Abstract
To assess the hypothesis that nitric oxide (NO) is critical in the pathogenesis of cerebral malaria, we analyzed those single nucleotide polymorphisms (SNPs) and microsatellite (MS) of the promoter region of inducible nitric oxide synthase (iNOS) gene which are known to enhance the NO production in vivo. A total of 428 (204 severe, 224 mild) adult patients living in the eastern part of India were analyzed. The single nucleotide substitutions −954G→C was found to be very rare, and −1173C→T was absent in this population. But interestingly, longer forms of MS were found to be significantly associated with severe malaria (OR = 2.89, 95% CI = 1.955–4.295, P < 0.0001), and the linear regression analysis revealed that the risk of severe malaria significantly increases as the summed repeat number in an individual increase (OR = 1.16, P = 0.0013). Further, the median plasma level of nitrate/nitrite (NOx) was observed to be high in mild patients compared to severe patients, and the level of parasitemia was significantly low among mild patients than severe ones. These findings suggest that the CCTTT repeats in iNOS may play a key role in the pathogenesis of severe malaria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malaria infects 300–500 million people annually, but fortunately, only a minority (1–2%) develops severe or fatal infection (Greenwood and Mulshingwa 2002). Why some young children and nonimmune hosts die, while others develop only a febrile illness, is not known. The ancient nature of relationship with malaria suggests that there might be balances in the host–parasite relationship that allow the majority of hosts to survive, while permitting the parasites to continue to propagate. Many factors such as host genetics, environmental determinants, socioeconomic conditions, and parasite properties (virulence) are thought to have roles in malaria pathogenesis (Miller et al. 2002). The oldest and best known, but still not fully understood, genetic factor for resistance is the sickle cell trait (Serjeant 1992). Other polymorphisms especially in immunorelevant genes have been associated with the clinical course or occurrence of malaria. Examples include polymorphisms in the tumor necrosis factor (TNF-α) promoter (Knight et al. 1999), the mannose-binding lectin (MBL) gene (Luty et al. 1998), major histocompatibility (MHC) genes (Hill et al. 1991), and the red blood cell (RBC) polymorphism (Agarwal et al. 2000). Recently, another gene involved in innate immunity, the inducible nitric oxide synthase (iNOS) has been found to play a crucial role in Plasmodium falciparum infection. The iNOS is one of the three distinct isoenzymes responsible for the catalytic oxidation of l-arginine yielding l-citrulline and the short-lived reactive free radical NO in various nucleated cells. A unique feature of iNOS is that when it is triggered by an immunologic and inflammatory stimulus, a sustained production of NO results. The in vitro, antiplasmodial activity of NO has been demonstrated (Mellouk et al. 1994), and high plasma level of NO has been found to be correlated with rapid in vivo parasite clearance (Chiwakata et al. 2000). There is also evidence that NO can inhibit adhesion of platelet, leukocyte, and P. falciparum-infected RBCs to endothelium, a process central to the pathogenesis of cerebral malaria (Serirom et al. 2003).
The gene-encoding iNOS is located on chromosome 17 and spans 37 kb(Chartrain et al. 1994). Several mutations in the promoter region of this gene have been found to be associated with its high level of expression in vivo and disease outcome in malarial infections, of course with some contradictions. In Gabon, −954G→C mutation was found to confer relative resistance to severe malaria, but not in Gambia and Tanzania (Kun et al. 1998; Hobbs et al. 2002; Burgner et al. 2003). Similarly, a protective role of iNOS −1173C→T against severe malaria has been demonstrated in Tanzania (Hobbs et al. 2002) but not in the Gambia (Burgner et al. 2003). Further, the CCTTT repeats present in −2.6 kb of the transcription site have been found to be associated with different clinical manifestations of P. falciparum malaria (Burgner et al. 1998; Ohashi et al. 2002). To investigate these apparently contradictory reports, we have made an attempt for the first time to study the association between the iNOS gene polymorphisms and disease severity in P. falciparum infection in Orissa, an eastern Indian state, with a unique epidemiological setting.
Materials and methods
Study area
The study was conducted from April 2006 and March 2007 in SCB Medical College and Hospital Cuttack, of Orissa. The state of Orissa is situated along the east coast and extends from 17°49′ N to 22°34′ N latitude and 81°28′ E to 87°29′ E longitude. The total population of the state is 36.8 million, 22.21% of them being aboriginal (Census of India 2001). The state is considered as hyperendemic for malaria, and the transmission is perennial with a seasonal peak (July to October). All four species of human malaria are found in Orissa, but >85% of all clinical malaria are due to P. falciparum (Ranjit 2006).
Patients
Clinically suspected malaria patients were screened for P. falciparum infection using both thick and thin film method. Severity of malaria was classified according to the definitions and associated characters published by the WHO (WHO 2000). Exclusion criteria were symptoms of mild or severe malaria with other acute infections including intestinal geohelminthic infections, chronic diseases like tuberculosis, leprosy, and malnutrition, and genetic disorders like haemoglobinopathies/thalassaemia and G6PD deficiency. About 2 ml of venous blood was collected in ethylenediaminetetraacetic acid (EDTA) vials from all enrolled (mild as well as severe) patients. Each patient was treated according to local guide lines, and care was provided until discharge from the hospital. The study was approved by the Ethical Committee of the Regional Medical Research Centre, Bhubaneswar.
DNA isolation
The human genomic DNA was purified from 200 μl of blood following the standard protocol (Sambrook and Russel 2001). In brief, blood cells were lysed with lysis buffer (10 mM Tris–HCl, pH 8.0; 0.1 M EDTA, pH 8.0; 0.5% sodium dodecyl sulfate, and 20 μg/ml pancreatic RNase) at 37°C for 1 h, and then proteinase K (100 μg/ml) was added, and the lysate was incubated at 54°C for 3 h. DNA was obtained by phenol–chloroform extraction and ethanol precipitation and then resuspended in 50 μl of DNase-free water.
PCR amplification and genotyping
The iNOS −954G→C polymorphism was identified by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) technique (Kun et al. 2001), and the −1173C→T polymorphism was detected by mutagenically separated PCR assays (Hobbs et al. 2002). The PCR products and restriction enzyme-digested fragments were resolved by electrophoresis on 2% Nuseive agarose containing ethidium bromide (0.5 μg/ml) and visualized under UV transilluminator. The pentanucleotide (CCTTT) microsatellite was analyzed by PCR amplification (Xu et al. 1997) followed by polyacrylamide gel electrophoresis (Hoefer SQ3 Sequencer, Amersham Biosciences, USA).
NOx assay
Plasma NO3 − plus NO2 − (NOx) was measured as NO2 − after enzymatic conversion of NO3 − to NO2 − by nitrate reductase according to the manufacturer’s instruction using a commercial method (R&D Systems, MN, USA) in 55 mild malaria cases and 45 cerebral malaria cases.
Statistical analysis
The frequencies of the microsatellite alleles were compared between severe and mild malaria patients using the χ 2 test, and linear regression analysis was carried out to examine the association of the summed repeat number of MS alleles with severity of malaria. Quantitative data were expressed as the mean ± SD and were compared by unpaired t test. Qualitative data were compared by Yates corrected χ 2 or Fisher’s exact test. The quantitative value of plasma NOx levels were compared by Mann–Whitney U test. Statistical analysis was performed using SPSS software.
Results
A total of 204 patients (mean age 34.2 years) with severe malaria and 224 patients (mean age 44.1 years) with mild malaria belonging to tribal communities were enrolled in this study. Mean parasite density was significantly high (P < 0.001) among the severe malaria patients (45,601.7/μl) compared to uncomplicated malaria patients (3,827.6/μl). In the severe group, all were cases of cerebral malaria. Of these, 6.8% had hyperparasitemia (>25,000 parasites/μl), 20.5% had scizontemia, 32.3% had generalized convulsions, and 85.3% had jaundice (Table 1).
Allele frequency of −954G→C and −1173C→T polymorphisms
Out of a total 204 severe malaria patients, only four individuals were found to harbor −954 G→C allele, and none of the patients in mild group had this mutation (Table 1). Thus, the frequency of −954 G→C allele becomes 0.00467 in the selected population. Statistical analysis also reveals that there is no association of this polymorphism with severe manifestation of the disease. Among the selected patients, no individual was found to have −1173C→T mutation.
Allele frequency of CCTTT microsatellite repeats polymorphism
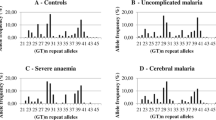
A total of eight different alleles (ten to17 repeats) of MS were found to be prevalent in this population. The frequency distribution of MS alleles was normal type ranging from 5.8% (ten repeats) to 1.6% (17 repeats) with a peak of 19.2% in repeat 13 (Fig. 1). Alleles of ≤13 repeats (short form) were found to be present in 66.1% (148 out of 224) of mild cases and 40.2% (82 out of 204) of severe cases, where ≥14 repeats (long form) were found to be present in 59.8% (122 out of 204) of patients with severe malaria and 33.9% (76 out of 224) of patients with mild malaria (Table 1). Statistical analysis reveals that longer forms of MS were significantly associated with severe malaria (OR = 2.89, 95% CI = 1.955–4.295, P < 0.0001). The linear regression analysis revealed that the risk of severe malaria significantly increases as the summed repeat number in an individual increases (OR = 1.16, P = 0.0013).
Plasma NOx
The median plasma level of NOx was observed to be significantly high (P < 0.0001) in mild malaria cases (75.4 mm/L; interquartile range 69.3–93.7 mm/L) compared to severe malaria (48.0 mm/L; interquartile range 39.4–81.3 mm/L) cases.
Discussion
Nitric oxide acts as an extra- and intercellular messenger participating in vascular homeostasis, neurotransmission, and defense against infectious agents (Nathan 1992). However, the role of NO in human malaria has generated much debate. Some studies have described the role of NO as protective (Mellouk et al. 1994; Chiwakata et al. 2000; Serirom et al. 2003), while others as pathogenic (Van Hensbroek et al. 1997). In the later studies, the fatal cerebral malaria has been attributed to the increased NO production by iNOS causing direct neurotoxicity or vasodilatation and raised cerebral pressure (Maneerat et al. 2000; Clark et al. 2003). During the present study, we have tried to find out the role of iNOS by analyzing the association of previously described iNOS-promoter variants responsible for increased NO production with clinical outcome of P. falciparum infection. This is the first study from Indian subcontinent and bears importance because the populations here are ethnically different from other parts of the world. Interestingly, the −954G→C and −1173C→T SNPs previously linked with severe malaria in some parts of Africa (Kun et al. 1998; Hobbs et al. 2002) are not present in our population who are also exposed to intense malaria transmission. Similar results have been observed among populations of Papua New Guinea and Thailand in Asia (Boutlis et al. 2003; Ohashi et al. 2002) and of Gambia and Ghana in Africa (Burgner et al. 2003; Cramer et al. 2004). Such conflicting results on association of malaria with these known SNPs in the promoter region of iNOS do not necessarily exclude the role of NO in malaria because a luciferase gene reporter assay has shown that the number of microsatellites repeat in the promoter region affects the level of iNOS transcription, as what seems to occur at the insulin gene minisatellite locus (Warpeha et al. 1999). We have observed a strong association of the short forms of CCTTT alleles with mild malaria and increased summed repeat number of MS as significant risk factor for severe malaria in our population. Further, we have also observed that the plasma level of NOx was significantly high among the uncomplicated/mild group of patients than severe ones (Fig. 2), and the level of parasitemia was significantly low in mild malaria cases compared to the severe group of patients. Therefore, it is possible that in the present situation, the short form of pentanucleotide MS repeat is increasing the NO level by influencing the level of iNOS transcription in the mild group of malaria patients. NO decreases expression of the cytokine inducible endothelial adhesion molecules, intercellular adhesion molecule-1 (ICAM 1), vascular cell adhesion molecule-1 (VCAM-1) and E-selectin, involved in cytoadherence and microvascualr sequestration of parasitized red cells (Turner 1997). If short forms of CCTTT results in enhanced iNOS expression and NO production in activated endothelium and local macrophages, then expression of adhesion molecules could be diminished, decreasing organ microvascular sequestration of P. falciparum-infected red blood cells and end-organ complications such as cerebral malaria. Further, the NO potently decreases macrophage production of tumor necrosis factor (Florquin et al. 1994). There is strong evidence that tumor necrosis factor, lymphotoxin, and other proinflammatory cytokines are directly related to the pathogenesis of severe malaria (Engwerda et al. 2002). Increased NO production due to the presence of the short form CCTTT alleles, possibly downregulating the production of proinflammatory cytokines in response to infection might explain the decreased risk of severe malaria in those with short-form CCTTT alleles. A similar result has been found in Thai population (Ohashi et al. 2002), whereas, shorter forms of the CCTTT repeat (≤10 repeats) and a reduced summed repeat length have been found to be associated with fatal cerebral malaria compared to cerebral malaria survivors, malarial anemia, and mild malaria in Gambian children (Burgner et al. 1998). Such contrasting result with Gambian study might be (1) because our study includes only adult cerebral survivors and (2) due to difference in ethnicity of the population, since previous studies have described that the evolutionary selection by malaria is not only very strong but also population specific at times which is evident at a global as well as local levels (Flint et al. 1998; Tishkoff and Williams 2002). Further, the distribution of MS variants among Africans show typical bimodal distribution with over-representation of nine and ten copies unlike Caucasians, where the CCTTT repeats show even distribution with a peak at 12 copies (Burgner et al. 2003; Cramer et al. 2004). The distribution of MS variants in our population is however even like the Caucasians, but the peak is at 13 copies (Fig. 1). Such variation in host genetics and disease phenotype may be due to different parasite strains and/or some other unidentified selection pressure. Since the MS repeats are potentially unstable and of unknown functional relevance (Burgner et al. 2003), presence of some other unknown mutation in the promoter region or the coding gene itself cannot also be ruled out in our population which might be giving protection against severe malaria. Our result warrants a more detailed analysis of iNOS and its promoter in malaria, which will provide novel preventive and therapeutic strategies against these major causes of death.
References
Agarwal A, Guindo A, Cissoko Y, Taylor JG, Coulibaly D, Kone A, Kayentao K, Djimde A, Plowe CV, Doumbo O, Wellems TE, Diallo D (2000) Hemoglobin C associated with protection from severe malaria in the Dogon of Mali, a West African population with a low prevalence of hemoglobin S. Blood 96:2358–2363
Boutlis CS, Hobbs MR, Marsh RL, Misukonis MA, Tkachuk AN, Lagog M, Booth J, Granger DL, Bockarie MJ, Mgone CS, Levesque MC, Weinberg JB, Anstey NM (2003) Inducible Nitric oxide synthase (NOS2) promoter CCTTT repeat polymorphism: relationship to in vivo nitric oxide production/NOS activity in an asymptomatic malaria-endemic population. Am J Trop Med Hyg 69:569–573
Burgner D, Xu W, Rockett K, Gravenor M, Charles IG, Hill AV, Kwiatkowski D (1998) Inducible nitric oxide synthase polymorphism and fatal cerebral malaria. The Lancet 352:1193–1194
Burgner D, Usen S, Rockett K, Jallow M, Ackerman H, Cervino A, Pinder M, Kwiatkowski DP (2003) Nucleotide and haplotypic diversity of the NOS2A promoter region and its relationship to cerebral malaria. Hum Genet 112:379–386
Census of India (2001) Provisional population totals, series-22. Orissa
Chartrain NA, Geller DA, Koty PP, Sitrin NF, Nussler AK, Hoffman EP, Billiar TR, Hutchinson NI, Mudgett JS (1994) Molecular cloning, structure and chromosomal localization of the human inducible nitric oxide synthase gene. J Biol Chem 269:6765–6772
Chiwakata CB, Hemmer CJ, Dietrich M (2000) High levels of inducible nitric oxide synthase mRNA are associated with increased monocyte counts in blood and have a beneficial role in Plasmodium falciparum malaria. Infect Immun 68:394–399
Clark IA, Rockett KA, Burgner D (2003) Genes, nitric oxide and malaria in African children. Trends Parasitol 19:335–337
Cramer JP, Mockenhaupt FP, Ehrhardt S, Burkhardt J, Otchwemah RN, Dietz E, Gellert S, Bienzle U (2004) iNOS promoter variants and severe malaria in Ghanian Children. Trop Med Int Health 9:1074–1080
Engwerda CR, Tracey L, Mynott TL, Sawhney S, De Souza JB, Bickle QD, Kaye PM (2002) Locally up-regulated lymph toxin alpha, not systemic tumor necrosis factor alpha, is the principal mediator of murine cerebral malaria. J Exp Med 195:1371–1377
Flint J, Harding RM, Boyce AJ, Clegg JB (1998) The population genetics of the hemoglobinopathies. Bailliere’s Clin Haematol 11:1–51
Florquin S, Amraoui Z, Dubois C, Decuyper J, Goldman M (1994) The protective role of endogenously synthesized nitric oxide in staphylococeal enterotoxin B-induced shock in mice. J Exp Med 180:1153–1158
Greenwood B, Mulshingwa T (2002) Malaria in 2002. Nature 415:670–672
Hill AV, Allsopp CE, Kwiatkowski D, Anstey NM, Twumasi P, Rowe PA, Bennett S, Brewster D, McMichael AJ, Greenwood BM (1991) Common west African HLA antigens are associated with protection from severe malaria. Nature 352:595–600
Hobbs MR, Udhayakumar V, Levesque MC, Booth J, Roberts JM, Tkachuk AN, Pole A, Coon H, Kariuki S, Nahlen BL, Mwaikambo ED, Lal AL, Granger DL, Anstey NM, Weinberg JB (2002) A new NOS2 promoter polymorphism associated with increased nitric oxide production and protection from severe malaria in Tanzanian and Kenyan children. The Lancet 360:1468–1475
Knight JC, Udalova I, Hill AVS, Greenwood BM, Peshu N, Marsh K, Kwiatkowski D (1999) A polymorphism that affects OCT-1 binding to the TNF promoter region is associated with severe malaria. Nat Genet 22:145–150
Kun JF, Mordmuller B, Lell B, Lehman LG, Luckner D, Kremsner PG (1998) Polymorphism in promoter region of inducible nitric oxide synthase gene and protection against malaria. The Lancet 351:265–266
Kun JF, Mordmuller B, Perkins DJ, May J, Mercereau-Puijalon O, Alpers M, Weinberg JB, Kremsner PG (2001) Nitric oxide synthase 2 (Lambarene) (G-954C), increased nitric oxide production, and protection against malaria. J Infect Dis 184:330–336
Luty AJF, Kun JFJ, Kremsner PG (1998) Mannose-binding lectin plasma levels and gene polymorphisms in Plasmodium falciparum malaria. J Infect Dis 178:1221–1224
Maneerat Y, Viriyavejakul P, Punpoowong B, Jones M, Wilairantana P, Pongponratn E, Turner G, Udomsangpetch R (2000) Inducible nitric oxide synthase expression is increased in the brain in fatal cerebral malaria. Histopathology 37:269–277
Mellouk S, Hoffman SL, Liu ZZ, de la Vega P, Billiar TR, Nussler AK (1994) Nitric Oxide mediated plasmodial activity in human and murine hepatocytes induced by gamma interferon and the parasite itself: enhancement by exogenous tetrahydrobiopterin. Infect Immun 62:4043–4046
Miller LH, Baruch DI, Marsh K, Doumbo OK (2002) The pathogenic basis of malaria. Nature 415:673–679
Nathan C (1992) Nitric oxide as a secretary product of mammalian cells. FASEB Journal 6:3051–3064
Ohashi J, Naka I, Patarapotikul J, Hananantachai H, Looareesuwan S, Tokunaga K (2002) Significant association of longer forms of CCTTT microsatellite repeat in the inducible nitric oxide synthase promoter with severe malaria in Thailand. J Infect Dis 186:578–581
Ranjit MR (2006) The epidemiology of malaria in Orissa. ICMR Bulletin 36:29–38
Sambrook J, Russell DW (2001) Molecular cloning—a laboratory manual, 3rd edition. Col Spring Harbor Laboratory Press, New York 1:6.4–6.11
Serirom S, Raharjo WH, Chotivanich K, Loareesuwan S, Kubes P, Ho M (2003) Anti-adhesive effect of nitric oxide on Plasmodium falciparum cytoadherence under flow. Am J Pathol 162:1651–1660
Serjeant GR (1992) Sickle cell disease. Oxford Medical, Oxford
Tishkoff SA, Williams SM (2002) Genetic analysis of African populations: human evolution and complex disease. Nat Rev Genet 3:611–621
Turner G (1997) Cerebral malaria. Brain Pathology 7:569–582
Van Hensbroek MB, Palmer A, Jaffar S, Schneider G, Kwiatkowski D (1997) Residual neurologic sequelae after childhood cerebral malaria. J Pediat 131:125–129
Warpeha KM, Xu W, Liu L, Charles IG, Patterson CC, Ah-Fat F, Harding S, Hart PM, Chakravarthy U, Hughes AE (1999) Genotyping and functional analysis of a polymorphic (CCTTT)n repeat of NOS2A in diabetic retinopathy. FASEB J 13:1825–1832
WHO (2000) WHO expert committee on malaria. Twentieth Report. World Health Organization Technical Report Series. Geneva No.892
Xu W, Liu L, Emson PC, Harrington CR, Charles IG (1997) Evolution of a homopurine-homopyrimidine pentanucleotide repeat sequence upstream of the human inducible nitric oxide synthase gene. Gene 204:165–170
Acknowledgements
The authors thankfully acknowledge Indian Council of Medical Research, New Delhi for financial support and to the Director RMRC, Bhubaneswar for providing necessary laboratory facilities for the study. We also acknowledge Council of Scientific and Industrial Research, New Delhi for providing fellowship to Mr. G Dhangadamajhi to carry out the research work. The authors are grateful to the patients who participated in the study.
Conflicts of Interest
The authors have no conflicts of interest connecting to the work reported in this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Statement of authorship
G DhangdaMajhi: conducted laboratory analysis of samples and performed statistical analysis of the data, B N Mohaptra: selection and provision of treatment to the patients, S K Kar: clinicopathological analysis and interpretation of data, M R Ranjit: conception/design of the study, execution, and interpretation of the data and drafting the paper.
Rights and permissions
About this article
Cite this article
Dhangadamajhi, G., Mohapatra, B.N., Kar, S.K. et al. The CCTTT pentanucleotide microsatellite in iNOS promoter influences the clinical outcome in P. falciparum infection. Parasitol Res 104, 1315–1320 (2009). https://doi.org/10.1007/s00436-009-1329-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-009-1329-9