Abstract
In forensic investigations, all immature stages of flies (egg, larvae, and puparium) can serve as entomological evidence at death scenes. These insects are primarily used to estimate the post mortem interval (PMI), but can also be involved in the analysis of toxic substances, determining manner of death, and in indicating relocation of a corpse in homicide cases. In this study, we present the morphology of the egg, larvae, and puparium of Hemipyrellia ligurriens, a blow fly species of forensic importance in Thailand. Examination was conducted using scanning electron microscopy (SEM). The egg stage was found to display a relatively wide plastron region (or median hatch line area) that spans almost the entire length of the egg. The median hatch line is oriented in an upright position. External chorionic sculpture of the egg is present in a hexagonal pattern whose reticular boundaries are slightly elevated. In the larval stages, the most prominent morphological changes were detected upon comparison of the first to the second instar; whereas, the differences between second and third instar larvae were less obvious outside of the increase in number of posterior spiracular slits. Most of the major differences involve body size and structure of the anterior and posterior spiracles. Each anterior spiracle in both the second and third instars projects five to seven papillae apically. Each posterior spiracular disc of a third instar exhibits a complete peritreme, three spiracular slits, and a prominent button that is ventromedially located. The puparium is coarctate and features a clustered bubble membrane comprised of ≈57 mammillate structures positioned dorsolaterally on each side of the first abdominal segment in young puparia. This feature is replaced by short, tubular respiratory horns in aged puparia. This study provides more detailed exposure of important morphological features that can be used for accurate identification of immature stages of H. ligurriens. Information presented can aid in forensic investigations involving this fly species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hemipyrellia ligurriens (Wiedemann) is a blow fly species currently considered to be of forensic importance since their larvae have been collected from human corpses (Chen et al. 2004; Lee et al. 2004; Sukontason et al. 2007a). Adult flies are also considered nuisance insects in market places and gardens within towns, as well as serving as mechanical vectors of pathogens due to their attraction to human excreta around human-occupied environments (Kurahashi et al. 1997). Larvae of this species have also been recorded as parasites of living animals in experimental conditions (Roy and Dasgupta 1971).
H. ligurriens has a fairly wide distribution in the many countries including the Philippines, China, Japan, Korea, Taiwan, India, Sri Lanka, Thailand, Malaysia (Malaya Borneo), Singapore, Indonesia (Sumatra, Java, Sulawesi, Ambon), New Guinea, and Australia (Kurahashi et al. 1997), but information pertaining to aspects of forensic importance of this species is limited (Ishijima 1967; Sukontason et al. 2007b). The morphology and fine structure of this species has received little attention in comparison to that of other blow fly genera (e.g., Chrysomya spp.). Ishijima (1967) provided some information on the adult and illustrated the third instar of H. ligurriens; whereas, some characters of the puparium have been recorded from light microscopy observations by Sukontason et al. (2007b). In light of the possibility of occurrence of this fly species in other forensic investigations in the future, there is an imperative need to study the biology of this fly (e.g., morphology, development, and succession in carcasses that may be applied to human corpse succession) in order to ensure successful outcomes of its application in forensic medicine. In particular, forensic entomology requires accurate identification of insect specimens as a critical prerequisite to its application. The present study expands the work of previous authors by using scanning electron microscopy (SEM) to describe fine details of the external morphology of all immature stages (egg, larvae, and puparium) of H. ligurriens, which are the stages usually in need of identification in forensic applications. Characteristics used to identify species will be highlighted.
Materials and methods
Fly rearing
All immature stages of H. ligurriens used in this study were obtained from a laboratory colony located at the Department of Parasitology, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand. Laboratory colonies are maintained at natural temperature and light/dark photoperiod for the area using methods previously described (Sukontason et al. 2004). Larvae are fed a fresh pork liver diet and adults are fed a 10% sugar solution mixed with a small amount of multivitamin syrup.
Scanning electron microscopy observation
For SEM observation, all larval instars were collected from the colony and washed several times with normal saline solution to remove surface artifacts and/or liver tissue. Larval specimens were killed by transferring them into a beaker containing water near the boiling point for ≈5 min. The dead larvae were then prefixed with a 2.5% glutaraldehyde mixture in phosphate-buffered saline (PBS), pH 7.4 at 4°C for 24 h. In addition, larvae were rinsed twice with PBS at 10-min intervals, and postfixed with 1% osmium tetroxide at room temperature for 3 days. Specimens were then rinsed twice with PBS and dehydrated with alcohol. The dehydration process involved the larvae being sequentially subjected to the following increased alcohol concentrations: 30%, 50%, 70%, 80%, and 90%. Larvae remained in each concentration of alcohol for 12 h during each step of the dehydration process. After treatment in the alcohol concentrations, specimens were placed in absolute alcohol for another two 12 h periods followed by treatment in acetone for two 12-h periods. Finally, the larvae were subjected to critical point drying, attached to double-stick tape on aluminum stubs and coated with gold in a sputter-coating apparatus in order for them to be viewed under a JEOL JSM-5910LV scanning electron microscope (SEM; Tokyo, Japan).
Egg specimens were processed for SEM as previously described for larvae. However, preparation of puparia was more simplistic. Puparia were cleaned by shaking them in a water bath for 1 h and then gently placing them onto double-stick tape on stubs and coating them with gold for 30 s in a sputter-coating apparatus (SPI-MODULE™ Coater Sputter, USA). This enabled viewing under a JEOL JSM-5910LV SEM using a high vacuum mode.
Results
Egg
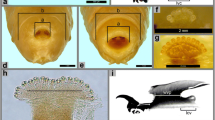
The egg of H. ligurriens is elongated and tapered at both anterior and posterior ends (Fig. 1a). The plastron region is located dorsally and is relatively wide with the hatch line upright in position (Fig. 1a,b,c). While both plastron and hatch line span almost the entire length of the egg, the plastron is bifurcated anteriorly to extend around and flank the micropyle, coming to an end below it (Fig. 1b). The upright hatch line and dorsal plastron display extreme similarity in ultrastructural appearance with both being composed of irregularly spaced plaques formed at the apices of pillars (Fig. 1c). Small pores were observed in the top of each plaque (Fig. 1d). Externally, the chorionic sculpture of H. ligurriens eggs appeared as a hexagonal pattern, with its reticular boundary slightly elevating like a net (Fig. 1e). Some parts of the eggshell that were observed were ruptured resulting in delamination of the chorion (Fig. 1f, rectangle). Delamination revealed the outermost exochorion, transverse middle layer of endochorion, and the transparent innermost chorionic layer. Higher magnification of the inner surface of the endochorion, where the innermost chorionic layer had peeled away, revealed arrangement of the inner surface into footlike structures that form approximate hexagonal patterns (Fig. 1g).
Scanning electron micrographs of egg of H. ligurriens. a Whole egg displaying elongated structure which tapers at both anterior (arrowhead) and posterior ends (arrow). The relatively wide plastron (P), located dorsally, spans almost the entire length of the egg. b Anterior end showing bifurcation of plastron terminating below micropyle (arrow). c Inner surface of hatch line (H) revealing similarity of appearance to plastron (P) which are both comprised of irregularly spaced plaques formed at apices of pillars. d Higher magnification of plastron highlighting irregularly spaced plaques formed at apices of pillars. e Hexagonal chorionic sculpture of egg with slightly elevated reticular borders. f Partial detachment of eggshell (rectangle) revealing its layers: outermost exochorion (E), transverse layer of endochorion (En), and transparent innermost chorionic layer (INL). g Inset shows how inner surface of endochorion is comprised of footlike structures arranged in approximate hexagonal patterns when innermost chorionic layer was detached from it
First instar
Under SEM observation, all larval stages of H. ligurriens display the typical muscoid-shaped vermiform larva that is pointed anteriorly and blunt in the posterior end. As seen in Fig. 2a, the first instar is small, measuring approximately 2 mm in length. Obvious features in the dorsal portion of the bilobate cephalic region are sensory structures known as the terminal organ and the dorsal organ (Fig. 2b, circle and Fig. 2c). Organs associated with feeding are the most obvious features of the ventral portion of the cephalic region. These are comprised of grouped spines, oral grooves, and the labium (Fig. 2b). On each side of the mouth opening, two lines of oral grooves are prominent and extend transversely from the dorsal region into the oral cavity (Fig. 2b). Two distinct types of sensillae (papillary sensillae and knobbed sensillae) were observed on each terminal organ (Fig. 2d). The papillary sensillae are arranged into clusters of sensillae. The dome-shaped dorsal organ is located dorsolaterally to the terminal organ, and higher magnification of the dorsal organ reveals minute longitudinal furrows throughout its surface (Fig. 2e). Extended mouthhooks were not seen in the first instar, but two groups of spines are situated mid-dorsally of the rudimentary transverse oral grooves (Fig. 2b,f). Each group of spines consists of clustered acute tipped spines in the upper half of the group and a cluster of bulbous round tipped spines in its lower half (Fig. 2f). The labium is tripartite, or comprised of three lobes, with the medial lobe being the largest (Fig. 2g). Small sensory structures were observed on the central region of the lateral labial lobes (Fig. 2g, higher magnification in Fig. 2h).
Scanning electron micrographs of first instar H. ligurriens. a Lateral view of vermiform larva showing typical muscoid shape that is pointed anteriorly (arrowhead) and blunt posteriorly (arrow). b Ventrolateral view of cephalic segment illustrating the terminal organ, dorsal organ at the back (circle), groups of spines (arrows), two lines of oral grooves (OG) and labium (L). c Lateral view of terminal organ (TO) and dorsal organ (DO). d Higher magnification of terminal organ displaying papillary sensillae (PS) and knobbed sensillum (KS). e Dome-shaped dorsal organ highlighting the longitudinal furrows within its surface. (f) Two groups of spines with each cluster consisting of apically oriented acute tipped spines (arrow) and bulbous round tipped spines in proximal position (arrowhead). g Tripartite labium displaying medial lobe as largest. Arrows indicate small sensory structures in lateral labial lobes. h Higher magnification of sensory structure in a lateral labial lobe. i Paired sensillae that each appear as a clustered group of three or four setae on the ventral region of thoracic segments. j Posterolateral view of 11th and 12th terminal abdominal segments showing smooth integument (IN) and pair of posterior spiracular discs (PSD) within slight depression in end of 12th segment. Arrows indicate peripheral rim of 12th segment bearing numerous fine setae. k Each posterior spiracular disc contains two straight spiracular slits (SS) that coalesce ventrally, have joined peritremes, and are interspaced with four bundles of broad and multibranched spiracular hairs (arrows). Below each posterior spiracular disc is a dome-shaped organ (arrowhead) that is dorsally spinose
While focusing on the thoracic segments, no apparent anterior spiracles were found at the posterior margin of the prothorax. Paired sensillae were present as clustered groups of three or four setae each on the anterior edge of the ventral surface of the mesothorax (Fig. 2i). The integument of the first instar is psilate and devoid of prominent tubercles (Fig. 2a,j). Observation of the posterior region in lateral view revealed the anterior and posterior margins of each segment bearing a few rows of posteriorly projecting acuminate spines (Fig. 2j). A pair of posterior spiracular discs is located in a slight depression in the caudal segment (Fig. 2j,k). The peripheral rim of the caudal segment bears numerous fine setae (Fig. 2j, arrows). Each posterior spiracular disc contains two straight spiracular slits that coalesce ventrally, have joined peritremes, and are interspaced with four bundles of broad and multibranched spiracular hairs (Fig. 2k). Below each posterior spiracular disc is a dome-shaped organ with numerous small spines on its dorsal surface (Fig. 2k, arrowhead).
Second instar
As would be expected, the second instar is larger in size than the first instar. In the cephalic region of the second instar, the dorsal organ, terminal organ, and ventral organ are present (Fig. 3a). Oral grooves appear in a transverse array to channel liquefied food toward the mouth opening (Fig. 3a). The ventral organ displays a raised coronate rim around a central opening (Fig. 3b). The anterior spiracles arise posterolaterally on the prothorax and are present as fan-shaped structures bearing a single row of five to seven papillae along the distal margin of each (Fig. 3c). While focusing on a lateral view of the last few abdominal segments, it was clear that most of the integument is psilate or smooth, while the caudal segment bears a single pair of posterior spiracles (Fig. 3d,e). Upon inspection at higher magnification, the integument beneath the posterior spiracles was discovered to be verrucate or covered with small rounded protuberances (Fig. 3e, asterisk). Each posterior spiracular disk contains two spiracular slits that, unlike in the first instar, are now more developed and have peritremes that are entirely separated (Fig. 3e), while the posterior spiracular hairs are no longer thick but are thin-branched (Fig. 3f, arrows).
Scanning electron micrographs of second instar H. ligurriens. a Lateral view of cephalic segment identifying dorsal organ (DO), ventral organ (VO), and oral grooves (OG). b Higher magnification of ventral organ highlighting its coronate rim (arrow). c Anterior spiracle with seven papillae. d Lateral view of terminal abdominal segments showing smooth integument and pair of posterior spiracular discs (PSD). e Paired posterior spiracular discs, each with two separate diagonally oriented slits (s). Asterisk indicates verrucate integument beneath posterior spiracular disc. f Higher magnification of left posterior spiracular disc showing two separated slits (s) and four groups of thin-branched posterior spiracular hairs (arrows)
Third instar
The body of the third instar also showed the typical muscoid shape, and prominent dorsal and terminal organs are present in the cephalic region (Fig. 4a). The oral groove area is well developed as an array of ridges between oral grooves leading into the mouth opening (Fig. 4a). The terminal organs lie ventrolateral to the dorsal organs (Fig. 4a) and house both papillary sensillae and a knobbed sensillum (Fig. 4b). The presence of papillary sensillae in groups of three is similar to what is seen in the first and second instars. The dorsal intersegmental spines between the prothorax and mesothorax of the third instar show the arrangement of spines into continuous individual rows, with each spine having a single sharp tip (Fig. 4c). The anterior spiracles located laterally at the posterior margin of the prothorax are prominent and comprised of a number of papillae ranging from five to seven (Fig. 4c). The caudal segment bears six pairs of small tubercles divided into dorsal and ventral sets around its peripheral rim, and the integument is verrucate in appearance (Fig. 4d,e, asterisk in Fig. 4f). The posterior spiracular discs are well-developed with each having three medially oriented slits whose peritremes are prominently separated from each other (Fig. 4f). The outer portion of the middle slit is slightly bent upward (Fig. 4f, arrow).
Scanning electron micrographs of third instar H. ligurriens. a Anteroventral view of cephalic segment showing dorsal organ (DO), terminal organ (TO), oral grooves (OG), and mouth opening (arrow). b Terminal organ containing both clustered papillary sensillae (PS) and knobbed sensillum (KS). c Dorsolateral view between prothorax and mesothorax indicating rows of intersegmental spines (S) and anterior spiracle (AS) comprised of five to seven papillae at end of prothorax. d Caudal view of dorsal portion of 12th segment showing small dorsal tubercles around peripheral rim. Inner dorsal tubercle (IDT), median dorsal tubercle (MDT), and outer dorsal tubercle (ODT). e Caudal view of ventral portion of 12th segment indicating small ventral tubercles around peripheral rim. Outer ventral tubercle (OVT), median ventral tubercle (MVT), and inner ventral tubercle (IVT). f Well-developed posterior spiracular discs each bearing three medially oriented slits (S) that are entirely separated from each other. Outer portion of middle slit is slightly bent dorsally (arrow). Asterisk indicates verrucate integument
Puparia
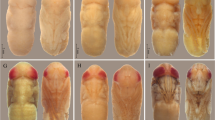
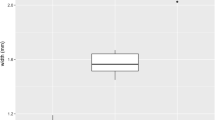
The puparium of H. ligurriens is of typical coarctate form in being thickly cylindrical in shape and composed of the hardened larval integument of the last larval instar (Fig. 5a). Puparia of H. ligurriens are 6.76 ± 0.2 mm in length and 2.7 ± 0.1 mm in width (n = 40). The first four segments (cephalic and three thoracic segments) of the anterior region of the puparium are gradually rounded anteriorly (Fig. 5a,b). An anterior spiracle is located on each lateral surface of the prothorax; whereas, a keel-like longitudinal ridge spans across the meso- and metathorax in the lateral positions as a continuing line (Fig. 5b,c, arrowheads). The cephalic segment is retracted into the prothorax during pupariation, thereby leaving the pair of anterior spiracles positioned at the anterior-most point of the lateral sides of the prothorax (Fig. 5b). In young puparia, a clustered bubble membrane comprised of ≈57 mammillate structures is observed dorsolaterally on each side of the puparium in the posterior end of the first abdominal segment (Fig. 5c, higher magnification in inset). Comparison of the mammillate structures of H. ligurriens with other fly species is summarized in Table 1.
Micrographs of puparium of H. ligurriens. a Light micrograph, (b–g) Scanning electron micrographs. a Whole puparium displaying typical coarctate form of thick cylindrical shape. b Anterior view indicating invaginated cephalic segment (arrow), pair of anterior spiracles (AS), and longitudinal keel-like ridge extending laterally across meso- and metathorax (arrowheads). c Dorsolateral view of anterior end of puparium showing anterior spiracle (AS) at prothorax, longitudinal keel-like ridge extending laterally across meso- and metathorax (arrowheads) and clustered bubble membrane located dorsolaterally at posterior end of first abdominal segment (arrow). Inset highlights clustered bubble membrane of young puparium comprised of ≈57 mammillate structures. d Short pupal respiratory horn protruding from center of bubble membrane in aged puparium. e Integument of last abdominal segment showing verrucate sculpture pattern that is slightly echinate and rows of intersegmental acuminate spines. f Posterior view showing broadly truncate caudal segment and pair of posterior spiracular discs (PSD) in dorsal portion of terminal end. g Higher magnification of posterior spiracular discs showing three straight spiracular slits (S) on each posterior spiracular disc and ventromedially located button (arrow). Asterisk indicates verrucate integument
In aged puparia, a short tubular respiratory horn protrudes from the center of the mammillate structures (Fig. 5d). The surface of the horn contains many longitudinal respiratory openings. The caudal segment of the puparium of H. ligurriens is broadly truncate (Fig. 5a,f) and bears a pair of posterior spiracular discs medially (Fig. 5f). The integument of the caudal segment bears a verrucate sculpture pattern (Fig. 5f,g) that is slightly echinate in appearance at higher magnification (Fig. 5e). Each posterior spiracular disc is comprised of three straight spiracular slits surrounded by separate peritremes, a distinct ventromedially located button (or ecdysial scar), and spiracular hairs (Fig. 5g).
Discussion
Given the number of fly species involved in the endeavors of forensic entomology, fast and reliable identification of the various developmental stages of species is a basic and crucial prerequisite for appropriate application in forensic investigations. In this study, we highlight the important characteristics of egg, larvae, and puparium of H. ligurriens based on SEM observation.
Egg of blow flies are frequently found deposited on human corpses, so it is important to define those characteristics that are distinctive features at this stage. Some of these include: length and width of the plastron, elevated median hatch line, and chorionic sculpture pattern. Results obtained from this study indicate that the characters observed for the eggs of H. ligurriens (wide plastron, length of plastron, upright median hatch line and slightly elevated hexagonal pattern of chorionic sculpture) were morphologically similar to those observed in Lucilia cuprina (Sukontason et al. 2007c), a species within the same tribe Luciliini of the subfamily Calliphorinae to which H. ligurriens belongs (Kurahashi et al. 1997). Therefore, these characteristics alone are not useful for differentiating between these two species. However, such features clearly differed from other blow fly species, such as Chrysomya megacephala, Chrysomya rufifacies, Chrysomya nigripes, or the phorid fly, Megaselia scalaris, all of which are commonly found in human corpses (Sukontason et al. 2007c). In order to distinguish between H. ligurriens and L. cuprina, it is suggested that egg specimens be hatched in the laboratory and reared at least to the third instar, a stage at which these species could be differentiated with confidence.
It is clearly seen from this study that the mouthhooks of the first instar H. ligurriens are absent, but instead, it shows that two groups of spines are situated dorsomedially of the oral cavity on the cephalic segment (see Fig. 2b,f). This feature is in agreement with similar morphological characters described in the first instars of L. cuprina (Sandeman et al. 1987), C. megacephala (Sukontason et al. 2003a) and C. rufifacies (Sukontason et al. 2003b), further suggesting that the morphology of these spines may help them to anchor to the substrate during locomotion, rake in food during the feeding process, and provide the ability to abrade or irritate skin resulting in myiasis (Sandeman et al. 1987). However, these spines are markedly different from the pair of maxillae found in the first instars of the bot fly, Dermatobia hominis (de Filippis and Leite 1997) or flesh fly, Parasarcophaga dux (Sukontason et al. 2003c). The tripartite labium observed in the first instar of H. ligurriens is virtually identical to those labia seen in third instars of other blow fly species, such as L. cuprina (Sandeman et al. 1987) and C. rufifacies (Sukontason et al. 2003b).
The dorsal organ is a morphological feature that appears essentially dome-shaped and remains undifferentiated in appearance in specimens of many fly species that have been observed. Examples of such specimens from various different families include: Calliphoridae, L. cuprina (Sandeman et al. 1987), Muscidae, Musca domestica (Chu-Wang and Axtell 1971), Sepsidae, numerous species (Meier 1995), Piophilidae, Piophila casei (Sukontason et al. 2001a), and Sarcophagidae, P. dux (Sukontason et al. 2003c). From these studies, it seems that the appearance of the dorsal organ might be a relatively constant character among species of immature flies. Interestingly, the longitudinal furrow patterns seen throughout the dorsal organ (Fig. 2e) are morphologically similar to those seen in the compound eye of M. domestica (Sukontason et al. 2008). Chu-Wang and Axtell (1971) proposed that the dorsal organ might have a sensory function serving as an olfactory receptor that aids house fly larvae in their existence. Likewise, the two distinct groups of papillae on the terminal organ of H. ligurriens (Fig. 4b) were similarly arranged in other calliphorids, e.g., L. cuprina (Sandeman et al. 1987); muscid, M. domestica (Chu-Wang and Axtell 1972); sepsids (Meier 1995); and piophilid, P. casei (Sukontason et al. 2001b). Based on evidence from research in an ultrastructural study on M. domestica, the papillary sensillae on the terminal organ may serve a dual function for both contact chemoreception and mechanoreception; whereas, the role of the knobbed sensillum is unknown (Chu-Wang and Axtell 1972). The function of the ventral organ is unknown, but the sensillum of this organ was assumed to function in both contact chemo- and mechanoreception in M. domestica by Chu-Wang and Axtell (1972).
The arrangement of the dorsal intersegmental spines between the prothorax and mesothorax in the third instar of H. ligurriens (Fig. 4c) is very bizarre and important for differentiation from the closely related species, L. cuprina. The continuous arrangement of spines within individual rows that was observed in H. ligurriens was distinctive from the groups of spines in rows that were revealed by light microscopy in L. cuprina (Sukontason et al. 2004). Moreover, this spine arrangement was markedly dissimilar from the blow flies, C. rufifacies (Sukontason et al. 2003b) and C. nigripes (Sukontason et al. 2005). In this regard, the dorsal intersegmental spine morphology between the prothorax and mesothorax of the third instar displays variation between species and is suggested to be included as a criterion for larval identification of forensically important flies.
It is widely known that both larvae and puparia of muscoid flies possess anterior and posterior spiracles that are used for respiration in terrestrial substrates. Our examination of posterior spiracular discs reveals fine detailed structures of H. ligurriens that differ from those of the blow fly species, C. megacephala (Sukontason et al. 2003a) and C. rufifacies (Sukontason et al. 2003b).
From the Oriental perspective, this study enhances the biological knowledge for identification of forensically important flies in this region of the world. This work shows the utility of applying an ultrastructural morphological approach to aid in the identification of H. ligurriens, a species that has immature stages that are morphologically very similar to other forensically important fly species. Studies are currently underway to assess the biology of this fly in Thailand. One important aspect of this species that needs more extensive study in the near future concerns the developmental rate of the various immature stages of this fly under different environmental conditions. This kind of information will be extremely useful and critical for determining post mortem intervals in applications to forensic investigations.
References
Chen WY, Hung TH, Shiao SF (2004) Molecular identification of forensically important blow fly species (Diptera: Calliphoridae) in Taiwan. J Med Entomol 41:47–57
Chu-Wang IW, Axtell RC (1971) Fine structure of the dorsal organ of the house fly larva, Musca domestica L. Z Zellforsch 117:17–34
Chu-Wang IW, Axtell RC (1972) Fine structure of the terminal organ of the house fly larva, Musca domestica L. Z Zellforsch 127:287–305
de Filippis T, Leite ACR (1997) Scanning electron microscopy studies on the first-instar larva of Dermatobia hominis. Med Vet Entomol 11:165–171
Ishijima H (1967) Revision of the third stage larvae of synanthropic flies of Japan (Diptera: Anthomyiidae, Muscidae, Calliphoridae and Sarcophagidae). Jpn J Sanit Zool 18:47–100
Kurahashi H, Benjaphong N, Omar B (1997) Blow flies (Insecta: Diptera: Calliphoridae) of Malaysia and Singapore. Raffles Bull Zool 5(Suppl):1–88
Lee HL, Krishnasamy M, Abdullah AG, Jeffery J (2004) Review of forensically important entomological specimens in the period of 1972–2002. Trop Biomed 21:69–75
Liu D, Greenberg B (1989) Immature stage of some flies of forensic importance. Ann Entomol Soc Am 82:80–93
Meier R (1995) Cladistic analysis of the Sepsidae (Cyclorrhapha: Diptera) based on a comparative scanning electron microscopic study of larvae. Syst Entomol 20:99–128
Roy P, Dasgupta B (1971) Behaviour of Chrysomya megacephala (Fabr.) and Hemipyrellia ligurriens (Weid.) as parasites of living animals under experimental conditions. South Afr J Med Sci 36:85–91
Sandeman RM, Collins BJ, Carnegie PR (1987) Scanning electron microscope study of L. cuprina larvae and the development of blowfly strike in sheep. Int J Parasitol 17:759–765
Siriwattanarungsee S, Sukontason KL, Kuntalue B, Piangjai S, Olson JK, Sukontason K (2005) Morphology of the puparia of the housefly, Musca domestica (Diptera: Muscidae) and blowfly, Chrysomya megacephala (Diptera: Calliphoridae). Parasitol Res 96:166–170
Sukontason KL, Sukontason K, Piangjai S, Choochote W, Vogtsberger RC, Olson JK (2001a) Scanning electron microscopy of the third-instar Piophila casei (Diptera: Piophilidae), a fly species of forensic importance. J Med Entomol 38:756–759
Sukontason KL, Sukontason K, Piangjai S, Choochote W, Vogtsberger RC, Olson JK (2001b) Scanning electron microscopy of the third-instar Piophila casei (Diptera: Piophilidae), a fly species of forensic importance. J Med Entomol 38:756–759
Sukontason KL, Sukontason K, Piangjai S, Boonchu N, Chaiwong T, Vogtsberger RC, Kuntalue B, Thijuk N, Olson JK (2003a) Larval morphology of Chrysomya megacephala (Fabricius) (Diptera: Calliphoridae) using scanning electron microscopy. J Vector Ecol 28:47–52
Sukontason KL, Sukontason K, Lertthamnongtham S, Kuntalue B, Thijuk N, Vogtsberger RC, Olson JK (2003b) Surface ultrastructure of Chrysomya rufifacies (Macquart) larvae (Diptera: Calliphoridae). J Med Entomol 40:259–267
Sukontason K, Sukontason KL, Piangjai S, Chaiwong T, Boonchu N, Kurahashi H, Vogtsberger RC (2003c) Larval ultrastructure of Parasarcophaga dux (Thomson) (Diptera: Sarcophagidae). Micron 34:359–364
Sukontason K, Sukontason KL, Ngern-klun R, Sripakdee D, Piangjai S (2004) Differentiation of the third instar of forensically important fly species in Thailand. Ann Entomol Soc Am 97:1069–1075
Sukontason KL, Vogtsberger RC, Boonchu N, Chaiwong T, Sripakdee D, Ngern-Klun R, Piangjai S, Sukontason K (2005) Larval morphology of Chrysomya nigripes (Diptera: Calliphoridae), a fly species of forensic importance. J Med Entomol 42:233–240
Sukontason KL, Kanchai C, Piangjai S, Boonsriwong W, Bunchu N, Sripakdee D, Chaiwong T, Kuntalue B, Siriwattanarungsee S, Sukontason K (2006a) Morphological observation of puparia of Chrysomya nigripes (Diptera: Calliphoridae) from human corpse. Forensic Sci Int 161:15–19
Sukontason KL, Narongchai P, Kanchai C, Vichairat K, Piangjai S, Boonsriwong W, Bunchu N, Sripakdee D, Chaiwong T, Kuntalue B, Siriwattanarungsee S, Sukontason K (2006b) Morphological comparison between Chrysomya rufifacies (Macquart) and Chrysomya villeneuvi Patton (Diptera: Calliphoridae) puparia, forensically important blow flies. Forensic Sci Int 164:230–234
Sukontason KL, Piangjai S, Bunchu N, Chaiwong T, Sripakdee D, Boonsriwong W, Vogtsberger RC, Sukontason K (2006c) Surface ultrastructure of the puparia of the blow fly, Lucilia cuprina (Diptera: Calliphoridae), and flesh fly, Liosarcophaga dux (Diptera: Sarcophagidae). Parasitol Res 98:482–487
Sukontason K, Narongchai P, Kanchai C, Vichairat K, Sribanditmongkol P, Bhoopat T, Kurahashi H, Chockjamsai M, Piangjai S, Bunchu N, Vongvivach S, Samai W, Chaiwong T, Methanitikorn R, Ngern-klun R, Sripakdee D, Boonsriwong W, Siriwattanarungsee S, Srimuangwong C, Hanterdsith B, Chaiwan K, Srisuwan C, Upakut S, Moopayak K, Vogtsberger RC, Olson JK, Sukontason KL (2007a) Forensic entomology cases in Thailand: a review of cases from 2000 to 2006. Parasitol Res 101:1417–1423
Sukontason KL, Ngern-klun R, Sripakdee D, Sukontason K (2007b) Identifying fly puparia by clearing technique: application to forensic entomology. Parasitol Res 101:1407–1416
Sukontason KL, Bunchu N, Chaiwong T, Kuntalue B, Sukontason K (2007c) Fine structure of the eggshell of the blow fly, Lucilia cuprina. J Insect Sci 7(Article 9)
Sukontason KL, Chaiwong T, Piangjai S, Upakut S, Moophayak K, Sukontason K (2008) Ommatidia of blow fly, house fly, and flesh fly: implication of their vision efficiency. Parasitol Res 103:123–131
Acknowledgements
We thank the Faculty of Medicine and Chiang Mai University for subsidizing publication cost. This publication is an output from research projects supported by the Thailand Research Fund (Project RMU4980007 to KLS).
Author information
Authors and Affiliations
Corresponding author
Additional information
This work has been presented in the 16th European Society of Vector Ecology held in Cambridge, UK, on 25–28 March, 2008.
Rights and permissions
About this article
Cite this article
Sukontason, K.L., Sribanditmongkol, P., Chaiwong, T. et al. Morphology of immature stages of Hemipyrellia ligurriens (Wiedemann) (Diptera: Calliphoridae) for use in forensic entomology applications. Parasitol Res 103, 877–887 (2008). https://doi.org/10.1007/s00436-008-1072-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-008-1072-7