were lower than in May 2007.
In calculations of Spearman correlations of the mean temperatures and the numbers of individuals of C. obsoletus s.l., C. pulicaris s.l., and total numbers of ceratopogonids, a significant correlation (p < 0,05) was evident at all locations except GE and DÜ. In DÜ, numbers of C. obsoletus s.l. and ceratopogonids correlated neither to the minimum nor the maximum temperatures, and the numbers of C. pulicaris s.l. did not correlate to the maximum temperature. In GE, none of the numbers of the three groups of the ceratopogonids correlated to minimum temperatures, and C. pulicaris s.l. did not correlate to mean temperatures. Plotting the numbers of individuals against the altitude (meters above sea level) seems to indicate a development of stronger populations of C. obsoletus s.l. at altitudes around 300 m (Fig. 9). For C. pulicaris s.l. and other Culicoides spp. or ceratopogonids, such tendency was not evident.
Discussion
UV light traps are a common method for insect-vector monitoring in the genus Culicoides (Baldet et al. 2004; Goffredo and Meiswinkel 2004; Purse et al. 2004; Miranda et al. 2004), but monitorings differ in the period of catching, e.g., once a week or several days per month. In addition, the period of time within the day is important. While most species of Culicoides show a maximum of activity at dawn (Olbrich 1987; Hill 1947), females of some hematophagous species are also active by day, e.g., Culicoides impunctatus, C. nubeculosus, and Culicoides vexans (Jobling 1953; Campbell and Pelham-Clinton 1960; Boorman and Goddard 1970). Specimens of these species were found also in the present investigation, but their low numbers can be due to the type of trap. Therefore, in further studies, different traps or attraction types should be used.
From Germany, 332 species of Ceratopogonidae are described, 57 of them belonging to the genus Culicoides (Havelka and Aguilar 1999). In the present investigation, 22 species were found in Rheinland-Palatinate and 12 in Saarland, but the diversity at the sampling sites differed strongly. Some of the species seem to be ubiquitous: species of the Obsoletus complex occur almost worldwide (Campbell and Pelham-Clinton 1960) and were found in Europe on the islands Cyprus, Mallorca, Minorca, and Corsica and in Italy, France, Great Britain, The Netherlands, and Germany (Hill 1947; Mellor and Pitzolis 1979; Olbrich 1987; Baldet et al. 2004; Miranda et al. 2004; Goffredo et al. 2004; Baldet and Delécolle 2006; Takken et al. 2006). The majority of investigations did not determine the specimens up to the species level in this complex, but C. obsoletus s.s. and Culicoides scoticus seem to be the most distributed species. These two species have been caught in the present investigation at all sampling sites. Culicoides chiopterus and Culicoides dewulfi were not found at one respectively two of the 12 sites. In the Pulicaris complex, C. pulicaris s.s. and Culicoides punctatus seem to be distributed ubiquitous in Germany (Olbrich 1987). Both species and Culicoides newsteadi were found at all locations in Rhineland-Palatinate and Saarland, Culicoides fagineus, C. impunctatus, and Culicoides lupicaris only at single locations.
The seasonal activity of ceratopogonids in Europe varies according to the region. In England, Culicoides spp. was active from April until end of October with maxima in June and September (Hill 1947) and in Lower Saxony (North Germany) from May to November with a maximum activity in August (Olbrich 1987). A bimodular abundance of C. obsoletus s.l. was reported for France with peaks in spring and autumn (Baldet et al. 2004). In the present investigation, one, two, or three peaks of abundances were evident at the different locations. The total number of individuals of C. obsoletus s.l. showed first a slight peak in June followed by maxima in August and October. Specimens of this complex were present during the whole winter.
Peaks of abundance can be caused by different generations per year, but in complexes of species, they can also be due to different species. In England, C. obsoletus s.s. was active from May to October with high abundances in June and at the end of September, while C. chiopterus showed nearly the same activity period with the highest abundance only in June (Hill 1947). In the Pulicaris complex, two species—C. pulicaris s.s. and C. punctatus—showed a similar appearance from April until October, mostly abundant in spring and late summer in Great Britain (Campbell and Pelham-Clinton 1960). In Germany, C. pulicaris s.s. was more abundant at the end of summer, while C. punctatus was present during the whole grazing season (Olbrich 1987). In Hesse, adults of C. pulicaris s.s. mainly hatched in May and of C. punctatus in August and October (Havelka 1976). This is identical with the maxima of abundance in the present investigation indicating that C. pulicaris s.s. may also be the most prevalent species of this complex in Rheinland-Palatinate and Saarland. Comparing the number of specimens of the different complexes, C. obsoletus s.l. always dominated, ranging from 96% of all ceratopogonids in April 2007 to 69% in May 2008. At the different locations, it ranged from 45% in TR to 95% in MW. C. pulicaris s.l. was much less abundant with 1.8% in August 2007 and 30% in May 2008. Percentages of the specimens of the Pulicaris complex from the total number of ceratopogonids differed between the locations from 0.2% in DÜ to 18% in GE.
Like in all insects, the activity of the ceratopogonids is strongly affected by biotic and abiotic factors, e.g., the temperature. This was examined in detail for C. variipennis, being active from 10°C to 30°C (Nelson and Bellamy 1971). In the present investigation, the total number of ceratopogonids was significantly correlated to minimum, maximum, and mean temperatures during the trapping period at nearly all locations. The highest numbers were caught in August at mean temperatures of 17.3°C during the trapping period.
Preference for an altitude range is known for C. imicola in Italy. There, it seems to prefer sparsely vegetated plateaus about 210 m (asl), and C. obsoletus s.l. mainly altitudes about 590 m, but still, more than 10% of catches were done >1,000 m (Conte et al. 2007). In Switzerland, C. obsoletus s.l. was present >1,100 m (Cagienard et al. 2006). Our data indicate a preference of C. obsoletus s.l. for altitudes about 300 m in Rhineland-Palatinate and Saarland, but influences of other abiotic or biotic factors, e.g., vegetation or soil (Conte et al. 2007), have to be considered in more detailed investigations.
The gender relation is noticeable. In previous studies, in North Rhine-Westphalia with UV light traps, the number of females of C. obsoletus was 2–40-fold higher than that of males (Mehlhorn et al. 2007). In the present investigation, nearly 50-fold more females than males of C. obsoletus s.l. and C. pulicaris s.l. have been caught. This difference might be due to the fact that only females are hematophagous, mating or swarming places are away from the host (Downes 1950; Mehlhorn et al. 2007), or that the ultraviolet light does not attract males so strongly as females. The increase in the percentages of engorged females in both complexes of Culicoides within the year can be explained by the lifespan of adults. Thereby, over the summer, the numbers of older females are increasing. Further studies are also necessary in this topic to clarify the natural lifespan for a calculation of the risk of BTV transmission.
References
Baldet T, Delécolle J-C (2006) Studies on Culicoides found in association with livestock in the bluetongue virus (BTV) affected region of northern France. CIRAD, France, Annex D to Appendix 9
Baldet T, Delécolle J-C, Mathieu B, de La Rocque S, Roger F (2004) Entomological surveillance of bluetongue in France 2002. Vet Ital 40:226–231
Becker P (1961) Observations on the life cycle and immature stages of Culicoides circumscriptus Kieffer (Diptera, Ceratopogonidae). Proc R Soc Edinb [Biol] 67:363–387
Boorman J (1993) Biting midges (Ceratopogonidae). In: Lane RP, Crosskey RW (eds) Medical insects and arachnids. Chapman & Hall, London, pp 289–309
Boorman J, Goddard P (1970) Observations on the biology of Culicoides impunctatus Goetgh. (Diptera: Ceratopogonidae) in southern England. Bull Entomol Res 60:189–198
Borkent A (2008) World species of biting midges (Diptera: Ceratopogonidae). Available: http://www.inhs.uiuc.edu/cee/FLYTREE/CeratopogonidaeCatalog.pdf. Accessed 01 March 2009
Cagienard A, Griot C, Mellor PS, Denison E, Stärk KDC (2006) Bluetongue vector species of Culicoides in Switzerland. Med Vet Entomol 20:239–247
Campbell JA, Pelham-Clinton EC (1960) A taxonomic review of the British species of Culicoides Latreille (Diptera: Ceratopogonidae). Proc R Soc Edinb [Biol] 67:181–302
Conte A, Goffredo M, Ippoliti C, Meiswinkel R (2007) Influence of biotic and abiotic factors on the distribution and abundance of Culicoides imicola and the Obsoletus complex in Italy. Vet Parasitol 150:333–344
Downes JA (1950) Habits and life-cycle of Culicoides nubeculosus Mg. Nature 166:510–511
Dyce AL (1969) The recognition of nulliparous and parous Culicoides (Diptera: Ceratopogonidae) without dissection. J Aust Entomol Soc 8:11–15
Goffredo M, Meiswinkel R (2004) Entomological surveillance of bluetongue in Italy: methods of capture, catch analysis and identification of Culicoides biting midges. Vet Ital 40:260–265
Goffredo M, Conte A, Meiswinkel R (2004) Distribution and abundance of Culicoides imicola, Obsoletus complex and Pulicaris complex (Diptera: Ceratopogonidae) in Italy. Vet Ital 40:270–273
Havelka P (1976) Limnologische and systematische Studien an Ceratopogoniden. Beitr Entomol 26:211–305
Havelka P, Aguilar M (1999) Ceratopogonidae. In: Schuhmann H, Bährmann R, Stark A (eds) Entomofauna Germanica 2—Checkliste der Dipteren Deutschlands. Studia Dipterologica. Suppl. 2. Ampyx-Verlag, Halle (Saale), pp 33–38
Hill M (1947) The life-cycle and habits of Culicoides impunctatus Goetghebuer and Culicoides obsoletus Meigen together with some observations on the life cycle of Culicoides obdibilis Austen, Culicoides pallidicornis Kieffer, Culicoides cubitalis Edwards and Culicoides chiopterus Meigen. Ann Trop Med Parasitol 41:55–115
Jobling B (1953) On the blood-sucking midge Culicoides vexans Stager, including the description of its eggs and the first-stage larva. Parasitology 43:148–159
Kettle DS, Lawson JWH (1952) The early stages of British biting midges Culicoides Latreille (Diptera: Ceratopogonidae) and allied genera. Bull Entomol Res 43:421–467
Lysyk TJ, Danyk T (2007) Effect of temperature on life history parameters of adult Culicoides sonorensis (Diptera: Ceratopogonidae) in relation to geographic origin and vectorial capacity for bluetongue virus. J Med Entomol 44:741–751
Mehlhorn H (2008) Ceratopogonidae. In: Mehlhorn H (ed) Encyclopedia of parasitology, vol 1, 3 rdth edn. Springer-Verlag, Heidelberg, pp 214–216
Mehlhorn H, Walldorf V, Klimpel S, Jahn B, Jaeger F, Eschweiler J, Hoffmann B, Beer M (2007) First occurrence of Culicoides obsoletus-transmitted bluetongue virus epidemic in Central Europe. Parasitol Res 101:219–228
Meiswinkel R, Gomulski LM, Delécolle J-C, Goffredo M, Gasperi G (2004) The taxonomy of Culicoides vector complexes—unfinished business. Vet Ital 40:151–159
Mellor PS (1990) The replication of bluetongue virus in Culicoides vectors. Curr Top Microbiol Immunol 162:143–161
Mellor PS, Pitzolis G (1979) Observations on breeding sites and light-trap collections of Culicoides during an outbreak of bluetongue in Cyprus. Bull Entomol Res 69:229–234
Mellor PS, Boorman J, Baylis M (2000) Culicoides biting midges: their role as arbovirus vectors. Annu Rev Entomol 45:307–40
Miranda MA, Rincón C, Borràs D (2004) Seasonal abundance of Culicoides imicola and C. obsoletus in the Balearic Islands. Vet Ital 40:292–295
Nelson RL, Bellamy RE (1971) Patterns of flight activity of Culicoides variipennis (Coquillett) (Diptera: Ceratopogonidae). J Med Entomol 8:283–291
Olbrich S (1987) Untersuchungen zur Biologie von Gnitzen der Gattung Culicoides Latreille (Diptera: Ceratopogonidae) an Weiderindern in Norddeutschland. Ergebnisse aus dem Freiland und dem Laboratorium. Ph-Diss., Fakultät Biologie, Tierärztliche Hochschule Hannover
Purse BV, Baylis M, Tatem AJ, Rogers DJ, Mellor PS, Van Ham M, Chizov-Ginzburg A, Braverman Y (2004) Predicting the risk of bluetongue through time: climate models of temporal patterns of outbreaks in Israel. Rev Sci Tech 23:761–775
Rawlings P (1996) A key, based on wing patterns of biting midges (genus Culicoides Latreille-Diptera: Ceratopogonidae) in the Iberian Peninsula, for use in epidemiological studies. Graellsia 52:57–71
Takken W, Verhulst N, Scholte E-J, Jacobs F, Jongema Y, de Koeijer A, van Roermand H, van Lammeren R (2006) Longitudinal sampling of Culicoides spp. in order to determine the risk of bluetongue outbreaks in the Netherlands. Wageningen, Netherlands (Annex A to Appendix 9)
Acknowledgements
We very much appreciate the funding of the project by the German federal ministry of food, agriculture, and user protection (BMELV) and the help of all farmers. We also thank A. Albring, D. Bellhoff, H. Boeddinghaus, I. Busch, R. Cassada, R. Conrad, A. Gajewski, J. Jesiek, L. Johrden, N. Kalweit, L. Klenner, M. Lucht, B. Meiser, M. Oldenburg, J. Pausch, T. Reichelt, J. Sareyka, I. Schalow, B. Schattling, S. Schulz, A. Schwarz, D. Segelcke, C. Soukou, and M. Stein for the separations of the different groups of insects and/or the evaluation of the results.
Author information
Authors and Affiliations
Corresponding author
Additional information
Bettina Vorsprach and Christian Karl Meiser contributed equally to this study.
Rights and permissions
About this article
Cite this article
Vorsprach, B., Meiser, C.K., Werner, D. et al. Monitoring of Ceratopogonidae in Southwest Germany. Parasitol Res 105, 337–344 (2009). https://doi.org/10.1007/s00436-009-1411-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-009-1411-3