Abstract
The present study was undertaken to investigate the inhibitory effects of fucoidan, a sulfated polysaccharide isolated from the edible brown seaweed Undaria pinnatifida, on the growth of Plasmodium parasites. In order to assess the anti-malarial activity of fucoidan, growth inhibition activities were evaluated using cultured Plasmodium falciparum parasites in vitro and on Plasmodium berghei-infected mice in vivo. Fucoidan significantly inhibited the invasion of erythrocytes by P. falciparum merozoites, and its 50% inhibition concentration was similar to those for the chloroquine-sensitive P. falciparum 3D7 strain and the chloroquine-resistant K1 strain. Four-day suppressive testing in P. berghei-infected mice with fucoidan resulted in a 37% suppressive effect versus the control group and a delay in death associated with anemia (P < 0.05). In addition, fucoidans had no toxic effect on RAW 264.7 cells. These findings indicate that fucoidans from the Korean brown algae U. pinnatifida inhibits the invasion of Plasmodium merozoites into erythrocytes in vitro and in vivo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The asexual erythrocyte cycle of malaria begins with the growth and proliferation of intraerythrocytic parasites to individual merozoites, which, on erythrocyte rupture, invade other erythrocytes via multiple adhesive interactions involving with host cell surface molecules (Cowman and Crabb 2006). Therefore, an understanding of the basic molecular mechanisms of asexual growth cycle, particularly the process of merozoite invasion into host red blood cells (RBCs), may accelerate the development of an effective therapeutic method and method for preventing malaria.
Recently, efforts to identify new drug candidates have raised interest in sulfated fucans (Berteau and Mulloy 2003; Smit 2004), which are known to act as coagulation modulators. Like heparin, fucoidan is a sulfated polysaccharide, and hence a negatively charged polysaccharide, and is mainly composed of l-fucose and ester sulfate. Several biological activities, e.g., anti-coagulant, anti-thrombotic, anti-bacterial, anti-viral, anti-inflammatory, and immunomodulating activities, have been attributed to the fucoidans (Berteau and Mulloy 2003; Ponce et al. 2003; Smit 2004). Fucoidan has also been shown experimentally to inhibit the in vitro invasion of Plasmodium falciparum merozoites into erythrocytes and to block the cytoadhesion of infected erythrocytes to various host receptors (Pancake et al. 1992; Xiao et al. 1996; Clark et al. 1997) or disrupt P. falciparum rosettes (Rowe et al. 1994). Furthermore, sulfate esters of fucoidan may have a biological effect on cellular recognition and adhesion via its specific binding to a cell surface ligand. Recently, it was reported that fucoidan extracted from the sporophylls of Undaria pinnatifida exhibits a potent inhibitory effect on cryptosporidiosis and viral diseases (Lee et al. 2004; Maruyama et al. 2007; Hayashi et al. 2008). Although fucoidan from U. pinnatifida has been studied in terms of its inhibitory effects on infections, the anti-malarial activity of fucoidan has not been previously investigated.
In the present study, we extracted three fractions of fucoidan (F1–F3) from the Korean brown seaweed U. pinnatifida and confirmed their inhibitory effects on the in vitro growth of P. falciparum parasites. In addition, the in vivo inhibitory effect of fucoidan was confirmed against the rodent malaria parasite Plasmodium berghei in mice.
Materials and methods
Isolation and purification of fucoidan from U. pinnatifida
Sporophylls of the brown algae U. pinnatifida were collected on the north east coast of the Republic of Korea (Gangwon Province) in February 2007. Seaweed samples were dried and milled to a powder. Seaweed samples were hydrolyzed with cellulose, pectinase, and arylsulfatase in the presence of CaCl2 (to separate alginic acid) and partially purified using cetylpyridinum chloride and ethanol precipitation as previously described (Vieira et al. 1991). The fucoidan so obtained was fractionated by anion-exchange chromatography using DEAE-Sephadex A-25 (Cl− form, Pharmacia, Uppsala, Sweden) using gradually increasing concentrations of sodium chloride (0–3.5 M). The three fractions obtained were dialyzed against pyridine acetate (0.1 M, pH 5.0–5.4) for 3 days, and carbohydrate and sulfate analyses by high performance anion-exchange chromatography were used to determine the fucose, monosaccharide, and uronic acid contents of the three fucoidan fractions. Three fractions of fucoidan (F-1, F-2, and F-3) were extracted from U. pinnatifida by hydrolytic enzymatic method.
Parasite culture and in vitro growth inhibition assays
P. falciparum parasites [3D7, chloroquine-sensitive strain (CQS) and K1, chloroquine-resistant strain (CQR)] were cultured in O+ human erythrocytes and maintained at a hematocrit level of 1% in complete medium at 37°C in an incubator as previously described (Trager and Jensen 1976). This complete medium contained RPMI 1640 medium containing l-glutamine and 25 mM HEPS buffer (Gibco, NY, USA), 0.225% NaHCO3 (Gibco), 0.5% AlbuMAX I (Gibco), 10 μg/ml of gentamicin (Gibco), and 50 mg/l of hypoxanthine (Sigma, St. Louis, USA). The parasite ring stage was synchronized using 5% sorbitol, as previously described (Lambros and Vanderberg 1979), and the late trophozoite and schizont stages were induced by incubating for additional incubation of 24 h.
The effects of the three fucoidan fractions on the invasion of erythrocytes with merozoites were assessed using growth inhibition assays. Flat-bottom 96-well cell culture plates (Costar, NY, USA) were first seeded with 190 μl of parasites (0.3% in final parasitemia) in the parasitized RBCs (pRBCs, trophozoite and schizont stages-rich) in 1.0% hematocrit. Different concentrations of fucoidan fractions were prepared with complete medium and chloroquine diphosphate (Sigma) and quinine hydrochloride (Sigma) were regarded as positive controls. Fucoidan and anti-malarial drugs serially diluted were transferred in triplicate (10 μl each) to pRBC suspensions in 96-well cell culture plates. Two hundred microliters of pRBCs and fresh RBCs (fRBCs) in suspension (1% hematocrit) were used as drug-free and negative controls, respectively. Culture plates were incubated for 40 h at 37°C in a modular chamber (Ipd/Appco, Vista, USA) in a 5% CO2, 5% O2, and 90% N2 atmosphere. Percent parasitemia was determined by counting under a microscope after incubation, and cultured parasites were keep in a freezer (−20°C) at least 3 h. Parasite lactate dehydrogenase (pLDH) enzymatic assays were performed as follows.
pLDH enzymatic assays
Fifty microliters of culture parasite samples were thawed completely and dissolved in 100 μl of pLDH assay buffer for 30 min at room temperature and samples in microtiter plate covered with aluminum foil. The assay buffer was composed of 100 mM Tris (Sigma, pH 8.0), 50 mM sodium l-lactate (Sigma), 0.25% Triton X-100 (Bio-Rad), 20 μg of nitro blue tetrazolium (Sigma), 5 μg of 3-acetylpyridine adenine dinucleotide (Sigma), and 0.1 U of diaphorase (Sigma). The absorbance at 620 nm was measured using a 96-well Spectra II spectrophotometer (Tecan, Grödig, Austria). Percent inhibition by each drug sample was defined as 100 − [(OD620 of drug treatment sample − OD620 of fresh RBCs only)/(OD620 of drug free sample − OD620 of fresh RBCs only) × 100].
Cytotoxicity testing
The cellular viabilities of RAW 264.7 cells (a murine macrophage cell line) that had been treated with fucoidan fractions were determined using MTT assays (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) as previously described (Habtemariam 2003). Briefly, cells were cultured in RPMI 1640 medium and harvested in the log phase of growth and seeded (5 × 103 cells/well in 100 μl volume) in 96-well microtitre plates. After 24 h of incubation at 37°C in 5% CO2 gas to allow cell attachment, cultures were treated with fucoidans (0, 1, 10, 25, 50, and 100 μg/ml, diluted in RPMI 1640 medium) for 24 h, and cell viabilities were assessed by measuring OD540 values using a 96-well Spectra II spectrophotometer.
Effect of fucoidan on the disease course of P. berghei ANKA in mice
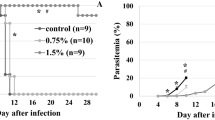
Based on growth inhibition results obtained in vitro, the activity of fucoidan F-3 was evaluated in vivo by treating mice infected with P. berghei ANKA, a model of cerebral malaria. BALB/c mice (6–8 weeks old) were divided into three groups [phosphate-buffered saline (PBS)-, chloroquine-, and fucoidan-treated groups; n = 7 mice in each group) and were intraperitoneally inoculated with P. berghei ANKA strain pRBCs. The test protocol was based on the 4-day suppressive test (Peters 1975). In brief, pRBCs were collected from an infected mouse and dissolved in PBS and test animals were infected by injecting a 0.2-ml suspension (1 × 106 parasites) intraperitoneally. From days 1 to 4 post-infection, 100 mg/kg of fucoidan (dissolved in 0.2 ml PBS) was administered to each mouse, whereas control group animals received 0.2 ml of PBS only, and animals in the standard group were treated with chloroquine (5 mg/kg). At 4 days post-infection, blood samples from all animals were smeared and stained with Giemsa; parasitemia was determined microscopically. Percentage inhibition was calculated as 100 − [(mean parasitemia treated/mean parasitemia control) × 100]. Survival times (in days) were recorded.
Statistical analysis
The 50% inhibitory concentrations (IC50) values of the compounds against malaria in vitro were analyzed by nonlinear dose–response curve fitting using SigmaPlot program version 10.0 (Systat Software, Point Richmond, USA). Percentage inhibitions and survival times in vivo were compared using the Student’s t test. Values of P ≤ 0.05 were considered to be statistically significant.
Results
Although the chemical structures of fucoidan and de-sulfated fucoidan have not yet been determined, the chemical properties of these natural compounds have been characterized.
The yields and compositions of the three fucoidan fractions purified from U. pinnatifida are shown in Table 1 and Fig. 1. All fucoidan fractions (molecular weight, about 15,000 kDa) contained fucose as the major component along with galactose and minor quantities of arabinose, rhamnose, glucose, xylose, and mannose, indicating that they were fucogalactans, with variable proportions of these sugar components. The fucoidan fraction F-1 that was eluted at the lowest NaCl concentration was rich in uronic acid and poor in sulfate groups. On the other hand, the fucoidan fraction F-3 that eluted at a higher concentration of NaCl had higher sulfate and lower uronic acid contents. The first fraction, F-1, was characterized by high percentage of uronic acid (21.1%) and a low sulfate content (11.4%). The second, F-2, and third, F-3, fractions were characterized by lower uronic acid levels (6.8% and 7.9%, respectively) and higher sulfate contents (20.6% and 31.2%, respectively).
Experimental results are presented in Fig. 1. All three fucoidan fractions demonstrated activity against P. falciparum, and fraction F-3 (with highest sulfate content) showed most anti-malarial activity, suggesting that sulfate content may play a pivotal role in the anti-malarial activity of fucoidans. The fact that fraction F-2 has less anti-invasion activity than F-3, although its fucose content was much higher, might be due to its uronic acid concentration, which also possesses anti-invasion properties (uronic acid content of fraction F-3 was 7.9%, while that of fraction F-2 was 6.8%). The mechanistic relations between anti-invasion activity and molecular weight and the ratios of fucose, uronic acid, and the sulfate group are not known.
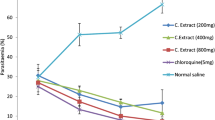
To access anti-malarial potency of fucoidan, its effects on the growth inhibition of P. falciparum parasite strains were examined (Table 2). The effects of fucoidan (F-1, F-2, and F-3) on the invasion of erythrocytes by merozoites (3D7 and K1 strain) were assessed using pLDH enzymatic assay. We found that all three fractions had a range of effects on the invasion of erythrocytes by merozoites (Table 2), and their inhibitions were concentration-dependent with a sigmoidal curve (Fig. 2). These findings concur with similar observed decreases in parasitemia in Fig. 2.
These studies determined that the IC50 values of fractions F-1, F-2, and F-3 were 9.17, 7.28, and 1.95 μg/ml in P. falciparum 3D7 stain and 7.03, 4.74, and 2.21 μg/ml in P. falciparum K1 strain, respectively (Table 2). The anti-malarial efficacy of F-3 was higher than those of F-1 and F-2.
Because in vitro studies demonstrated that fucoidan inhibits the invasion of erythrocytes by P. falciparum, we evaluated the efficacy of the third fraction on parasitemia and survival in mice infected with P. berghei ANKA. After a 4-day suppressive test in vivo, fucoidan F-3 had a suppressive effect of 37.12 ± 0.37% in P. berghei-infected mice at 100 mg/kg, while chloroquine (the positive control) had a suppressive effect of 94.37 ± 0.94% at 5 mg/kg at fourth days (Table 3). Fucoidan F-3 was found to have lower anti-malarial activity than chloroquine, but it inhibited parasitemia significantly versus the PBS-treated controls (P < 0.05). Furthermore, the survival times of mice treated with fucoidan F-3 were significant longer about 4 days than those of the control groups (P < 0.05; Table 3).
Discussion
The growth inhibitory effects of fucoidan derived from U. pinnatifida seaweed were investigated on malaria infection in vitro and in vivo in this study. The in vitro anti-malarial efficacy of fucoidan described here was tenfold higher than that reported earlier, i.e., the IC50 value for fucoidan (molecular weight, 18,000 kDa) from Fucus vesiculosus against P. falciparum FCR-3 parasite was 22 μg/ml with 42.3% inhibition (Clark et al. 1997). An in vivo animal experiment performed during a previous study reported found that fucoidan does not significantly reduce parasitemia (Xiao et al. 1996). However, in the present study, fucoidan was found to have a significant effect.
The amount of fucose in fucoidans extracted using hydrolytic enzymes was 2.8- to 3.7-fold higher than that obtained from mekabu (15.6%) by acidic hydrolysis (Maruyama et al. 2003). Shimizu et al (2005) reported that high-molecular-weight fucoidan extracted from Okinawa mozuku changed the ratio of CD4+/CD8+ and increased the ratio of cytotoxic T cells in mice splenocytes. In the present study, all fractions had higher molecular weights than those previously reported (Lee et al. 2004) from the same source; the molecular weight of fucoidan determined by their study was 9,000 kDa. In addition, its sulfur content was estimated at 31.2% in fucoidan fraction F-3; this value was higher than the 10.4% sulfur content described by Lee et al. (2004).
Our in vitro experiments showed that fucoidan has no dose-dependent inhibitory effect on parasite growth from the ring to the schizont stage. However, in growth inhibition assay for whether fucoidan interfere invasion of the new merozoite to fRBCs, it was effectively prevented by fucoidan within a few days.
The results of our in vivo experiments (Table 3) showed that fucoidan suppressed the invasion of erythrocytes by P. berghei merozoites. Moreover, infected mice treated with fucoidan for 4 days had lower parasitemia during the entire course of infections than untreated mice. These treated mice died later during the anemia phase of the infection. The above effect can be explained by reduced parasitemia due to treatment. Thus, fucoidan may inhibit the adhesive interaction between merozoites and RBCs and direct binding of the sulfate of fucoidan to cell surface ligands of host cells, thereby inhibiting parasite invasion.
In this study, the IC50 of fucoidan in a mouse model was determined to be 100 mg/kg per mouse. However, eye hemorrhages and death occurred in five mice during the treatment period on either 250 or 500 mg/kg of fucoidan. These adverse reactions might have been caused by the anti-coagulatory effect of fucoidan. Therefore, for anti-malarial therapy, the application of fucoidan at doses as high as those mentioned above is not recommended, even though these doses suppress parasite growth.
In a different study, fucoidan could inhibit the invasion of human Duffy-positive and rhesus erythrocytes by Plasmodium knowlesi, analogous to the results reported in this study (Dalton et al. 1991). This finding is consistent with that described in earlier reports which demonstrate that the inhibitory effect of fucoidan on the invasion can be simply considered as a result of nonspecific electrostatic interactions rather than a consequence of specific cytoadhesion receptors (Xiao et al. 1996). But another experiment suggests that the inhibition of invasion is not due only to nonspecific ionic interactions between fucoidan and a parasite protein but, instead, a particular conformation of the anion(s) is required for effective inhibition (Clark et al. 1997).
In present study, the growth-inhibitory activity of fucoidan from the brown seaweed U. pinnatifida was evaluated on P. falciparum parasite strains by pLDH enzymatic assay and was found to highly inhibit the invasion of erythrocytes by merozoites. In addition, fucoidan was found to slightly inhibit parasitemia in P. berghei-infected mice. No report has been issued on a molecule with an affinity for fucoidan on the surfaces of free merozoites of Plamodium parasites. Further investigations of growth inhibition caused by fucoidan would be helpful in providing an understanding of the process of merozoite invasion. In particular, the identity of the molecule with an affinity for fucoidan is of interest.
References
Berteau O, Mulloy B (2003) Sulfated fucans, fresh perspectives: structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology 13:29R–40R
Clark DL, Su S, Davidson EA (1997) Saccharide anions as inhibitors of the malaria parasite. Glycoconj J 14:473–479
Cowman AF, Crabb BS (2006) Invasion of red blood cells by malaria parasites. Cell 124:755–766
Dalton JP, Hudson D, Adams JH, Miller LH (1991) Blocking of the receptor-mediated invasion of erythrocytes by Plasmodium knowlesi malaria with sulfated polysaccharides and glycosaminoglycans. Eur J Biochem 195:789–794
Habtemariam S (2003) Cytotoxic and cytostatic activity of erlangerins from Commiphora erlangeriana. Toxicon 41:723–727
Hayashi K, Nakano T, Hashimoto M, Kanekiyo K, Hayashi T (2008) Defensive effects of a fucoidan from brown alga Undaria pinnatifida against herpes simplex virus infection. Int Immunopharmacol 8:109–116
Lambros C, Vanderberg JP (1979) Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol 65:418–420
Lee JB, Hayashi K, Hashimoto M, Nakano T, Hayashi T (2004) Novel antiviral fucoidan from sporophyll of Undaria pinnatifida (Mekabu). Chem Pharm Bull (Tokyo) 52:1091–1094
Maruyama H, Tamauchi H, Hashimoto M, Nakano T (2003) Antitumor activity and immune response of Mekabu fucoidan extracted from sporophyll of Undaria pinnatifida. In Vivo 17:245–249
Maruyama H, Tanaka M, Hashimoto M, Inoue M, Sasahara T (2007) The suppressive effect of Mekabu fucoidan on an attachment of Cryptosporidium parvum oocysts to the intestinal epithelial cells in neonatal mice. Life Sci 80:775–781
Pancake SJ, Holt GD, Mellouk S, Hoffman SL (1992) Malaria sporozoites and circumsporozoite proteins bind specifically to sulfated glycoconjugates. J Cell Biol 117:1351–1357
Peters W (1975) The chemotherapy of rodent malaria, XXII. The value of drug-resistant strains of P. berghei in screening for blood schizontocidal activity. Ann Trop Med Parasitol 69:155–171
Ponce NM, Pujol CA, Damonte EB, Flores ML, Stortz CA (2003) Fucoidans from the brown seaweed Adenocystis utricularis: extraction methods, antiviral activity and structural studies. Carbohydr Res 338:153–165
Rowe A, Berendt AR, Marsh K, Newbold CI (1994) Plasmodium falciparum: a family of sulphated glycoconjugates disrupts erythrocyte rosettes. Exp Parasitol 79:506–516
Shimizu J, Wada-Funada U, Mano H, Matahira Y, Kawaguchi M, Wada M (2005) Proportion of murine cytotoxic T cells is increased by high molecular weight fucoidan extracted from Okinawa mozuku (Cladosiphon okamuranus). J Health Sci 51:394–397
Smit AJ (2004) Medicinal and pharmaceutical uses of seaweed natural products: a review. J Appl Phycol 16:245–262
Trager W, Jensen JB (1976) Human malaria parasites in continuous culture. Science 193:673–675
Vieira RP, Mulloy B, Mourao PA (1991) Structure of a fucose-branched chondroitin sulfate from sea cucumber. Evidence for the presence of 3-O-sulfo-beta-d-glucuronosyl residues. J Biol Chem 266:13530–13536
Xiao L, Yang C, Patterson PS, Udhayakumar V, Lal AA (1996) Sulfated polyanions inhibit invasion of erythrocytes by plasmodial merozoites and cytoadherence of endothelial cells to parasitized erythrocytes. Infect Immun 64:1373–1378
Acknowledgments
This work was supported by Inter-campus Research Funds from the Kangwon National University (2007). We are grateful to Dr. Lucio H. Freitas-Junior (Institut Pasteur Korea, Seoul, Korea), Dr. Takafumi Tsuboi (Ehime University, Matsuyama, Japan) and Dr. Yeon-Soo Han (Jeonman National University, Gwangju, Korea) for their kind gifts of Plasmodium falciparum and Plasmodium berghei parasite strains, respectively.
Author information
Authors and Affiliations
Corresponding author
Additional information
J.-H. Chen and J.-D. Lim contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, JH., Lim, JD., Sohn, EH. et al. Growth-inhibitory effect of a fucoidan from brown seaweed Undaria pinnatifida on Plasmodium parasites. Parasitol Res 104, 245–250 (2009). https://doi.org/10.1007/s00436-008-1182-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-008-1182-2