Abstract
Tubulins are heterodimeric molecules responsible for the polymerization of microtubules in apicomplexan parasites. The α-tubulin, a subcellular structural protein of Eimeria acervulina, was cloned and expressed in Escherichia coli as an α-tubulin-GST fusion protein. Immunogenicity of the recombinant protein was studied in chickens by subcutaneous injection of 50, 100, or 150 μg of the protein with or without Freund’s adjuvant. Immunization with 150 μg α-tubulin-GST protein in combination with Freund’s adjuvant conferred partial protection against E. acervulina oocyst challenge, as shown by a 36% reduction in oocyst shedding, a marked decrease in intestinal lesion score and a significant increase in body weight gain in comparison with the nonimmunized controls. The results suggest that α-tubulin protein may be used as an effective vaccine antigen for the control of Eimeria infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Avian coccidiosis is a major parasitic disease in chickens. The disease threatens the health of more than 35 billion chickens produced in the world each year (Blake et al. 2004) and results in worldwide economic losses estimated to exceed £500 million per annum (Shirley et al. 2007). Coccidiosis in poultry can be caused by any of seven species of Eimeria, which are obligate intracellular protozoan parasites, and is clinically and pathologically manifested as intestinal hemorrhage, malabsorption, diarrhea, reduced bodyweight gain, and even mortality.
In most countries, prophylactic chemotherapy using ionophores and synthetic drugs is still the main method for the control of coccidiosis (Chapman 1997). However, the long-term use of these preventative drugs induces drug resistance. To overcome the emergence of drug-resistant parasites, attenuated live coccidial vaccines have been developed and are used worldwide (Lillehoj et al. 2000). However, live vaccines are not always effective and are associated with risks of reversion to highly virulent strains in the host. Therefore, with the progress of molecular biology, recombinant subunit vaccines or DNA vaccines that elicit specific immunity are eminently preferable as alternatives (Dalloul and Lillehoj 2005). Recent studies have demonstrated various levels of protection by vaccination with recombinant antigen or DNA vaccines (Ding et al. 2005, 2004; Kopko et al. 2000; Wu et al. 2004; Xu et al. 2006).
Proteins as effective vaccine antigens are generally involved in essential parasite functions. Surface-expressed and apical complex-associated proteins are the key targets selected for antibody binding, as these proteins are involved in Eimeria invasion into the intestinal epithelia. The apical complex is a specialized subcellular structure that consists of the conoid, polar ring, subpellicular microtubules, and secretory organelles containing micronemes, rhoptries, and dense granules. Secretory organelle-related proteins have been extensively studied, but there has been little research on cytoskeletal proteins such as tubulin, as immunogens, which may offer good protection against Eimeria infection.
Tubulin molecules are heterodimers of an α-subunit and a β-subunit and are responsible for the polymerization of microtubules. The microtubules are of particular importance in Eimeria biology, as they are critical components of the subpellicular lattice, a network of microtubules, which maintain the shape and structure of the organism and are involved in parasite motility and cell division (Morrissette and Sibley 2002; Russell and Burns 1984).
α-Tubulin (GenBank accession no. X88776) and β-tubulin gene of Eimeria tenella have been cloned (Zhu and Keithly 1996); however, there have been no further studies on these molecules. In this study, α-tubulin of E. acervulina was cloned and expressed in Escherichia coli and its immunogenicity studied in chickens. The immunity induced by the recombinant α-tubulin protein was assessed by the lymphocyte proliferation assay. The protective effects of immunization were evaluated by parasitological criteria (mortality, intestinal lesion scores, and oocyst excretion) and growth performance after a challenge with E. acervulina oocyts.
Materials and methods
Animal care and management
The use of chickens in our experiments was approved by the Animal Ethics Committee of China Agricultural University. One-day-old broiler chickens were purchased from the Institute of Animal Science, Chinese Academy of Agricultural Sciences and reared in wire cages in a coccidia-free environment. The chickens were provided with food and water ad libitum.
Parasite source and maintenance
E. acervulina BJ strain was maintained in the Parasitology Laboratory of the College of Veterinary Medicine, China Agricultural University. Sporulated oocysts of E. acervulina BJ strain were stored in 2.5% potassium dichromate solution at 4°C and passaged through chickens every 3 months as previously described (Wu et al. 2004). The oocysts were collected in saturated sodium hypochlorite and cleaned using 5% sodium hypochlorite solution before being washed three times with phosphate-buffered saline (PBS) to remove sodium hypochlorite and counted using a hemocytometer (Hausser Scientific, USA).
Construction of α-tubulin plasmid and expression of recombinant α-tubulin protein
Total RNA was extracted from 2 × 108 sporulated oocyts using the Trizol reagent according to the manufacturer’s instructions (Invitrogen, USA). The first strand cDNA was synthesized by the conventional method using Oligo (dT)16 primers, Moloney murine leukemia virus reverse transcriptase (Promega), and a ribonuclease inhibitor (RNasin, Promega). Based on the publicly available sequence of α-tubulin (GenBank accession no. X88776), primers were designed as follows: forward primer 5′-GTGGATCCGGTATCCAAATCGGAAAT-3′ and reverse primer 5′-CCGGAATTCATCTCCATATCCTTCTTC-3′ (sites for digestion by BamHI and EcoRI are underlined). The E. acervulina α-tubulin gene was amplified using the ExTaq polymerase chain reaction (PCR) system (TaKaRa, Dalian, China) using cDNA as the template. The amplified α-tubulin gene was purified and cloned into the pGEM-T easy vector (Promega). The recombinant plasmid vector, designated as pGEM-α-T, was transformed into JM109 competent cells for cloning. The open reading frame (ORF) of the α-tubulin protein predicted using the DNAstar software was obtained from the pGEM-α-T cloning vector by PCR using 5′-TAGGATCCCAGGTTCCCCGCTGCGTG-3′ as the forward primer and 5′-CCGGAATTCATCTCCATATCCTTCTTC-3′ as the reverse primer (BamHI and EcoRI restriction sites are underlined). The insert was recovered and ligated into the pGEX-6p-1 expression vector with the GST protein tag (Amersham Pharmacia Biotech). This was designated as pGEX-α-T. The amino acid sequence had a transmembrane region between the 6th and 19th amino acids as predicted on the TMHMM website and the DNAstar software, hindering protein expression. Therefore, the transmembrane region was removed using the second pair of primer with the sequence beginning at the 58th nucleic acid base.
The recombinant pGEX-α-T, was transformed into BL21 (DE3) E. coli cells using the standard methods (Sambrook and Russel 2001). Briefly, a positive bacterial clone containing the recombinant plasmid was cultured in 3 ml liquid Luria–Bertani (LB) media with 50 μg/ml ampicillin at 37°C overnight. This culture was transferred to 300 ml of LB media containing 50 μg/ml ampicillin and was further grown in an incubator at 37°C until the OD600 reached 0.5 to 0.6. For protein expression, isopropyl-β-d-thiogalactopyranoside (IPTG; AMRESCO) was added to a final concentration of 1 mM/ml and further cultured for another 6 h. Using an ultrasonicator (Scientz, Ningbo, China), bacterial cells were lyzed and pelleted by centrifugation at 4,000×g for 8 min.
The expressed α-tubulin-GST fusion protein was separated on a 12% (w/v) polyacrylamide gel by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) according to the manufacturer’s instructions (Bio-Rad, California, USA) and analyzed by Western blot with mouse anti-GST monoclonal antibodies (NeoMarkers, Fermont, CA, USA). The band was visualized using diamino benzidine (AMRESCO) and horseradish peroxidase.
The solubilized α-tubulin-GST protein was purified by affinity chromatography using a Glutathione Sepharose 4B column and 10 mM glutathione elution buffer according to the manufacturer’s instructions (Amersham Biosciences). The purity of the protein was assayed by SDS-PAGE, and the protein concentration was spectrophotometrically estimated at A 280nm and A 260nm.
α-Tubulin ORF sequence analysis
For α-tubulin nucleic acid sequence comparison, we have carried out by neighbor-joining method (Saitou and Nei 1987) sequence analysis of our reported α-tubulin protein (AY488134), α-tubulin of Neospora caninum (AF508031), Toxoplasma gondii (M20024), Cryptosporidium parvum (XM_625871), P. berghei (XM_671223), Babesia bovis (XM_001611364), and Schistosoma mansoni (M80214), and the β-tubulin of E. tenella (U19268). The phylogenetic tree was constructed using the Kimura two-parameter method (Kimura 1980).
Immunization
Ten groups of chickens (n = 10) were immunized with two doses of tubulin-GST protein, GST, PBS, or live oocysts 10 days apart. Three groups (groups III–V) were injected subcutaneously with 0.1 ml α-tubulin-GST in combination with an equal volume of complete Freund’s adjuvant (CFA) as the first dose and with incomplete Freund’s adjuvant (IFA) for the booster dose. In addition, three groups (groups VI–VIII) were immunized with α-tubulin-GST only. As controls, two groups received two subcutaneous doses of PBS (groups I and II), while one group received subcutaneously purified GST protein with CFA for the first immunization dose and IFA for the booster dose (group IX), and one (group X) received orally 300 E. acervulina sporulating oocysts as the live vaccine control (Table 1).
Blood (2 ml) was collected randomly from three chickens of each group except for group II by cardiac puncture before immunization and 10 days after the first and boost injection for the lymphocyte proliferation assay (described below). The immunization experiments were repeated three times, and results from all three experiments were analyzed.
Lymphocyte proliferation assay
Lymphocytes were isolated using a previously described method (Du et al. 2005). Briefly, lymphocytes were separated by gradient centrifugation and washed three times with RPMI1640 (Gibco) containing 3% fetal calf serum (FCS). Viable cells were counted using the Trypan-blue method (Strober 2001).
For the lymphocyte proliferation assay, 96-well flat-bottomed plates (Costar, USA) were used. Lymphocytes (1 × 105 cells in a 100-μl culture medium RPMI1640 containing 100 U/ml penicillin, 100 U/ml streptomycin, and 5% FCS) were incubated with 20 μl α-tubulin (final concentration, 10 μg/ml), 20 μl ConA (final concentration: 10 μg/ml) as positive control, or 20 μl PBS as negative control in triplicate at 37°C in 5% CO2 atmosphere for 56 h. Ten microliters of 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT, 5 mg/ml, AMRESCO) solution was added to each well and incubated for 4 h. The reaction was stopped by the addition of 100 μl of dimethyl sulfoxide, and the optical density was measured in an enzyme-linked immunosorbent assay reader (Bio-Rad) at 570 nm.
Evaluation of immune protection
Ten days after the booster dose, each group was divided into two subgroups of five chickens each. Each subgroup of five chickens was housed in one cage. Chickens of subgroup A were challenged with 103 sporulated oocysts and subgroup B with 8 × 104 sporulated oocysts by gavage and were observed daily for clinical signs and mortality. Feces were collected from subgroup A between days 5 and 8 after oocyst inoculation. Total oocysts in feces were counted using the McMaster method modified by Talebi and Mulcahy (1995). Each sample was counted three times. Chickens of subgroup B were killed 5 days after oocyst inoculation, and duodenal lesions were scored according to Johnson and Reid (1970) by double-blind examination (minimum score 0, maximum score 4). All chickens were weighed immediately before oocyst inoculation and 8 days (subgroup A) or 5 days (subgroup B) post inoculation to determine weight gain during infection. The immunization, challenge, and assessment schedule is schematically illustrated in Fig. 1.
Schematic outline of immunization, challenge infection with E. acervulina and immunoprotection assessment schedule. Chickens were immunized with purified α-tubulin-GST fusion protein or GST protein by subcutaneous injection on days 10 and 20. Five chickens (subgroup A) of each group were orally inoculated with 103 sporulated oocysts of E. acervulina, and five chickens (subgroup B) of each group were orally inoculated with 8 × 104 sporulated oocysts of E. acervulina on day 30. Body weight gain was assessed between days 30 and 38 (subgroup A) or days 30 and 35 (subgroup B). Feces were collected between days 35 and 38 in subgroup A for oocyst counting. Duodenal lesions of subgroup B chickens were scored on day 35
Statistical analysis
Data were statistically analyzed by analysis of variance and Student’s t-test. Difference between groups was considered significant if the p value was less than 0.05 (p < 0.05).
Results
Expression of the recombinant α-tubulin protein
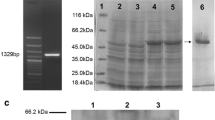
Figure 2 shows the α-tubulin gene of E. acervulina that is determined to be of 1,937 bp. BLASTed sequence result showed that the sequence shared 99.5% identity with the previously reported E. acervulina α-tubulin (GenBank accession no. X88776). The obtained α-tubulin gene sequence has been deposited in the GenBank (accession no: AY488134). The coding sequence analyzed by the DNAstar software was of 1,248 bp, corresponding to 416 amino acids, with a molecular weight of 46 kDa.
The α-tubulin gene was successfully amplified using the primers designed based on the coding sequence of E. acervulina α-tubulin (GenBank accession no. X88776) and was ligated to pGEX-6p-1 as confirmed by PCR and restriction enzyme digestion. Maximal expression of α-tubulin protein was obtained after 6 h of incubation with 1 mM IPTG. SDS-PAGE of the whole cell preparation of the recombinant pGEX-α-T-BL21 strain showed a clear band of 69 kDa for the α-tubulin-GST fusion protein (Fig. 3). This is consistent with the predicted molecular weight of α-tubulin, which is estimated to be 43 kDa after removal of the 6th–19th amino acid region + 26 kDa for GST. Only the GST protein was expressed in the bacteria transformed with the empty pGEX-6p-1 plasmid vector (Fig. 3, lane 3). The expressed α-tubulin-GST fusion protein was purified and confirmed by Western blotting with monoclonal antibodies directed against the GST-fused α-tubulin protein (Fig. 3, lane 8).
SDS-PAGE and Western blot of the whole cell preparation of α-tubulin recombinant E. coli BL21 (DE3) and purified α-tubulin-GST fusion protein. Lane 1 MW marker. Lane 2 BL21 (DE3) transformed with the pGEX-6p-1 vector. Lane 3 BL21 (DE3) transformed with the pGEX-6p-1 vector and cultured in the presence of 1 mM IPTG. Lane 4 BL21 (DE3) transformed with the pGEX-α-T plasmid. Lane 5 BL21 (DE3) transformed with the pGEX-α-T plasmid and cultured in the presence of 1 mM IPTG. Lane 6 purified α-tubulin-GST fusion protein. Lane 7 purified GST protein (from E. coli harboring nonrecombinant pGEX-α-T plasmid). Lane 8 Western blot of α-tubulin-GST protein
The phylogenetic tree of the tubulin protein family is shown in Fig. 4. The α-tubulin of E. acervulina was similar to α-tubulin of other apicomplexan parasites such as B. bovis, C. parvum, and N. caninum but different from the E. tenella β-tubulin. There was also some homology of E. acervulina α-tubulin with α-tubulin of nonapicomplexan parasites such as S. mansoni, indicating that α-tubulin is conserved in Eukaryota.
Phylogenetic tree of tubulin. The unrooted phylogenetic tree was inferred from ORF sequence alignment of tubulin proteins to E. acervulina α-tubulin using the Clustal W algorithm program. The tree was constructed by the neighbor-joining alignment method. Topology supports from 1,000 bp replicates are presented as percentage values next to internal nodes. The branch lengths are in the same units as those of the evolutionary distances computed using the Kimura two-parameter method. In brackets are accession numbers of the coding gene for each protein
Ex vivo lymphocyte proliferation in response to α-tubulin
As T lymphocyte proliferative responses are generally related to the cell-mediated immunity to parasite infection, α-tubulin antigen specific T lymphocyte proliferation was evaluated. ConA induced proliferation of peripheral lymphocytes from all groups, with OD570 nm values of about 2 for all groups. As shown in Fig. 5, lymphocyte proliferation responses to α-tubulin for the groups immunized with α-tubulin without adjuvant was slightly higher than that before immunization, but none of the OD570nm values were statistically significant compared to the control. Similar results were observed for the groups immunized with 50 or 100 μg α-tubulin protein with adjuvant. In contrast, 150 μg α-tubulin with adjuvant induced good immune responses, and the OD570nm value of this group was significantly higher (p < 0.05) than values of all other groups.
Immunoprotection
There was no mortality in the control or immunized groups. Oocyst production, body weight gain, and intestinal lesion scores are shown in Table 1. Fecal oocyst output in the α-tubulin-GST immunized groups was slightly reduced compared to that of the unchallenged control group (group I). The highest reduction was recorded in the live oocyst group (97% relative to the challenged control group). Oocyst production in the groups receiving α-tubulin-GST plus adjuvant (III, IV, V) was reduced by 28–36%, and the reduction in the 150 μg + adjuvant group (36%) was statistically significant (p < 0.05) compared to the challenged control group (II). A very small reduction in oocyst output was observed in the groups immunized with α-tubulin without adjuvant (VI, VII, VIII; 13–28%) or GST protein with adjuvant (IX; 12.7%); the small decrease was not significantly different from the challenged control group.
Mean body weight gains in 5 days in the subgroups challenged with 8 × 104 oocysts were small, ranging from 44.22 g to 55.36 g, and there was no significant intergroup difference. Among the groups inoculated with 103 oocysts, the nonimmunized, challenged group (group II) gained the least weight (55.47 g) in 8 days after oocyst inoculation. Body weight gains in all α-tubulin plus adjuvant groups (III, IV, and V), unchallenged control group (I), and the live oocyst-immunized chickens (X) were higher (p < 0.05) than that of the challenged control group (II). Immunization with the nonadjuvanted α-tubulin-GST fusion protein or with adjuvanted GST protein did not protect chickens from the coccidial infection-induced reduction in body weight gain.
The nonimmunized, challenged control chickens (group II) had the highest lesion score, while no lesions were observed in the unchallenged control group (I) and negligible lesions were recorded in the live oocyst group (X). The chickens immunized with 100 or 150 μg α-tubulin protein with adjuvant or 150 μg α-tubulin protein without adjuvant (IV, V, and VIII) had significantly less duodenal lesions (mean lesion score: 0.3 to 0.67 cf. 2 for the challenged control group). Duodenal lesions in the chickens immunized with 50 μg α-tubulin protein with or without adjuvant (III and VI) were not statistically different from lesions in the challenged control group. As expected, a relatively high duodenal lesion score (1.33) was recorded in the group immunized with the GST protein (IX), which provided no protection from coccidial challenge.
Discussion
The α-tubulin gene of the Beijing strain E. acervulina was cloned and expressed as a fusion α-tubulin protein in E. coli BL21. The α-tubulin gene sequence has 99.5% identity with the reported sequence of α-tubulin from the same Eimeria species (GenBank accession no. X88776). The small difference in nucleotide sequence (0.5%) suggests minor genetic differences between the two strains. In addition, the α-tubulin showed 77.2% identity with the N. caninum α-tubulin, which is consistent with the previously reported finding that α-tubulin is a highly conserved protein (Siverajah et al. 2003).
Identification of parasite antigens that could induce protective immunity against natural infection is critical in the development of an effective recombinant protein vaccine (Vermeulen 1998). The antigenicity of E. acervulina proteins has been studied by Jenkins et al. (1990, 1988). Surface proteins designated as cSZ-1 and MA1 from the sporozoite (Jenkins et al. 1988) and EAMZp30–47 from the merozoite (Jenkins et al. 1990) induced T lymphocyte activation, but they were not recognized by sera obtained from E. acervulina infected chickens, suggesting the absence of a humoral epitope on the E. acervulina proteins. In contrast, recombinant antigens M16 constructed from the cDNA shared by sporozoites and merozoites (Castle et al. 1991, O’Lorcain et al. 1996) and cMZ-8 from the merozoite (Jenkins et al. 1988) from the same Eimeria species possessed both T and B cell epitopes. The above findings were conducted using in vitro experiments, and none has been studied in animals. Experiments in live animals are necessary for the determination of immunoprotective effects of potential subunit or DNA vaccine candidates.
It is widely known that T lymphocytes are major immune components against intracellular parasitic infections (Dalloul and Lillehoj 2006; Lillehoj 1998; Trout and Lillehoj 1996). It has also been shown that immunoprotection in Eimeria infection is mainly mediated by T cell immune response (Lillehoj 1998; Trout and Lillehoj 1996). Therefore, for an ideal vaccine against Eimeria, the presence of a conserved protein with a membrane domain epitope to elicit cellular immune response is important. In our study, the recombinant α-tubulin-stimulated ex vivo lymphocyte proliferation in chickens immunized with the high dose of α-tubulin protein (150 μg) in combination with an adjuvant was significantly greater than that in other groups, suggesting that immunization of chickens with sufficiently high doses of the α-tubulin protein induced antigen-specific cellular immunity.
Body weight gain, mortality, intestinal lesion score, and oocyst output were used as the main criteria for the assessment of the efficacy of α-tubulin protein as a vaccine against E. acervulina infection in chickens. The measurement of oocyst excretion and duodenal lesions simultaneously in the same group of chickens was impracticable. Scoring of lesions caused by E. acervulina necessitates the killing of chickens at the time of maximum intestinal damage; however, this is incompatible with the assessment of oocyst output, which requires the challenged chickens to be kept alive (Talebi and Mulcahy 2005). In addition, as a large number of oocysts are produced from each inoculated oocyst, the challenge dose of oocysts needs to be carefully selected to avoid the crowding effect. Therefore, for intestinal lesion scoring, each chicken was challenged with 8 × 104 oocysts, while for the examination of oocyst output, each chicken was inoculated with a smaller number of oocysts (1 × 103). The reduction of oocyst shedding was greater in α-tubulin + adjuvant groups than those immunized with α-tubulin alone; this is consistent with findings reported in the scientific literature that the Freund’s adjuvant enhances the immunogenicity of many antigens (Wallach et al. 1995).
The principal finding in our study is that recombinant α-tubulin protein was immunogenic and provided some protection against coccidian infection in chickens. As cytokines were found to increase the antigenicity of coccidia DNA vaccines (Lillehoj et al. 2000, 2005, 2004), using cytokines as adjuvant may improve immunogenicity of the α-tubulin protein. In addition, anti-coccidial vaccines containing multiple proteins may be more immunogenic than a monovalent vaccine. The immunogenicity of the α-tubulin protein in combination with other eimerian proteins is currently under evaluation in our laboratory.
Reference
Blake DP, Hesketh P, Archer A, Carroll F, Smith AL, Shirley MW (2004) Parasite genetics and the immune host: recombination between antigenic types of Eimeria maxima as an entree to the identification of protective antigens. Mol Biochem Parasitol 138:143–152
Castle MD, Jenkins MC, Danforth HD, Lillehoj HS (1991) Characterization of a recombinant Eimeria acervulina antigen expressed in sporozoite and merozoite developmental stages. J Parasitol 77:384–390
Chapman HD (1997) Biochemistry, genetic and applied aspects of drug resistance in Eimeria parasites of the fowl. Avian Pathol 26:221–244
Dalloul RA, Lillehoj HS (2005) Recent advances in immunomodulation and vaccination strategies against coccidiosis. Avian Dis 49:1–8
Dalloul RA, Lillehoj HS (2006) Poultry coccidiosis: recent advancements in control measures and vaccine development. Expert Rev Vaccines 5:143–163
Ding X, Lillehoj HS, Quiroz MA, Bevensee E, Lillehoj EP (2004) Protective immunity against Eimeria acervulina following in ovo immunization with a recombinant subunit vaccine and cytokine genes. Infect Immun 72:6939–6944
Ding X, Lillehoj HS, Dalloul RA, Min W, Sato T, Yasuda A, Lillehoj EP (2005) In ovo vaccination with the Eimeria tenella EtMIC2 gene induces protective immunity against coccidiosis. Vaccine 23:3733–3740
Du A, Hu S, Wang S (2005) Eimeria tenella: ginsenosides-enhanced immune response to the immunization with recombinant 5401 antigen in chickens. Exp Parasitol 111:191–197
Jenkins MC, Lillehoj HS, Dame JB (1988) Eimeria acervulina: DNA cloning and characterization of recombinant sporozoite and merozoite antigens. Exp Parasitol 66:96–107
Jenkins MC, Lillehoj HS, Barta JR, Danforth HD, Strohlein DA (1990) Eimeria acervulina: cloning of a cDNA encoding an immunogenic region of several related merozoite surface and rhoptry proteins. Exp Parasitol 70:353–362
Johnson J, Reid WM (1970) Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Exp Parasitol 28:30–36
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Kopko SH, Martin DS, Barta JR (2000) Responses of chickens to a recombinant refractile body antigen of Eimeria tenella administered using various immunizing strategies. Poult Sci 79:336–342
Lillehoj HS (1998) Role of T lymphocytes and cytokines in coccidiosis. Int J Parasitol 28:1071–1081
Lillehoj HS, Lillehoj EP (2000) Avian coccidiosis. A review of acquired intestinal immunity and vaccination strategies. Avian Dis 44:408–425
Lillehoj HS, Choi KD, Jenkins MC, Vakharia VN, Song KD, Han JY, Lillehoj EP (2000) A recombinant Eimeria protein inducing interferon-gamma production: comparison of different gene expression systems and immunization strategies for vaccination against coccidiosis. Avian Dis 44:379–389
Lillehoj HS, Min W, Dalloul RA (2004) Recent progress on the cytokine regulation of intestinal immune responses to Eimeria. Poult Sci 83:611–623
Lillehoj HS, Ding X, Quiroz MA, Bevensee E, Lillehoj EP (2005) Resistance to intestinal coccidiosis following DNA immunization with the cloned 3-1E Eimeria gene plus IL-2, IL-15, and IFN-gamma. Avian Dis 49:112–117
Morrissette NS, Sibley LD (2002) Cytoskeleton of apicomplexan parasites. Microbiol Mol Biol Rev 66:21–38 table of contents
O’Lorcain P, Talebi A, Mulcahy G (1996) B-cell epitope mapping within the MA16 antigenic sequence found in Eimeria acervulina merozoites and sporozoites. Vet Parasitol 66:147–157
Russell DG, Burns RG (1984) The polar ring of coccidian sporozoites: a unique microtubule-organizing centre. J Cell Sci 65:193–207
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Sambrook J, Russell DW (2001) Molecular cloning: A laboratory manual, 3rd edition. Cold Spring Harbor Laboratory Press, New York
Shirley MW, Smith AL, Blake DP (2007) Challenges in the successful control of the avian coccidia. Vaccine 25:5540–5547
Siverajah S, Ryce C, Morrison DA, Ellis JT (2003) Characterization of an alpha tubulin gene sequence from Neospora caninum and Hammondia heydorni, and their comparison to homologous genes from Apicomplexa. Parasitology 126:561–569
Strober W (2001) Trypan blue exclusion test of cell viability. Current Protocols in Immunology. Wiley, New York
Talebi A, Mulcahy G (1995) Correlation between immune responses and oocyst production in chickens monospecifically infected with Eimeria maxima. Avian Pathol 24:485–495
Talebi A, Mulcahy G (2005) Partial protection against Eimeria acervulina and Eimeria tenella induced by synthetic peptide vaccine. Exp Parasitol 110:342–348
Trout JM, Lillehoj HS (1996) T lymphocyte roles during Eimeria acervulina and Eimeria tenella infections. Vet Immunol Immunopathol 53:163–172
Vermeulen AN (1998) Progress in recombinant vaccine development against coccidiosis. A review and prospects into the next millennium. Int J Parasitol 28:1121–1130
Wallach M, Smith NC, Petracca M, Miller CM, Eckert J, Braun R (1995) Eimeria maxima gametocyte antigens: potential use in a subunit maternal vaccine against coccidiosis in chickens. Vaccine 13:347–354
Wu SQ, Wang M, Liu Q, Zhu YJ, Suo X, Jiang JS (2004) Construction of DNA vaccines and their induced protective immunity against experimental Eimeria tenella infection. Parasitol Res 94:332–336
Xu SZ, Chen T, Wang M (2006) Protective immunity enhanced by chimeric DNA prime-protein booster strategy against Eimeria tenella challenge. Avian Dis 50:579–585
Zhu G, Keithly JS (1996) The beta tubulin gene of Eimeria tenella. Mol Biochem Parasitol 76:315–319
Acknowledgment
The authors thank Prof. Xun Suo for providing experimental instruments and facilities and Ms. Chong Deng and Mr. Jingpeng Han for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ding, J., Bao, W., Liu, Q. et al. Immunoprotection of chickens against Eimeria acervulina by recombinant α-tubulin protein. Parasitol Res 103, 1133–1140 (2008). https://doi.org/10.1007/s00436-008-1106-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-008-1106-1