Abstract
Two deltamethrin resistance-associated serine protease genes (NYD-tr and NYD-ch) were isolated from Culex pipiens pallens in our previous study. To study the function of NYD-Tr and NYD-Ch in the metabolism of deltamethrin, we constructed the recombinant plasmid pET32a(+)/NYD-tr and pET32a(+)/NYD-ch with a 6× histidine tag. Sodium dodecyl sulfate polyacrylamide gel electrophoresis and Western blot analyses of the recombinant proteins revealed that the molecular weights of NYD-Tr and NYD-Ch are 42 and 50 kDa. Enzyme activity assay indicated that the recombinant NYD-Tr and NYD-Ch had the corresponding features of trypsin and chymotrypsin. Using BApNA as the substrate, NYD-Tr gave optimal activity between pH 9.0 and 10.5, while NYD-Ch was optimally active over the range of pH 8.0–11.0 using the S(Ala)2ProPhe-pNA as the substrate. Then, we investigated the metabolism of deltamethrin by NYD-Tr and NYD-Ch. Our results showed that NYD-Tr and NYD-Ch could hydrolyze deltamethrin. The acute oral toxicity of the metabolite to Wistar rats was much lower than deltamethrin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vector-borne diseases affect two thirds of the world’s population and kill millions annually, and the cost of combating these diseases and loss of productivity has crippled the economic growth of endemic countries (Riehle and Jacobs-Lorena 2005).
Mosquitoes are the most important vectors. Current mosquito control strategies depend primarily on synthetic insecticides (Sobotka and Styczynska 1991) and have changed little over the decades. Unfortunately, the vectors have developed resistance against many commonly used pesticides all over the world (WHO 1992). Indeed, the continuous development of resistance threatens the long-term viability of insecticide-based products (Denholm et al. 2002). After the use of dichlorodiphenyltrichloroethane was phased out in the late 1970s, organophosphates and carbamates were employed as the main insecticides for mosquito control until pyrethroids were introduced between 1981 and 1985(Wiktelius and Edwards 1997; Nauen 2007). Synthetic pyrethroids are the most effective insecticides and widely used all over the world. Like other insecticides, resistance to pyrethroid has already appeared in many vectors and continues to increase at an alarming rate(Cui et al. 2006). Besides that, no new class of chemicals has emerged for mosquito control since the development of the pyrethroids more than 20 years ago(N’Guessan et al. 2007).
Metabolic resistance (degradation of the active ingredient by detoxification enzymes) is one of the major forms of resistance mechanisms. Previous studies have reported that CYP450 and carboxylesterases are involved in enzyme-based detoxification for the pyrethroid resistance in insects (Diaz et al. 2004; Heidari et al. 2005; Rodpradit et al. 2005). As resistance is a multigenic phenomenon, systematic studies to characterize the enzymes involved in the hydrolysis of pyrethroids are still lacking.
We have isolated two serine protease genes from C. pipiens pallens that are significantly upregulated in deltamethrin-resistant strain through a combination of suppression subtractive hybridization (SSH) and complementary DNA (cDNA) microarrays (Wu et al. 2004). NYD-tr and NYD-ch gene expressed 4.57 and 3.49-fold higher in the resistant strain than in the susceptible strain. Using rapid amplification of cDNA ends (RACE) from the constructed cDNA library, we obtained full-length cDNA of these two sequences (GenBank/NCBI AY034060, GenBank/NCBI AF468495). Stable transfection of these two genes into mosquito C6/36 cells conferred protective effects (Gong et al. 2005), while RNAi-mediated knockdown made C6/36 cells more sensitive to deltamethrin (Gong et al., unpublished). These results suggested that trypsin- and chymotrypsin-like proteases might be directly involved in the metabolism of deltamethrin. In vitro study of enzymatic activities of NYD-tr and NYD-ch would shed light into their functions. Here, we report the recombinant expression, purification, and characterization of these two proteins.
Materials and methods
Chemicals
Deltamethrin ((S)-alpha-cyano-3-phenoxybenzyl-(1R,cis)-2,2-dimethyl-3-(2,2-dibromvinyl)-cyclopropanecarboxylate) was purchased from Roussel Uclaf Company (Paris, France, purity higher than 98%). Enzymes, substrates, and inhibitors (all obtained from Sigma Chemical Co., Poole, Dorset, UK) were used in these studies as follows: α-N-benzoyl-dl-arginine β-nitroanilide (BApNA), N-succinyl-alanine-alanine-proline-phenylalanine-p-nitroanilide (S(Ala)2ProPhe-pNA), phenylmethylsulfonyl fluoride (PMSF), N-α-tosyl-l-lysine chloromethyl ketone (TLCK), N-α-tosyl-l-phenylalanine chloromethyl ketone (TPCK), and soybean trypsin inhibitor (SBTI). All other chemicals were of analytical grade.
Animals
Fourteen female and 14 male Wistar rats with body weight of 180–200 g were obtained from Shanghai Slac Laboratory Animal Co. [certificate no. SCXK (Hu) 2003–0003]. Animals were kept in a barrier-sustained animal room, and the certificate number of environmental condition was SCXK (Su) 2002-0031. Stainless steel wire mesh cages were used. Each cage had five rats of the same sex, and allowed food and water ad libitum. The animal facility was maintained at 22 ± 1°C, 50% ± 10% humidity, with a 12-h light/dark cycle. Animals were acclimatized to the testing environment for 7 days after receipt. All animal handling procedures followed NIH (the US National Institutes of Health) guidelines and were approved by the Nanjing Medical University Animal Care and Use Committee.
Construction of the expression vector
Because the initial NYD-Tr expression did not generate significant amount of stable protein in Escherichia coli BL21 (DE3), this sequence was reengineered for expression by inserting deleted signal peptides (19 peptides) coding sequences into the pET-32a(+) vector. Recombinant plasmid of NYD-Ch gene contained the whole open reading frame (ORF) of the gene. The NYD-tr sense primer (5′-CGGAATTCGACGTCCCGTACCAGGTGTC-3′) contained EcoRI restriction site, and its antisense primer (5′-ACTAGCGGCCGCTTAAACCTTGGCCACCTTTT-3′) contained a NotI restriction site. The NYD-ch sense primer (5′-CGGAATTCAACATGGCATCGAAACTGACGGT-3′) contained a EcoRI restriction site, and its antisense primer (5′-ACTAGCGGCCGCTTA AGCATCCTGACGCAGCTG-3′) contained a NotI restriction site. Recombinant plasmid pcDNA3.1(+)-NYD-tr/NYD-ch (constructed by our laboratory, containing the whole open reading frame of NYD-tr and NYD-ch gene, respectively) was used as template. Polymerase chain reaction (PCR) conditions were as follows: 95°C for 5 min, 95°C for 40 s, 58°C for 40 s, 72°C for 100 s, 30 cycles, and 72°C final extension for 7 min. PCR products were ligated with pGEM-T Easy vector. The two genes were respectively subcloned into pET32a(+), resulting in the recombinant plasmids of pET32a(+)/NYD-tr and pET32a(+)/NYD-ch. The positive clone was verified by PCR and sequencing. The recombinant plasmids were transformed in the E. coli BL21(DE3). Plasmid pET-32a(+) was employed as a negative control.
Expression of the NYD-tr and NYD-ch genes
Expression was carried out using E. coli BL21(DE3). The transformed cells with plasmids of pET32a(+)/NYD-tr and pET32a(+)/NYD-ch were established. Inocula were prepared by transferring a transformant into a culture tube containing 2 ml Luria–Bertani (LB) medium with ampicillin. Cultures were initially grown overnight at 37°C, then inoculated into 100 ml LB medium with ampicillin, which were shaken at 200 rpm until the OD600 was approximately 0.6; IPTG (1 mM) was added and the cultures were incubated at 37°C for 4 h to induce the protein. Bacteria were harvested by centrifugation at 6000×g for 10 min. Harvested cells were suspended in 5 ml buffer A0 (20 mM Tris/HCl buffer, pH 7.9, 0.5 M NaCl, 10% glycerol, 8 M urea) and 1 mM PMSF, disrupted by sonication in an ice bath, and centrifuged at 12,000×g for 15 min, and the supernatant was used as source of crude protein. The expression of NYD-tr or NYD-ch gene was assessed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) followed by Coomassie brilliant blue staining.
Purification of the recombinant NYD-Tr and NYD-Ch
The recombinant proteins NYD-Tr and NYD-Ch contain a His×6 tag at the N terminus, which can be purified by nickel–nitrilotriacetic acid (Ni–NTA) affinity column chromatography. The crude proteins obtained from the induced bacteria were applied onto the Ni-NTA column, which was pre-equilibrated with buffer A0. Then, the column was washed with buffers A0, A1, A2, A3, A4, and A5 (containing 0, 20, 40, 60, 100, 500 mM imidazole, respectively) until no more protein was eluted. The elution of the protein from Ni–NTA column was determined by Coomassie brilliant blue staining of SDS-PAGE.
Collected elutions of high protein concentration were pooled, desalted on dialysis bag (Pharmacia), which was previously boiled with buffer B (2% Na2CO3, 1 mM EDTA) and washed by ddH2O. The protein was dialysed with phosphate-buffered saline containing urea of different concentrations (6, 5, 4, 3, 2, 1, and 0 M) for 3 h, respectively, at 4°C; finally the protein was dehydrated by PEG8000 (Amresco). Protein concentration was measured by BCA™ protein assay kit (Pierce).
Enzyme activity assays
The activity of NYD-Tr was assayed by using 1 mM BApNA as substrates based on the methods described by Ortego et al. (1996). Except as otherwise stated, these activities were carried out at their optimal pH in 1 ml reaction buffer and incubated at 30°C. The reaction was incubated for 30 min, stopped by the addition of 500 ul of 30% acetic acid, centrifuged at 10,000×g for 5 min, and the supernatant monitored spectrophotometrically at 405 nm. The activity of NYD-Ch was assayed as described above, except that the substrate was 1 mM S(Ala)2ProPhe-pNA.
The optimal pH of NYD-Tr and NYD-Ch for activity with BApNA and S(Ala)2ProPhe-pNA was assayed, respectively, by using universal buffer with pH values ranging from 5.5 to 12.0. Reaction buffers were 0.1 M phosphate (pH 5.5–7.0), 0.1 M Tris–HCl (pH 7.0–9.0), 0.1 M glycine–NaOH (pH 9.0–11.0), and 0.1 M Na2HPO4–NaOH (pH 11.0–12.0). All buffers contained 0.15 M NaCl and 5 mM MgCl2 except Na2HPO4–NaOH that only contained 0.15 M NaCl, as Mg2+ would form Mg(OH)2 precipitation at high pH.
For inhibitor studies, the purified proteins were assayed in the presence of the following specific serine protease inhibitors: PMSF, SBTI, TLCK, and TPCK. Each inhibitor and corresponding concentrations were given in Table 1. Inhibitors were preincubated at 30°C with the enzymes diluted in buffer at the pH optimal for each proteolytic activity of 10 min before the addition of substrate. All inhibitors were added into 100 μl of 0.15 M NaCl. For each enzyme, the activity in the absence of inhibitor was used as the value of full activity. The effects of the inhibitors on recombinant NYD-Tr and NYD-Ch were also compared with the effects on commercially purchased bovine trypsin and chymotrypsin.
Degradation experiments
The degradation reactions of deltamethrin by expressed protein NYD-Tr and NYD-Ch were as follows: Deltamethrin (10 μg/ml) was dissolved in 0.1 M Tris–HCl buffer (pH 8.0), and then protein NYD-Tr and NYD-Ch (10 mg) were added. The total reaction volume was 1 ml. After 10 min of incubation at 30°C, 1 ml acetone was added to the incubation mixture to stop the reaction and then extract the deltamethrin and metabolites. Two milliliters of cyclohexane were added to the sample; the tubes were vortexed for 1 min and centrifuged at 2,000×g for 10 min. The supernatant was transferred to a new centrifuge tube, and the pellet was washed with 2 ml cyclohexane again. The organic phase was dried in a nitrogen evaporator with a stream of 0.5 kPa N2 at room temperature. According to the above procedure and dosage, different concentrations of deltamethrin were catalyzed.
UV-vis spectrophotometry
Spectra of analytes were obtained using a Cary 5000 UV-vis spectrophotometer (Varian, USA) according to the method described by Modi and LaCourse (2006). Experiments were carried out in 3 ml UV-transparent cuvettes using Cary Win UV Bio Pack software (version 3.0). Reactions were run at 30°C using a circulating water bath and a thermostated cell, and the internal standard chymotrypsin was conducted by following the same procedures except in the absence of deltamethrin.
Acute oral toxicity study
The acute oral toxicity study was conducted using the main test of up and down procedure according to OECD/OCDE Test Guidelines on Acute Oral Toxicity under a computer-guided Statistical Programme–AOT425statPgm, version 1.0 (AOT 2001), and the assumed Sigma value was at 0.5 (Adeneye et al. 2006). Acute toxicity study was carried out in vivo. The mixture after deltamethrin metabolic reaction and deltamethrin were prepared using corn oil and administered per os using gastric tube. The rats were fasted of rat chow overnight before dosing on each occasion. The main test consists of a single-ordered dose progression in which rats are dosed one at a time at a minimum of 48-h intervals. The first rat receives a dose a step below the level of the best estimate of the LD50. If the rat survives, the dose for the next rat is increased 3.2 times the original dose; if it dies, the dose for the next animal is decreased by a similar dose progression. Each rat was observed each time for the first 5 min after loading for signs of regurgitation and then kept in a cage. Each was watched for every 15 min in the first 4 h after dosing, then every 30 min for the successive 6 h, and then daily for the successive 38 h for the short-term outcome and the remaining 12 days for the long-term possible lethal outcome, which in this case, was “death.” Behavioral manifestations of acute oral toxicity were also noted. All observations were systematically recorded with individual records being maintained for each rat. Dosing is stopped when one of these criteria is satisfied, at which time an estimate of the LD50 and a confidence interval are calculated for the test based on the status of all the rats at termination. The LD50 is calculated using the method of maximum likelihood.
Results
Construction of the expression vector
The PCR products were detected by 1% agarose electrophoresis (Fig. 1), which indicated the products could be the sequences of NYD-tr and NYD-ch (729 and 816 bp). The results of sequencing indicated that the sequences of NYD-tr and NYD-ch gene in recombinant plasmid were correct.
Expression and purification of the recombinant NYD-Tr and NYD-Ch
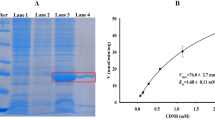
The recombinant plasmids pET-32a(+)/tr and pET-32a(+)/ch were transformed into E. coli BL21(DE3). The pET-32a(+)/tr and pET-32a(+)/ch were expressed successfully after induction (Figs. 2 and 3). SDS-PAGE and Western blot analysis indicated that the molecular weights of the expressed products were about 42 and 50 kDa, respectively. The recombinant protein of NYD-Tr and NYD-Ch, being expressed as His-tagged fusion proteins, were purified by using Ni–NTA agarose affinity chromatography (Figs. 2, 3, and 4).
Activity of NYD-Tr and NYD-Ch proteins toward synthetic substrates
To determine the substrate preference of NYD-Tr and NYD-Ch proteins, the enzyme was incubated with two different synthetic substrates, respectively (BApNA for NYD-Tr, S(Ala)2ProPhe-pNA for NYD-Ch). The maximum activity of NYD-Tr was obtained with BApNA, NYD-Ch with S(Ala)2ProPhe-pNA (Figs. 5 and 6).
Influence of pH on NYD-Tr and NYD-Ch activity
The optimum pH for NYD-Tr and NYD-Ch activities were analyzed by measuring the hydrolysis of BApNA and S(Ala)2ProPhe-pNA in a pH ranging from 5.5 to 12 (Figs. 5 and 6). The activity of both enzymes decreased when pH was below 8. NYD-Tr and NYD-Ch activity toward BApNA and S(Ala)2ProPhe-pNA was optimal at alkaline pH. The optimum activity of NYD-Tr toward BApNA occurred when pH range from 9 to 10.5 and was reduced above pH 11. The optimum activity of NYD-Ch toward S(Ala)2ProPhe-pNA ranged from pH 8 to 11 and was also reduced above 11.
Sensitivity of NYD-Tr and NYD-Ch to inhibitors
Data on the effect of some inhibitors on the NYD-Tr and NYD-Ch are presented in Table 1. NYD-Tr and NYD-Ch was strongly inhibited by TLCK and TPCK, respectively. PMSF and SBTI can inhibit the activity of NYD-Tr and NYD-Ch.
Deltamethrin metabolic reactions catalyzed by NYD-Tr and NYD-Ch
The hydrolytic activities of NYD-Tr and NYD-Ch toward deltamthrin were examined by UV-vis spectrophotometry assay following the methods of Modi and LaCourse (2006), and the obtained spectra of deltamethrin and metabolic products were not overlapping. The peak absorbance of deltamethrin was at 264 nm, the absorbance of deltamethrin metabolic products catalyzed by recombination NYD-Tr were at 249 and 292 nm, respectively, and absorbance of deltamethrin metabolic products catalyzed by recombination NYD-Ch were at 250 and 296 nm, respectively (Fig. 7).
The spectra of deltamethrin and metabolic products. The spectra of deltamethrin and metabolic products were not overlapping in different concentrations, the absorbance of deltamethrin was at 264 nm (a), the absorbance of deltamethrin metabolic products catalyzed by recombination protein NYD-Tr were at 249 and 292 nm, respectively (b), and absorbance of deltamethrin metabolic products catalyzed by recombination protein NYD-Ch were at 250 and 296 nm, respectively (c)
Acute oral toxicity study
The short- and long- term outcome of the main test of up and down procedure of deltamethrin are shown in Table 2, and that of the mixture after deltamethrin metabolic reaction are shown in Table 3. The toxic symptoms were hyperspasmia, diaphoresis, salivate, and myasthenia of posterior limb. The onset of the symptoms was observed 2–3 h after administration, and the surviving rats recovered in 2–3 days. The LD50 of deltamethrin and the mixture after deltamethrin metabolic reaction in male and female rats were 55 and 310.2 mg/kg, respectively.
Discussion
Serine proteases are involved in a wide range of physiological functions, including digestion of dietary protein, blood coagulation, immune respond, signal transduction, hormone activation, and development (Krem et al. 2000). Trypsin and chymotrypsin are two kinds of widely studied serine proteases. In insects, the most abundant and best-studied group of serine proteases including these two enzymes are expressed in the larval midgut, and they are supposed to be involved in the digestion of dietary protein. In addition, they have been implicated in the process of pathogen establishment in several vector insects (Yan et al. 2001). Ramalho-Ortigao et al. (2003) reported that trypsin and chymoptrpsin were also involved in the relationship between vector and parasite. In sand flies, these proteases have been shown to be a potential barrier to the growth and development of Leishmania within midgut (Ramalho-Ortigao et al. 2003). There is a possible role of trypsin and chymotrypsin in the proteolytic remodeling that occurs in the gut during the molt from larval to pupal (Herrero et al. 2005). Trypsin and alpha-chymotrypsin, which shared similar properties of the insect midgut alkaline proteases, rapidly degraded the polh (polyhedrin) protein in vitro, leaving only the toxic Cry1Ac protein behind (Seo et al. 2005). We also observed that NYD-Tr and NYD-Ch were associated with deltemathrin resistance of C. pipiens pallens (Gong et al. 2005).
After the expression and purification of NYD-Tr and NYD-Ch, we got the actual molecular weight of NYD-Tr and NYD-Ch, which were similar to trypsins and chymotrypsins from other insects and mammals (Lehane and Billingsley 1996; Barrett et al. 1998).
The active sites of chymotrypsin and trypsin have much in common with respect to their specificities, both enzymes catalyze the hydrolysis of a series of substrates such as benzoylalamine and β-nitrophenylacetate (Hartley and Kilby 1954; Ravin et al. 1954). However, their substrate specificities are different. Trypsin favors basic residues like lysine and arginine; chymotrypsin favors aromatic residues like phenylalanine, tyrosine, and tryptophan (Ma et al. 2005). Knowledge of the conformation of the substrate when located at the active center of an enzyme is essential for understanding the enzyme specificity, as the conformation adopted by the substrate discloses the stereochemistry of the active center in view of its complementarity to the substrate (Beirão et al. 2001). Compounds that meet this criteria include BApNA, which is specific for NYD-Tr activity (Hayakawa et al. 1980) and S(Ala)2ProPhe-pNA for NYD-Ch activity (Blocher et al. 1999). They were accordingly used in this work to determine the specificity of the purified enzymes. The results demonstrated that the expressed NYD-Tr and NYD-Ch had corresponding tryptic and chymotryptic activity.
Using protease inhibitors to determine mechanistic class or specificity is complicated by the cross-reactions that many inhibitors have different types of proteases (Walkera et al. 1998). Most of the serine protease inhibitors are known to inhibit more than one type of serine protease. SBTI has been known as a double-headed inhibitor possessing trypsin- and chymotrypsin-binding activities (Wu and Laskowski 1955; Ryan et al. 1965), and PMSF is a general protease inhibitor (Mayer et al. 1982). However, TLCK is highly specific for trypsin activity and TPCK for chymotrypsin activity (Mayer et al. 1982; Singh et al. 2001). PMSF was a very effective inhibitor for NYD-Tr and NYD-Ch, providing evidence that two proteins have the activity of serine proteases. NYD-Tr was inhibited strongly by TLCK but not TPCK; NYD-Ch was inhibited strongly by TPCK but not TLCK; SBTI inhibited both NYD-Tr and NYD-Ch activity. All these information confirmed that NYD-Tr and NYD-Ch had trypsin and chymotrypsin characteristics, respectively.
The NYD-Tr and NYD-Ch had high pH optima, NYD-Tr substrates were optimally hydrolyzed over a broader pH (9–10.5) range, while NYD-Ch substrates were optimally hydrolyzed over a broader pH (8–11). This is similar to the pH optima found in studies of the serine proteases from other lepidopteran larvae and consistent with the high pH of the midgut lumen (Jongsma et al. 1996; Lehane and Billingsley 1996; Fredholt et al. 1999).
To further elucidate the role of NYD-Tr and NYD-Ch in deltamethrin-resistance, we tested whether deltamethrin could be directly degraded by these recombination proteins in vitro through the detection of spectra change of UV-vis spectrophotometry. Comparing the UV-vis spectrophotometry profile of the deltamethrin and reaction products generated by NYD-Tr and NYD-Ch, we found that deltamethrin could be metabolized by both enzymes. To explore the toxicity of the deltamethrin metabolic products catalyzed by NYD-Tr and NYD-Ch, we tested the acute oral toxicity of deltamethrin and metabolic products to Wistar rats, respectively. Our results showed that the mixture after deltamethrin metabolic reaction was still toxic to Wistar rats, but the toxicity was much lower than deltamethrin. Considering that the reaction was not complete, i.e., the mixture from the reaction still contained an amount of deltamethrin, it would be impossible to tell if the metabolic products have no toxicity or low toxicity to the Wistar rats. In either case, treatment of NYD-Tr and NYD-Ch could reduce the toxicity of deltamethrin. These data provide mechanistic information of the correlation between trypsin and chymotrypsin upregulation and deltamethrin resistance.
The carboxylesterases are members of the serine hydrolyse superfamily of esterases and catalyze the hydrolysis of esters, amides, and thioesters. Carboxylesterases possess a triad of amino acid residues (serine, histidine, and glutamic acid) that are essential for activity (Ross et al. 2006). Compared with carboxylesterases, trypsin and chymotrypsin are also members of the serine proteases superfamily and have a catalytic triad of serine, histidine, and aspartate within the S1 binding pocket, which hydrolyzes peptides and ester bonds. Further studies are needed to identify whether functions of NYD-Tr and NYD-Ch proteins are identical to carboxylesterases.
In conclusion
Two serine proteases, NYD-Tr and NYD-Ch, from deltamethrin-resistant C. pipiens pallens were successfully cloned and expressed in E. coli. Their enzymatic activities have been characterized. These two enzymes have hydrolysis activities for deltamethrin. Our work showed that the NYD-Tr and NYD-Ch could be considered as attractive candidates for the development of biocatalyst in the future practical use. The elucidation of the detailed pesticide degradation mechanisms of these enzymes is in progress.
Abbreviations
- NYD-tr:
-
trypsin-like gene isolated from Culex pipiens pallens
- NYD-ch:
-
chymotrypsin-like gene isolated from Culex pipiens pallens
- PMSF:
-
phenylmethylsulfonyl fluoride
- SBTI:
-
soybean trypsin inhibitor
- TLCK:
-
N-α-tosyl-l-lysine chloromethyl ketone
- TPCK:
-
N-α-tosyl-l-phenylalanine chloromethyl ketone
- CYP450:
-
cytochrome P450
- SSH:
-
suppression subtractive hybridization
- BApNA:
-
α-N-benzoyl-dl-arginine β-nitroanilide
- S(Ala)2ProPhe-pNA:
-
N-succinyl-alanine-alanine-proline-phenylalanine-p-nitroanilide
- EDTA:
-
ethylene diamine tetraacetic acid
- UV-vis spectrophotometry:
-
ultraviolet-visible spectrophotometry
- LD50:
-
median lethal dose
References
Adeneye AA, Ajagbonna OP, Adeleke TI, Bello SO (2006) Preliminary toxicity and phytochemical studies of the stem bark aqueous extract of Musanga cecropioides in rats. J Ethnopharmacology 105:374–379
Barrett AJ, Rawlings ND, Woessner JF (1998) Handbook of proteolytic enzymes. Academic, San Diego
Beirão LH, Mackie IM, Teixeira E, Damian C (2001) Purification and characterisation of trypsin-like enzyme from the pyloric caeca of cod (Gadus morhua) II. Braz Arch Biol Technol 44:33–40
Blocher M, Walde P, Dunn IJ (1999) Modeling of enzymatic reactions in vesicles: the case of alpha-chymotrypsin. Biotechnol Bioeng 62:36–43
Cui F, Raymond M, Qiao CL (2006) Insecticide resistance in vector mosquitoes in China. Pest Manag Sci 62:1013–1022
Denholm I, Devine GJ, Williamson MS (2002) Evolutionary genetics. Insecticide resistance on the move. Science 297:2222–2223
Diaz C, Rodriguez MM, Fresneda M, Bisset JA (2004) Determination of glutathione-S-transferase activity in Culex quinquefaciatus strains in Cuba and other Latin American countries. Rev Cubana Med Trop 56:111–116
Fredholt K, Ostergaard J, Savolainen J, Friis GJ (1999) Alpha-chymotrypsin-catalyzed degradation of desmopressin (dDAVP): influence of pH, concentration and various cyclodextrins. Int J Pharm 178:223–229
Gong MQ, Shen B, Gu Y, Tian HS, Ma L, Li XL, Yang MX, Hu Y, Sun Y, Hu XB, Li J, Zhu CL (2005) Serine proteinase over-expression in relation to deltamethrin resistance in Culex pipiens pallens. Arch Biochem Biophys 438:53–62
Hartley BS, Kilby BA (1954) The reaction of p-nitrophenyl esters with chymotrypsin and insulin. Biochem J 56:288–297
Hayakawa T, Kondo T, Yamazaki Y, Iinuma Y, Mizuno R (1980) A simple and specific determination of trypsin in human duodenal juice. Gastroenterol Jpn 15:135–139
Heidari R, Devonshire AL, Campbell BE, Dorrian SJ, Oakeshott JG, Russell RJ (2005) Hydrolysis of pyrethroids by carboxylesterases from Lucilia cuprina and Drosophila melanogaster with active sites modified by in vitro mutagenesis. Insect Biochem Mol Biol 35:597–609
Herrero S, Combes E, Van Oers MM, Vlak JM, de Maagd RA, Beekwilder J (2005) Identification and recombinant expression of a novel chymotrypsin from Spodoptera exigua. Insect Biochem Mol Biol 35:1073–1082
Jongsma MA, Peters J, Stiekema WJ, Bosch D (1996) Characterization and partial purification of gut proteinases of Spodoptera exigua Hubner (Lepidoptera: Noctuidae). Insect Biochem Mol Biol 26:185–193
Krem MM, Rose T, Di Cera E (2000) Sequence determinants of function and evolution in serine proteases. Trends Cardiovasc Med 10:171–176
Lehane MJ, Billingsley PF (1996) Biology of the insect midgut. Chapman & Hall, London
Ma W, Tang C, Lai L (2005) Specificity of trypsin and chymotrypsin: loop-motion-controlled dynamic correlation as a determinant. Biophys J 89:1183–1193
Mayer M, Neufeld B, Finci Z (1982) Inhibition of serine proteases by steroids. Biochem Pharmacol 31:2989–2992
Modi SJ, LaCourse WR (2006) Monitoring carbohydrate enzymatic reactions by quantitative in vitro microdialysis. J Chromatogr A 1118:125–133
Nauen R (2007) Insecticide resistance in disease vectors of public health importance. Pest Manag Sci 63:628–633
N’Guessan R, Boko P, Odjo A, Akogbeto M, Yates A, Rowland M (2007) Chlorfenapyr: a pyrrole insecticide for the control of pyrethroid or DDT resistant Anopheles gambiae (Diptera: Culicidae) mosquitoes. Acta Trop 102:69–78
Ortego F, Novillo C, Castañera P (1996) Characterization and distribution of digestive proteases of the stalk corn borer, Sesamia nonagrioides Lef. (Lepidoptera: Noctuidae). Arch Insect Biochem 33:163–180
Ramalho-Ortigao JM, Kamhawi S, Rowton ED, Ribeiro JM, Valenzuela JG (2003) Cloning and characterization of trypsin- and chymotrypsin-like proteases from the midgut of the sand fly vector Phlebotomus papatasi. Insect Biochem Mol Biol 33:163–171
Ravin HA, Bernstein P, Seligman AM (1954) A colorimetric micromethod for the estimation of chymotrypsin activity. J Biol Chem 208:1–15
Riehle MA, Jacobs-Lorena M (2005) Using bacteria to express and display anti-parasite molecules in mosquitoes: current and future strategies. Insect Biochem Mol Biol 35:699–707
Rodpradit P, Boonsuepsakul S, Chareonviriyaphap T, Bangs MJ, Rongnoparut P (2005) Cytochrome P450 genes: molecular cloning and overexpression in a pyrethroid-resistant strain of Anopheles minimus mosquito. J Am Mosq Control Assoc 21:71–79
Ross MK, Borazjani A, Edwards CC, Potter PM (2006) Hydrolytic metabolism of pyrethroids by human and other mammalian carboxylesterases. Biochem Pharmacol 71:657–669
Ryan CA, Clary JJ, Tomimatsu Y (1965) Chicken chymotrypsin and Turkey trypsin. Ii. Physical and enzymic properties. Arch Biochem Biophys 110:175–183
Seo JH, Yeo JS, Cha HJ (2005) Baculoviral polyhedron–Bacillus thuringiensis toxin fusion protein: a protein-based bio-insecticide expressed in Escherichia coli. Biotechnol Bioeng 92:166–172
Singh J, Vohra RM, Sahoo DK (2001) Purification and characterization of two extracellular alkaline proteases from a newly isolated obligate alkalophilic Bacillus sphaericus. J Ind Microbiol Biotechnol 26:387–393
Sobotka W, Styczynska B (1991) Review of the methods of control of pathogenetic arthropods of medical and veterinary importance. Wiad Parazytol 37:167–171
Walkera AJ, Forda L, Majerusb MEN, Geogheganc IE, Birchc N, Gatehousea JA, Gatehouse AMR (1998) Characterisation of the mid-gut digestive proteinase activity of the two-spot ladybird (Adalia bipunctata L.) and its sensitivity to proteinase inhibitors. Insect Biochem Mol Biol 28:173–180
WHO (1992) Vector resistance to pesticides. Fifteenth Report of the WHO Expert Committee on Vector Biology and Control. World Health Organ Tech Rep Ser 818:1–62
Wiktelius S, Edwards CA (1997) Organochlorine insecticide residues in African Fauna: 1971–1995. Rev Environ Contam Toxicol 151:1–37
Wu FC, Laskowski M (1955) Action of the naturally occurring trypsin inhibitors against chymotrypsins alpha and beta. J Biol Chem 213:609–619
Wu HW, Tian HS, Wu GL, Langdon G, Kurtis J, Shen B, Ma L, Li XL, Gu Y, Hu XB, Zhu CL (2004) Culex pipiens pallens: identification of genes dierentially expressed in deltamethrin-resistant and -susceptible strains. Pestic Biochem Physiol 79:75–83
Yan J, Cheng Q, Li CB, Aksoy S (2001) Molecular characterization of two serine proteases expressed in gut tissue of the African trypanosome vector, Glossina morsitans morsitans. Insect Mol Biol 10:47–56
Acknowledgment
The authors thank Professors Yuyin Feng and Renzhen Zhao for their technical help during the study. This project was supported by grants from the Chinese Natural Science Research Council (no. 30671827 to Changliang Zhu), the Specialized Research Fund for the Doctoral Program of Higher Education of China (no. 20050312004 to Changliang Zhu), Academic Natural Science Foundation of Jiangsu Province (no. 03KJB180082 to Bo Shen; no. 07KJD180137 to Donghui Zhang), and Science and Technology Development Foundation of Nanjing Medical University (no. NY0501 to Yan Sun; no. 06NMUZ005 to Donghui Zhang).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, Q., Zhou, D., Sun, L. et al. Expression and characterization of two pesticide resistance-associated serine protease genes (NYD-tr and NYD-ch) from Culex pipiens pallens for metabolism of deltamethrin. Parasitol Res 103, 507–516 (2008). https://doi.org/10.1007/s00436-008-0997-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-008-0997-1