Abstract
DEAD box proteins are putative RNA unwinding proteins found in organisms ranging from mammals to bacteria. We have identified a novel immunodominant cDNA clone, BmL3-helicase, encoding DEAD box RNA helicase by immunoscreening of a larval stage cDNA library of Brugia malayi. The cDNA sequence exhibited strong sequence homology to Caenorhabditis elegans and C. briggsae RNA helicase, a prototypic member of the DEAD (Asp-Glu-Ala-Asp) box protein family. The clone also showed similarity with RNA helicase of Wolbachia, an endosymbiotic bacterium of filarial parasite. It was overexpressed as ∼50 kDa His-tag fusion protein, and ATP hydrolysis assay of recombinant enzyme showed that either ATP or dATP was required for the unwinding activity, indicating BmL3-helicase as an ATP/dATP-dependent RNA helicase. The recombinant protein also demonstrated cross-seroreactivity with human bancroftian sera. The presence of BmL3-helicase in various life stages of B. malayi was confirmed by immunoblotting of parasite-life-cycle extracts with polyclonal sera against the BmL3-helicase, which showed high levels of expression in microfilaria, L3, and adult (both male and female) stages. In the absence of an effective macrofilaricidal agent and validated anti-filarial drug targets, RNA helicases could be utilized as a rational drug target for developing agents against the human filarial parasite.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lymphatic filariasis caused by Wuchereria bancrofti, Brugia malayi, and Brugia timori is an incapacitating disease transmitted by mosquitoes (Culex, Aedes, and Anopheles). About 120 million cases occur worldwide in tropical and sub-tropical countries, and 1.1 billion people are at risk (WHO 1997; Michael 2000), exacting a heavy socioeconomic toll in developing nations (Ottesen et al. 1997; Frank et al. 1996; Helmy et al. 2000). Present treatments for lymphatic filariasis include annual doses of albendazole plus diethylcarbamazine (DEC), albendazole plus ivermectin, or use of DEC-fortified salt, treatments which are directed principally at the larval forms of the parasites (microfilariae) and have limited or no action on adult worms (Ismail 1998; Sharma 2002).
The life cycle of filarial parasites includes microfilariae, larval (L2, L3, and L4), and adult male or female stages. During L3, the infective stage, the parasite gains entry into vertebrate host through vector mosquito bite; therefore, both vector-stage and vertebrate-stage L3 development are associated with the expression of a characteristic set of genes which can be used as potential drug targets in attempts to block or prevent the infection (Ibrahim et al. 1992). Immunological screening of cDNA libraries of B. malayi and other nematodes has been used to find specific immunoreactive genes involved in the host’s immune response or generation of protective immunity. The recently released draft genome sequence of B. malayi also presents opportunities for a systems-based approach to understanding the molecular basis of parasitism and identifying and characterizing novel proteins with unique functions which can be used as parasite intervention targets (Elodie et al. 2007).
In the present study, we explored a novel class of proteins called DEAD box RNA helicases which have not yet been identified in filarial nematodes. The DExD/H box families of RNA helicases are a multifunctional group of proteins involved in unwinding of inter- and intra-molecular base-paired regions, which is a required step in many biochemical processes. They are principally ATP-dependent helicases and utilize ATP as their energy source, thus participating in a highly specific way in a variety of processes involving RNA. All members of the DEAD box protein family share eight conserved motifs including the characteristic “DExD/H” sequence (V/I)-L-D-E-AD-X-(M/L)-L-X-X-G-F (where x can be any amino acid) (Walker et al. 1982; Andersen et al. 2006; Jankowsky and Bowers 2006). DExH/D proteins, a subgroup of the Super family 2 (SF2) helicases, have been implicated in every step of RNA metabolism, including nuclear transcription, pre-mRNA splicing, ribosome biogenesis, nucleocytoplasmic transport, translation, RNA decay, organellar gene expression, and protein displacement from RNA. In the present study, we have overexpressed and characterized a novel immunodominant cDNA clone encoding DEAD box RNA helicase isolated by immunoscreening of larval stage cDNA library of B. malayi.
Materials and methods
Immunoscreening of cDNA library
Infective larvae were recovered from gently crushed Aedes aegypti mosquitoes and cleaned in Ringer’s solution. Larvae were exposed to Cobalt60 irradiation at dose of 25 krad and inoculated subcutaneously to mastomys (∼100 larvae) three times at 4-week intervals (Weil et al. 1992) until the animals developed high antibody titer to L3. Serum antibody titer was assessed by ELISA using somatic soluble antigen of L3 as mentioned earlier (Verma et al. 2007). A B. malayi L3 cDNA library (SAW94WL-BmL3 library) constructed in the lambda UniZap XR vector (Stratagene) kindly provided by Prof. S.A. Williams, Smith College, Northampton, Massachusetts, USA was used for immunoscreening with high titer pooled L3 resistant serum (Verma et al. 2007). Nucleotide sequencing of the 5′ and 3′ ends of the inserts using universal T3 and T7 primers was performed at the UDSC Department of Biochemistry, University of Delhi, South Campus, New Delhi, India, using automatic sequencer ABI Prism (Version 3.0. Model 3100). The open reading frame (ORF) was deduced using an ORF Finder at the National Center for Biotechnology Information (NCBI; Bethesda, MD). Sequences were compared with nucleotide and protein sequences available in non-redundant databases and B. malayi draft genome using the basic local alignment search tool (BLAST, version 2; NCBI). Comparison with expressed sequence tag (EST) sequences was performed using tBLASTn (NCBI) and NemaBLAST (Washington University BLAST, version 2) and multiple sequence alignment by CLUSTAL-W algorithm.
Cloning, expression, and purification studies of cDNA clone
Sub-cloning, overexpression, and solubility studies of recombinant BmL3-helicase
BmL3-helicase cDNA insert in pBluescript vector was sub-cloned into expression plasmid vector pET 28a+ (Novagen) and transformed into competent E. coli BL21 (DE3) strain for expression studies. BL21 (DE3) cells containing pET28a-BmL3-helicase construct were inoculated and allowed to grow (37°C, 220 rpm) to obtain expression of recombinant protein. Cultures in the logarithmic phase (OD600 = 0.5–0.6) were induced for 3 h with 1.0, 0.5, and 0.2 mM of isopropyl β-d-thiogalactopyranoside (IPTG). Induced cells were harvested by centrifugation (7,000 rpm, 5 min) and lysed in sample buffer (0.313 M Tris–HCl, pH 6.8, 50% glycerol, 10% SDS, and 0.05% bromophenol blue) for analysis on 10% SDS-PAGE (Laemmli et al. 1970) along with uninduced vector control culture. To determine the solubility of recombinant protein, the cell pellet was resuspended in lysis buffer (50 mM Tris–HCl, pH 7.5; 200 mM NaCl; 100 mM DTT), sonicated at 10 db/10 s in a Soniprep150 sonicator in cold, and centrifuged (14,000 rpm, 30 min), yielding supernatant containing soluble fraction and a remaining pellet containing insoluble fraction. Both fractions were then analyzed in parallel on 10% SDS-PAGE and visualized by Coomassie blue staining.
Localization and purification of recombinant BmL3-helicase
Purified protein was run on 10% SDS-PAGE, transferred onto nitrocellulose membrane, blocked with 5% non-fat dry milk at 4°C, incubated with 1:1,000 anti-His-tag antibody (Qiagen, Germany) for 2 h, and reincubated with 1:10,000 anti-mouse IgG-HRP (Sigma) for another 2 h at room temperature (R/T) to visualize the recombinant protein band after adding DAB. The soluble fraction of cell-lysate containing the overexpressed recombinant protein was purified under nondenaturing conditions based on its N-terminal His-tag by affinity chromatography with Ni-nitrilotriacetic acid resin (Ni-NTA; Qiagen, Germany). Column was equilibrated with 60 mL of binding buffer containing 10 mM imidazole. The bound protein was eluted by applying a gradient of 10–250 mM imidazole and 10% glycerol in elution buffer. The fractions containing single band of recombinant protein were pooled, dialyzed (50 mM Tris–HCl, pH 7.5; 50 mM NaCl) for 12 h, and concentrated to 1 ml volume using Amicon ultra filters (Millipore, USA) with 33 kDa cut off. The protein concentration was determined by Bradford’s method using bovine serum albumin as standard [3].
Immunoblot assay
Seroreactivity of BmL3-helicase with human bancroftian sera
Reactivity of antibody present in the sera of infected animals and human subjects with the parasite-recombinant helicase was observed in blots. For bancroftian serum samples, blood were collected from subjects staying in an area on the outskirts of Lucknow, India, where W. bancrofti is highly endemic. Samples were categorized as endemic normal, asymptomatic microfilaremic carriers with symptoms or without symptoms, and amicrofilaremic symptomatic sera. Microfilarial presence or absence was determined in 2 ml of night blood sample after membrane filtration (Singh et al. 1997). Endemic normals were microfilaria negative and free from any clinical filarial symptoms when followed for 5 consecutive years. Sera from humans living in a filaria-free zone (Jammu and Kashmir, India) served as non-endemic normal control. Purified recombinant protein along with prestained molecular weight marker was run on a preparative 10% SDS-PAGE, transferred to nitrocellulose membrane and processed for immune-recognition with human (1:200) and rodent (1:200) sera pools from ten subjects of each category. Rodent sera were obtained from B. malayi-infected and normal uninfected mastomys. Goat anti-human and anti-mouse IgG-HRP (1:10,000 dilutions each) was used as secondary antibodies, and reaction was developed by DAB.
Immunization of mice with recombinant BmL3-helicase and production of polyclonal antibodies
Male BALB/c mice weighing 10–15 g maintained in the CDRI animal facility were used for immunization with BmL3-helicase. A group of 10 BALB/c mice were vaccinated subcutaneously with the recombinant protein (25μg/animal) prepared in Freund’s complete/incomplete adjuvant in three doses at 2-week intervals. Control groups received an equivalent volume of adjuvant alone. The animals were euthanized by terminal anesthesia 1 week after the last injection. Pre-immunized sera were collected from the retro-orbital plexus before immunization and also on day 7 at the time of autopsy. Serum antibody titration was done by ELISA. All the animal-handling procedures and experimental protocols were duly approved by the Institutional Animal Ethics Committee.
Preparation of soluble antigens of B. malayi and stage-specific expression of BmL3-helicase
Different life-cycle stages of B. malayi maintained experimentally in our laboratory were used to prepare soluble antigens. The mf, L3, and adult male and female stages of B. malayi were homogenized, sonicated in phosphate buffer saline (pH 7.4) in the presence of protease inhibitor cocktail (Sigma, USA), and pelleted by centrifugation at 10,000×g for 15 min at 4°C. The soluble fraction in the supernatant was collected and protein was estimated by Bradford’s method. Levels of expressed BmL3-helicase protein were evaluated in soluble antigenic preparations of B. malayi using an immunoblot analysis. Briefly, 10 μg of B. malayi antigens derived from the life-stages described above were resolved on 10% SDS-PAGE, transferred onto nitrocellulose membranes, and probed with mouse anti-BmL3-helicase serum (1:200 dilutions) and HRP-labeled goat anti-mouse IgG as secondary antibody (Sigma, USA) at 1:1,000 dilutions. The membrane was developed using 3′-3′ diaminobenzidine (DAB) and H2O2. Purified BmL3-helicase was used as positive control.
ATPase assay
RNA and DNA substrates for ATPase assay
The substrates used for ATPase assay are listed in Table 1.
ATP hydrolysis assay
To determine the enzymatic activities of the bacterially expressed recombinant BmL3-helicase, we examined the ATPase activity of the recombinant enzyme by measuring the released inorganic phosphate during ATP hydrolysis using a direct colorimetric assay (Chan et al. 1986; Pugh et al. 1999; Youliang and Zhi-Ren 2002) with some modifications. The method is based on the quantification of the blue–green complex formed between malachite green (MG) molybdate and free phosphate at 630 nm. A typical ATPase assay was carried out in 50 μl reaction volumes, containing 20 mM Tris–HCl, pH 7.5, 200 mM NaCl, 1 mM MgCl2, 5 mM DTT, 1–2 µg of appropriate RNA, 40 units of RNase A, 4 mM NTP, and 10 μl of recombinant helicase. The ATPase reactions were incubated at 37°C for 30 min, 20 µl of malachite green-molybdenum reagent (prepared freshly) was added, and reactions were further incubated at R/T for 5 min. The absorption (A) at 630 nm was then measured on a microplate reader (Infinite M200, Tecan, Switzerland), and concentrations of inorganic phosphate were determined by matching the A 630 nm in a standard curve of A 630 nm versus known standard phosphate concentrations.
Nucleotide sequence accession number
The B. malayi super family-II DNA/RNA helicase-like mRNA sequence as described in this study was submitted to GenBank and was assigned an accession number EF409381.
Results
Immunoscreening
About 2 × 105 recombinant phages were screened using B. malayi L3-resistant mouse polyclonal serum. Five strongly positive clones were picked up and purified for further analysis. The insert size of one of the clone BmL3-helicase was 1.16 kb. Sequencing and homology matching (using BLASTx at NCBI) of the immunodominant clone (1.16 kbp) indicated similarity with the DEAD/DEAH box helicase family proteins of B. malayi (GenBank accession no. EDP36872), Caenorhabditis briggsae (GenBank accession no. CAE62876) and C. elegans (GenBank accession no. AAB71200) giving a very significant e value of 4 × 10−97. Interestingly, the sequence also showed similarity with RNA helicase of Wolbachia TRS strain of B. malayi, an endosymbiotic bacterium of B. malayi. The amino acid sequence of BmL3-helicase contains eight conserved motifs represented in Fig. 1, which are present in all helicase superfamily proteins and considered important for their functioning.
Multiple sequence alignment of the deduced amino acid sequence of B. malayi BmL3-helicase with proteins of other species using Clustal-W software. Alignment, of BmL3-helicase amino acid sequence with Caenorhabditis briggsae (CAE62876) and C. elegans (AAB71200) and Wolbachia TRS strain of B. malayi (EDP36872). The putative motifs involved in ATPase and helicase activity are represented along with their functional roles. Regions of identity (asterisk), strong similarity (colon) and weak similarity (period) are indicated
Expression and localization of recombinant BmL3-helicase
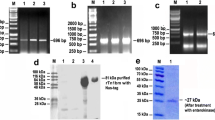
The cDNA of BmL3-helicase was found to be in frame with pET28a (Novagen) bacterial expression vector producing hexa-Histidine tag fusion proteins. The BmL3-helicase cDNA clone in E. coli BL-21(DE3) host cells encodes a functional protein of about 50-kDa. BmL3-helicase was optimally expressed at 37°C for 3 h after induction with 0.5 mM IPTG. The majority of recombinant protein expressed was found in the soluble fraction. The expressed protein was purified using Ni-NTA affinity column and eluted to obtain single band native protein. The expression of recombinant protein was also confirmed by western blotting with anti-His antibody. A single band of ∼50 kDa showed reactivity with anti-His antibody, which confirms expression of a single protein by the insert-vector construct (Fig. 2).
Purified recombinant His-tag BmL3-helicase and western blot analyses of bacterially expressed His-tag BmL3-helicase by anti-His antibody. The BmL3-helicase/pET28a+ construct transformed into BL21 (DE3) E. coli cells for expression studies. BmL3-helicase was purified by Ni-NTA affinity chromatography: fractions of protein eluted with 250 mM imidazole as observed on 10% SDS-PAGE after Coomassie blue staining. The Western blot experiments were carried out with 300 ng of purified 6× His-tag BmL3-helicase, anti-His antibody was diluted to 1:1,000 and developed with DAB (3′, 3′ diaminobenzidine)
Cross-seroreactivity with human bancroftian sera
Western blot analysis was done with lysate of transformed BL21 (DE3) induced with 0.5 mM IPTG at 37°C for 3 h. The strong reactivity of purified BmL3-helicase could be observed with the antibodies present in the pooled human bancroftian sera of various disease categories from the endemic area along with sera from B. malayi-infected mastomys. Sera of humans from the filaria-free zone or from uninfected mastomys did not react with the protein as shown in Fig. 3.
Cross-reactivity of BmL3-helicase with pooled (ten patients each) human bancroftian sera belonging to various categories in western blots. Endemic normal (lane 1), microfilaremic asymptomatic (lane 2), amicrofilaremic symptomatic (lane 3), microfilaremic symptomatic (lane 4), pooled (5 animals) B. malayi-infected mastomys (lane 5), irradiated L3 immunized mastomys (lane 6), anti-His antibody (lane 7), normal mastomys serum (lane 8) and non-endemic control (lane 9). Protein molecular weight marker indicated on right
Stage-specific expression of BmL3-helicase in different life-cycle stages of B. malayi
Immunoblot analysis on the soluble protein extracts of L3, microfilariae, and adult male and female parasite stages revealed that the expression levels of helicase enzyme were high in all stages (Fig. 4). These findings suggest that BmL3-helicase is expressed highly in all three stages of the B. malayi life cycle.
Presence of BmL3-helicase protein in various life-cycle stages of B. malayi. Soluble extracts (10 µg/lane) from microfilariae, L3, adult (male and female) stages of the parasite were resolved on 10% SDS-PAGE, transferred to nitrocellulose membrane and probed with mouse anti-BmL3-helicase antibody. HRP-labeled goat anti-mouse IgG (Sigma, USA) was used as the secondary antibody and the reactive bands were detected by DAB substrate. Recombinant BmL3-helicase was used as positive control
ATPase assay
The ATPase assays demonstrated that the recombinant protein hydrolyzed ATP in a polynucleotide-dependent manner (Fig. 5a). As a control, no ATP hydrolysis was observed in the same assay using BSA. Our experiments also showed that the BmL3-helicase enzyme hydrolyzed dNTPs apart from ATP. BmL3-helicase led to more hydrolysis of dATP and dTTP in comparison to dCTP and dGTP. To examine the nucleic acid-dependent ATPase activity of the bacterially expressed recombinant Bm-RNA-helicase, we carried out ATPase assays in the presence of ssRNA, dsRNA (Qiagen), dsDNA (pBluescript, linearized with EcoRI) or ssDNA (oligonucleotide of 22 bp). ATPase activity of the bacterially expressed BmL3-helicase was triggered by both the ssRNA and the dsRNA and, to a much lesser extent, by the ssDNA and dsDNA (Fig. 5b). The ATPase activity of the protein was almost doubled in the presence of the dsRNA compared with that in the presence of the same molar amounts of the ssRNA. Thus, these results indicate that ATPase activity of the recombinant BmL3-helicase is strongly stimulated by dsRNA.
ATPase activities of the recombinant His-tag BmL3-helicase. The ATPase activity is expressed as µM (concentrations) of released inorganic phosphate in the 50 µl of reaction volume. a ATPase activity is measured in the presence of 1 µg of yeast total RNA (Fermentas). The ATP hydrolysis reactions were carried out at room temperature for 30 min containing, 4 mM ATP alone (A); 4 mM ATP and 2.5 µg of BSA (B); 4 mM ATP and 2.5 µg of His-tag BmL3-helicase (C); 4 mM ATP and 2.5 µg of His-tag BmL3-helicase with RNA (D); 4 mM of dATP (E), dTTP (F), dCTP (G) and dGTP (H) with 2.5 µg of His-tag BmL3-helicase. b Dependence of ATPase activity of BmL3-helicase on the nucleic acids. Assays were carried out with 4 mM ATP and 10 µl of helicase (2.5 µg) in 50 µl reactions containing 0.5 µg of Yeast RNA (ssRNA), 0.1 µg of synthetic dsRNA, 0.2 µg of 24-nt synthetic DNA oligonucleotides (ssDNA), and 1 µg of pBluescript linearized by EcoRI (dsDNA)
Discussion
The expression and purification of recombinant BmL3-helicase carried out using an E. coli expression system showed that the protein was an ATP-dependent RNA helicase. The results also showed that the ATPase activity of the recombinant protein was polynucleotide-dependent and markedly enhanced by dsRNA. This is the first description involving a protein of the DEAD box family of RNA helicase in any filarial species. RNA helicases have been identified as potential drug target candidates in herpes simplex virus (HSV), hepatitis C virus (HCV), and malarial parasites (Tuteja 2007; David 2003); however, they have not yet been explored in filarial nematodes.
Immunoscreening of cDNA expression libraries and phage display libraries using patients’ sera especially from endemic normal or monoclonal antibodies (Gnanasekar et al. 2004) is one of the most sought after approaches in the search for filarial vaccine candidates/drug targets (Rao et al. 2000; Kobayashi et al. 2007; Shibuia et al. 2001; Feng et al. 2007; Nisbet et al. 2008; Merriweathera et al 2001). BmL3-helicase shows about 58% similarity with C. elegans RNA helicase, 78% with B. malayi RNA helicase and 48% with Wolbachia sequence generated from draft genome; it also contains nine conserved motifs responsible for ATP hydrolysis, RNA substrate binding and unwinding activities. Wolbachia of filarial nematodes are the obligate intracellular alpha-proteobacteria and are present in all life stages of filarial worms and proteins encoded by it have been considered as potent drug target for anti-filarial therapy (Taylor et al. 2000). Interestingly, the B. malayi 2-day-old irradiated larval stage cDNA library also contains ESTs of ATP-dependent RNA helicase strongly indicate that the genes encoding DEAD box RNA helicases have an even distribution. The human filarial parasite B. malayi has the genome size of ∼90 megabase (Mb), and about 11,500 protein-coding genes are predicted to be present. The DEAD Box RNA helicases are the 16th most abundant domains in B. malayi, C. elegans, and C. briggasae, as revealed by the recently released B. malayi draft genome data (Elodie et al. 2007).
Further analysis of a subset of randomly selected clones sequenced from the porcine nodule nematode Oesophagostomum dentatum male-specific cDNA library demonstrated that ESTs of DEAD box RNA helicase were present as major spermproteins (MSPs) from a male-specific cDNA library, suggesting their role in development, sexual differentiation, and reproduction of parasitic nematodes (Alasdair et al. 2004). This also indicates that filarial helicase may possibly be exploited for interfering with worm fertility and reproduction to block filarial transmission. Recently, an RNA binding motif in RNA helicase A having a DExH helicase domain of Xenopus laevis was found to be involved in the unwinding of duplex of nucleic acids (Cheng et al. 2005), explaining the importance of DEAD box domain in the enzyme. The C. elegans glh-1 and cgh-1 genes also contain regions of conserved sequence present in RNA helicases, and these are predicted to be a single copy gene correlated with gametogenesis and protection from physiological germline apoptosis (Deborah and Karen 1993; Rosa et al. 2001). RNAi studies with RNA helicase in C. elegans established its role in causing embryonic lethality and female sterility in worms (Kamath et al. 2003) suggesting the biological importance of helicase-like genes in cellular functions, embryonic development and/or larval growth. Various other studies have also shown that helicases are indispensable enzymes; in yeast, the loss of one DEAD box gene cannot be supplemented by overexpression of another family member, which further suggests that each helicase gene is independently essential (Story et al. 2001). ATP hydrolysis activity of BmL3-helicase was done to corroborate that the recombinant protein contains functional domains. It has been shown earlier that the ATPase activity of so-called DEAD/DExH box of ATPases is polynucleotide-dependent (Youliang and Zhi-Ren 2002). We also found that ATP hydrolysis by BmL3-helicase is substrate dependent. The amount of ATP hydrolysis increased with addition of RNA to BmL3-helicase as compared to BSA and BmL3-helicase alone. We observed an increase in ATP hydrolysis in the presence of other substrates like dsDNA and ssDNA.
BmL3-helicase also showed strong reactivity with sera of various categories (asymptomatic microfilaremic carriers, symptomatic with or without mf) of human parasite W. bancrofti and B. malayi-infected rodent sera. Sera of endemic normal persons staying in a filaria-endemic area with no parasitological or clinical signs of lymphatic filariasis during a long 5-year follow-up also reacted very strongly with this recombinant protein, indicating that BmL3-helicase is also implicated in immune protection studies. In a filaria-endemic area, endemic normals are consistently exposed to infective mosquito bites and in general exhibit hyper-responsiveness to filarial antigens and are considered to be putatively immune. The non-endemic normal human serum collected from filaria-free state Jammu and Kashmir, India and uninfected mastomys serum did not show any reactivity with the BmL3-helicase showing filaria-specific reactivity. To further elucidate the antigenic property of BmL3-helicase, a polyclonal serum was raised in BALB/c mice and high antibody titers were observed in ELISA, indicating the presence of antigenic epitopes in the expressed protein. The stage-specificity of recombinant protein was seen by immunoblot using soluble somatic extracts of various life-cycle stages of B. malayi. BmL3-helicase was found to highly express in all the major life stages, i.e., L3, both sexes of adult stages, and microfilariae, revealing that an immune response to B. malayi helicase is developed once the infective larvae are inoculated into the vertebrate host. The response is maintained with further larval development and transformation into subsequent life stages, emphasizing the importance of this enzyme as a diagnostic antigen. It would be interesting to further observe if this enzyme is secretary in nature which is quite expected.
Because little is known about DEAD box RNA helicase in filarial nematodes, further exploration of the BmL3-helicase enzyme is warranted to gain information on its functions in parasite viability, reproduction, and host–parasite interactions. This enzyme is considered a rational target for structure-based molecular design (Tuteja 2007; David 2003). A study on the three-dimensional structure of the protein will shed more light on the important conserved motifs, further revealing the structural features of this protein and its ATP-dependent RNA unwinding property (Christine et al. 1993). Even if the eight characteristic motifs are well conserved between all the helicases (Gorbaleny and Koonin 1993), the DEAD box proteins may be specifically sorted out using peculiarities in their motifs. Figure 1 presents the residues best conserved in the eight motifs of the DEAD family. Their involvement in the biochemical functions and their interactions with substrates have been demonstrated by site-directed mutagenesis. Among the DEAD box proteins, nearly full-length proteins crystal structure of only two organisms (Methanococcus jannaschii and Homo sapiens) are available (Hogbom et al. 2007). The DEAD Domain or Walker B Motif is known to be an ATP Binding domain and is indispensable for ATPase and helicase activity (Fig. 1). Also, the aspartate in the DEAD box motif seems to play an important role in coupling the ATPase and helicase activities (Gorbaleny and Koonin 1993). The presence of these motifs is further evidenced by the in vitro assays for respective activities as already discussed. The position of both domains in proximity as shown in our model suggests strict coordination with respect to the cascade of events after ATP binding followed by unwinding of dsRNA.
Since very few validated drug targets are available for filariasis, BmL3-helicase could possibly be utilized as a potential target for the human filarial parasites W. bancrofti and B. malayi. Although a number of filarial proteins/enzymes have been cloned in the recent past and some of these have been suggested as possible drug targets, studies have not been followed up to further validate their potential (Geldhof et al. 2006). Parasite helicase can be specifically targeted using a specific antibody or dsRNA to validate its potential as a drug target. RNAi methods have been successful in the case of helminths including C. elegans and B. malayi, using dsRNAs of various proteins and enzymes (Geldhof et al. 2006; Kamath et al. 2003). The information on chemical inhibitors of RNA helicase is meager and, therefore, structure-based drug design might help in synthesizing compounds which could target this enzyme by specifically inhibiting the synthesis of parasite helicase without affecting the host enzyme.
In summary, the present findings describe a DEAD box RNA helicase as a novel group of proteins not previously unveiled in filarial parasites. In the absence of information on filarial parasite helicases, detailed additional study is warranted to explore the mechanistic function of all the RNA helicases of B. malayi. Extensive evaluation is essential before these enzymes can be adopted as legitimate drug targets for designing therapies against filariasis. The results of the present study are quite encouraging, and application of RNAi technique would further assess the role of these enzymes in parasite biology and filarial survival.
References
Alasdair JN, Pauline C, Robin BG (2004) Molecular biology of reproduction and development in parasitic nematodes: progress and opportunities. Int J Parasitol 34:125–138
Andersen CB, Ballut L, Johansen JS, Chamieh H, Nielsen KH, Oliveira CL, Pedersen JS, Seraphin B, Le Hir H, Andersen GR (2006) Structure of the Exon junction core complex with a trapped DEAD-box ATPase bound to RNA. Science 313:1968–1972
Bradford MA (1976) Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Chan KM, Delfert D, Junger KD (1986) A direct colorimetric assay for Ca+2-stimulated ATPase Activity. Anal. Biochem 157:375–380
Cheng Z, Coller J, Parker R, Song H (2005) Crystal structure and functional analysis of DEAD-box protein Dhh1p. RNA 1258–1270
Christine SR, Eugene S, James HM-Tt, Garson KL, Philip JR, Irwin DK, Fred EC (1993) Structure-based inhibitor design by using protein models for the development of antiparasitic agents. Proc Natl Acad Sci 90:3583–3587
David NF (2003) Helicases as antiviral drug targets. Drug News Perspect 16:355
Deborah LR, Karen LB (1993) glh-1, a germ-line putative RNA helicase from Caenorhabditis, has four zinc fingers. Proc Natl Acad Sci 90:9300–9304
Elodie G et al (2007) Draft genome of the filarial nematode parasite Brugia malayi. Science 317:1756
Feng J, Zhan B, Liu Y, Liu S, Williamson A, Goud G, Loukas A, Hotez P (2007) Molecular cloning and characterization of Ac-MTP-2, an astacin-like metalloprotease release. Mol Biochem Parasitol 152(2):132–138
Frank GR, Tripp CA, Grieve RB (1996) Molecular cloning of a developmentally regulated protein isolated from excretory–secretory products of larval Dirofilaria immitis. Mol Biochem Parasitol 75:231–240
Geldhof P, Visser A, Clark D, Saunders G, Britton C, Gilleard J, Berriman M, Knox D (2006) RNA interference in parasitic helminths: current situation, potential pitfalls and future prospects.1–11
Gnanasekar M, Rao KVN, He Y-X, Mishra PK, Nutman TB, Kaliraj P, Ramaswamy K (2004) Novel phage display-based subtractive screening to identify vaccine candidates of Brugia malayi. Infect Immun 72(8):4707–4715
Gorbaleny AE, Koonin EV (1993) Helicases: amino acid sequence comparisons and structure–function relationships. Curr Opin Struct Biol 3:419–429
Helmy H, Weil GJ, Faris R, Gad AM, Chandrashekar R, Ashour A, Ramzy RM (2000) Human antibody responses to Wuchereria bancrofti infective larvae. Parasite Immunol 22:89–96
Hogbom M, Collins R, van den Berg S, Jenvert RM, Karlberg T, Kotenyova T, Flores A, Karlsson HGB, Holmberg SLH (2007) Crystal structure of conserved domains 1 and 2 of the human DEAD-box Helicase DDX3X in complex with the mononucleotide AMP. Mol Biol 372:150–159
Ibrahim MS, Richie TL, Scott AL (1992) Surface-associated antigens of Brugia malayi L2 and L3 parasites during vector-stage development. Mol Biochem Parasitol 52:97–110
Ismail MM (1998) Efficacy of single dose combinations of albendazole, ivermectin and diethylcarbamazine for the treatment of bancroftian filariasis. Trans of the Royal Soc of Trop Med and Hyg 92:94–97
Jankowsky E, Bowers H (2006) Remodeling of ribonucleoprotein complexes with DExH/D RNA helicases. Nucleic Acids Res 34:4181–4188
Kamath R S, Fraser A G, Dong Y, Poulin G, Durbin R, Gotta M, Kanapink A, Bot N L, Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R et al (2003) Systematic functional analysisof the Caenorhabditis elegans genome using RNAi. Nature 421
Kobayashi Y, Ishizaki S, Shimakura K, Nagashima Y, Shiomi K (2007) Molecular cloning and expression of two new allergens from Anisakis simplex. Parasitol Res 100(6):1233–1241
Laemmli UK, Molbert E, Showe M, Kelenberger E (1970) Form-determining function of genes required for the assembly of the head of bacteriophage T4. J Mol Biol 49:99–113
Merriweathera A, Guenzlerb V, Brennerb M, Unnasch TR (2001) Characterization and expression of enzymatically active recombinant filarial prolyl 4-hydroxylase. Mol Biochem Parasitol 116(2):185–197
Michael E (2000). The population dynamics and epidemiology of lymphatic Filariasis. Imperial College Press, London. 1: 41–82
Nisbet AJ, Halliday AM, Parker L, David SW, Kenyon F, Knox DP, Huntley JF (2008) Psoroptes ovis: identification of vaccine candidates by immunoscreening. Exp Parasitol 120(2):194–199
Ottesen EA, Duke BO, Karam M, Behbehani K (1997) Strategies and tools for the control/ elimination of lymphatic filariasis. Bulletin of WHO 75:491–503
Pugh GE, Nicol SM, Fuller-Pace FV (1999) Interaction of the Escherichia coli DEAD box protein DbpA with 23S ribosomal RNA. J Mol Biol 292:771–778
Rao UR, Salinas G, Mehta K, Klei TR (2000) Identification and localization of glutathione-S-transferase as a potential target enzyme in Brugia species. Parasitol Res 86:908–915
Rosa EN, Eun YS, Yuji K, Andrew S, Blackwell TK (2001) cgh-1, a conserved predicted RNA helicase required for gametogenesis and protection from physiological germline apoptosis in C. elegans. Development 128:3221–3232
Sharma DC (2002) New goals set for filariasis elimination in India: news. The Lan Infec Dis 2:389
Shibuia A, Takamotob M, Shib Y, Komiyamaa A, Suganeb K (2001) Cloning and characterization of a novel gene encoding keratin-like protein from nematode Nippostrongylus brasiliensis. Biochimica et Biophysica Acta (BBA)—Gene Structure and Expression 1522(1):59–61
Singh U, Misra S, Murthy PK, Katiyar JC, Agarwal A, Sircar AR (1997) Immunoreactive molecules of Brugia malayi and their diagnostic potential. Serodiag Immunotherap Infect Dis 8:207–212
Story RM, Li H, Abelson JN (2001) Crystal structure of a DEAD box protein from the hyperthermophile Methanococcus jannaschii. Proc Natl Acad Sci 98:1465–1470
Taylor MJ, Bandi C, Hoerauf AM, Lazdins J (2000) Wolbachia bacteria of filarial nematodes: a target for control? Science S0169-4758(00)01661-6
Tuteja R (2007) Helicases-feasible antimalarial drug target for Plasmodium falciparum. FEBS J 274:4699–4704
Verma SK, Bansal I, Vedi S, Saxena JK, Katoch VM, Bhattacharya SM (2007) Molecular cloning, purification and characterization of myosin of human lymphatic filarial parasite Brugia malayi. Parasitol Res 102:481–489
Walker JE, Saraste M, Runswick MJ, Gay NJ (1982) Distantly related sequences in the alpha-and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J 1:945–951
Weil GJ, Li BW, Liftis F, Chandrashekar R (1992) Brugia malayi: antibody responses to larval antigens in infected and immunized jirds. Exp Parasitol 74:315–323
WHO (1997) Lymphatic filariasis: Reasons for hope. Document No.WHO/CTD/FIL/97.4, Geneva
Youliang H, Zhi-Ren L (2002) The ATPase, RNA unwinding, and RNA binding activities of recombinant p68 RNA helicase. J Biol Chem 277:12810–12815
Acknowledgments
The author acknowledges University Grants Commission, New Delhi, India, for financial assistance in the form of Senior Research Fellowships to M.S. We are grateful to Mr. A. K. Roy and R. N. Lal for their excellent technical assistance in maintenance of B. malayi infection in laboratory.
Author information
Authors and Affiliations
Corresponding author
Additional information
Nucleotide sequence reported in this paper is available in the GenBank™, EMBL, and DDBJ databases under the accession number EF409381.
Rights and permissions
About this article
Cite this article
Singh, M., Srivastava, K.K. & Bhattacharya, S.M. Molecular cloning and characterization of a novel immunoreactive ATPase/RNA helicase in human filarial parasite Brugia malayi . Parasitol Res 104, 753–761 (2009). https://doi.org/10.1007/s00436-008-1251-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-008-1251-6