Abstract
Our objective was to determine the mean production of entomopathogenic nematodes (EPNs) infective juveniles (IJs) from Alphitobius diaperinus and Galleria mellonella larvae and the possible morphometric changes of emergent IJs. Heterorhabditis riobravus and Steinernema carpocapsae nematodes were placed on 20 larvae of each host individually located in Petri dishes, which were maintained in an environmental control chamber. After death, each larva was individually transferred to White traps where they remained for a maximum of 20 days in environmental control chambers. With IJ multiplication, the water from each trap was separately collected, emergent IJs were counted, and mean production was calculated for each host species. Relative populations of each nematode species emerging from each host were randomly selected and miscegenated. Then, 50 IJs from each host/species were randomly selected for morphometric studies. Significant difference was seen between the two EPN species for mean IJ production values from G. mellonella larvae (P = 0.0048) but not from A. diaperinus larvae (P = 0.8883). Significant differences were also seen in total body length and width between the emergent H. riobravus and S. carpocapsae IJs (P = 0.0002).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Entomopathogenic nematodes (EPNs) of the Steinernema (Steinernematidae) and Heterorhabditis (Heterorhabditidae) genus parasitize many different insect species (de Doucet et al. 1998) and have been the subject of many biological control research studies (Geden et al. 1985; Berry et al. 1997; Vasconcelos et al. 2004).
Galleria mellonella Linnaeus 1758 (Lepidotera: Pyralidae), known as the greater wax moth, has been widely used for experimentation because of its high susceptibility to EPNs of the Steinernematidae and Heterorhabditidae families (Martin 1997). Many of the experiments using different EPNs strains have been able to maintain their stocks using last instar larvae of G. mellonella in the infection process. Breeding this Lepidopteron in the laboratory is expensive and requires intense management.
Many factors can affect the multiplication potential of EPNs within their hosts; these include the virulence of symbiont bacteria, concomitant infection with another microorganism, species of host used, and number of penetrated infective juveniles (IJs). According to Kaya and Koppenhöfer (1996), when this number exceeds the ideal level within the host, intraspecific exploitative competition can occur between developing nematodes, which can reduce the total emergent progeny from the cadaver. If the number of IJs established in the host exceeds its capacity to support them, no progeny are obtained. Thus, resultant competition between nematodes in the host can be antagonistic to the survival of naturally occurring populations or to the establishment of applied nematodes.
Geden et al. (1985), using Alphitobius diaperinus pupae and adults as EPN larvae hosts, emphasized successful Steinernema feltiae nematode reproduction in this beetle. Therefore, starting from the principle that the re-establishment of a new IJ population after infected host death could actually occur in the application sites is fundamental in evaluating the actual multiplication rate of these methods before determining initial applied dosage. Moreover, because of its easy and cheap breeding management, this coleopteran could be a new alternative for in vivo EPN multiplication.
Therefore, the objective of this study was to determine mean production of EPN species in A. diaperinus and G. mellonella larvae and pinpoint possible changes in some morphometric aspects of emergent IJs from each host species, as little is known about their postmultiplication morphological changes in different host species or even in different orders of insects.
Materials and methods
Our experiment was conducted in the Laboratory of Parasitology of Embrapa Dairy Cattle, located in the city of Juiz de Fora, southeastern Minas Gerais state, Brazil, during the period of May to December, 2005.
Sourcing and handling the caterpillars
Greater wax moth caterpillars, G. mellonella, were bred in the laboratory for nematode multiplication. They were produced from adults recently collected from G. mellonella pupae, which had been kept in 24 × 25 cm diameter plastic mating chambers. Folded pieces of paper were placed in these chambers for the adults to lay their eggs on. These pieces of paper with the eggs were then transferred to 30-cm-diameter aluminum egg trays with galvanized steel mesh covers for ventilation. Artificial feed was added to these trays for the hatched caterpillars. The feed was consisted of: 120 g honey, 120 g glycerol, 200 g milk, 60 g yeast or brewer’s yeast, 100 g wheat germ, 100 g wheat flour, and 120 g wheat bran. The material was kept in a temperature chamber at 27 ± 1°C and greater than 80% relative humidity (RH).
Obtaining EPN strains from the caterpillars
Seventh instar caterpillars, weighing approximately 0.26 g, were taken from the breeding trays and used for multiplication of the two nematode species used in this experiment.
Two milliliters of distilled water with IJs was sprinkled in the 9-cm-diameter Petri dishes, which had been prepared with two equal-sized sterilized sheets of filter paper. Ten G. mellonella caterpillars were liberated in these dishes, and then the dishes with caterpillars and nematodes were wrapped in plastic film to avoid evaporation and other organisms entering. These were kept in a temperature chamber at 28°C for 48 h. After death, the larvae were distributed in White traps (Kaya and Stock 1997) to obtain H. riobravus and S. carpocapsae IJs.
Obtaining and handling lesser mealworms
The colony of A. diaperinus used in this experiment were initially obtained from poultry installations at the Aviculture sector of Viçosa Federal University—MG, Brazil. The specimens were then taken to the Embrapa Dairy Cattle Parasitology Laboratory in Juiz de Fora—MG, Brazil, where species breeding was performed under temperature and humidity control in environmental control chambers. The coleoptera populations were reared in a substrate of material collected from chicken litter—sawdust, feces, feathers, and chicken feed. They were periodically fed chicken feed and banana skins were also added to the substrate.
The experiment
Initially, 310 A. diaperinus and 167 G. mellonella larvae were collected from the two host colonies and weighed. From these, mean in vitro-reared larva weights were calculated for each host species. Twenty beetle larvae and 20 moth larvae with weights corresponding or approximating the two colony means were randomly selected.
Each larva was placed in its own isolated 5-cm-diameter sterilized Petri dish with a substrate of two sterilized filter papers. To each of these was added a similar inoculum containing 1,600 IJs of both EPNs species suspended in aqueous solution. These were then placed in environmental control chambers at 25 ± 1°C and greater than 80% RH. When the larvae died, they were individually transferred to adapted mini-White traps (Kaya and Stock 1997) and again placed in environmental control chambers at 25 ± 1°C and greater than 80% RH for a maximum of 20 days.
When IJs were observed in the water of each White trap, it was periodically collected using a plastic pipette and individually stored in 25-cm2 flasks typically used for tissue culture. This procedure was followed for a maximum of 20 days for total IJ multiplication to occur within the larvae of both host species. Throughout this period, counts were made under an optical microscope of the number of IJs emerging from each cadaver.
From these individual production values, mean IJ production levels were calculated for both host species to determine their multiplication potentials. At the end of counting, some flasks from each nematode species and its host were randomly selected from the rest, and their contents mixed together. The four resulting species/host (A—IJs from H. riobravus/G. mellonella; B—IJs from H. riobravus/A. diaperinus; C—IJs from S. carpocapsae/G. mellonella, and D—IJs from S. carpocapsae/A. diaperinus) were first analyzed to determine each mean IJs population.
Then, 50 IJs from each species/host, corresponding to 0.037 (A), 0.14 (B), 0.037 (C), and 0.17% (D) of each population, were randomly selected by suction using a plastic pipette and mounted on slides for morphometric measurements with an Olympus BX 41 optical microscope and ocular micrometer (400× magnification). A small drop of Lugol’s solution was applied to those IJs still living to aid morphometric analysis. Measurements were total body length (TBL) and total body width (TBW) of IJs from each host.
Mean host EPN production potentials and mean morphometric data from the emergent nematodes were compared using the Student’s t test. Differences were considered significant when P < 0.05. Results were interpreted using SAEG software (UFV 2000).
Results and discussion
Multiplication potential
H. riobravus had a 2.5-time more effective multiplication potential in the lepidopteron, the host traditionally used for this function, than S. carpocapsae. The explanation for this could be linked to the host’s natural evolutional characteristics for each species of nematode, the density-dependent effect, and also the type of searching and infecting IJs behavior on the filter paper substrate.
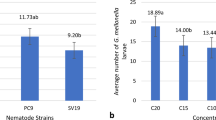
There was a significant difference between IJs mean production values from the two EPN species obtained from G. mellonella moth larvae (P = 0.0048). The same was not true using the A. diaperinus beetle larvae as the host (P = 0.8883; Table 1); Data showed that the difference in number of larvae obtained from the two EPN species in A. diaperinus did not influence the mean production values attained in this host.
When the host is colonized by EPNs, a certain number of IJs are needed to overcome host defenses (Gaugler et al. 1994; Wang et al. 1994) and guarantee mating (in Steinernematidae). However, invasion by many nematodes may seriously impede their development, survival, and reproduction in host individuals (Selvan et al. 1993). This density-dependent effect had been seen with S. carpocapsae (Selvan et al. 1993), Steinernema glaseri (Zervos et al. 1991; Koppenhöfer and Kaya 1995), and Heterorhabditis spp. (Molyneux et al. 1983; Selvan et al. 1993). However, Heterorhabditis bacteriophora is much less susceptible to intraspecific competition than S. carpocapsae and S. glaseri (Selvan et al. 1993; Koppenhöfer and Kaya 1995).
According to Peters (1996), S. carpocapsae has already become naturally isolated from insects belonging to the following orders: Coleoptera, Hymenoptera, Diptera, and Lepidoptera. We have found no reports on the natural hosts of H. riobravus. It is important to emphasize that most EPN species produced today in laboratories are of different strains because of the many multiplications in different host types. Apparently, this condition could interfere with attempts to biologically control naturally occurring pests and even those classified as exotic. However, these studies provide information that could predict important future aspects concerning nontarget species through applications in the field.
Because of the smaller size of the A. diaperinus larva, about 10.8 times smaller than the moth larva, results in terms of production for both nematode species in each host were as expected. However, if we ignore these obvious anatomical and physiological differences between the two host species and start considering them as an “identical source” of body mass for the parasite, one can infer from Table 2 that comparative IJs production of S. carpocapsae and H. riobravus from A. diaperinus larvae is, respectively, 1.9 and 0.71 the number from G. mellonella, or that the coleopteron larva produced nearly twice as many S. carpocapsae IJs and approximately 30% less H. riobravus IJs than the lepidopteron larva. Therefore, the number of A. diaperinus larvae needed to produce the same number of H. riobravus and S. carpocapsae IJs as G. mellonella would be 236 and 77, respectively.
As it is considered a pest by poultry farmers and a carrier of different pathological agents to birds, A. diaperinus is constantly the target of attempted chemical control (Vaughan and Turner 1984; Salin et al. 2003). Our results show that larvae of this species can be eliminated by biological control using EPNs. In addition, the same host species can be a viable laboratory alternative for EPNs multiplication because of its low cost and ease of breeding compared to the traditional G. mellonela.
H. bacteriophora nematodes bred in vitro (culture media) were smaller and produced fewer eggs than those previously grown in vivo (Zioni et al. 1992). Hatab et al. (1998) studying S. glaseri nematodes also verified that the number of IJs produced per mg dry weight could actually vary with culture method. These authors found that the number of IJ produced from coleopteron Popillia japonica larvae and lepidopteron G. mellonella larvae were 1.267 ± 67 and 1.346 ± 47 per mg of host. In contrast, numbers from liquid and solid culture media were: 409 ± 41 and 350 ± 37 per mg dry culture, respectively.
Parasites considered obligatory depend on their hosts for nutrients. Hatab et al. (1998) stated that lipid composition, in terms of quantity and quality, for EPNs is strongly influenced by the host or culture medium on which they are bred. The high lipid content of IJs is a reflection of their dependency on lipids as their main energy source and food reserve. Similar to other predatory insects, EPNs depend on their prey as a source of cholesterol or usable sterols (Svoboda et al. 1978; Morrison and Ritter 1986).
Morphometric analysis
The morphometry of IJs from Steinernematidae and Heterorhabditidae is important for describing and identifying species. However, according to Nguyen and Smart (1995), morphometric results from in vitro cultures must not be used taxonomically or for identification. They say that in addition to the culture medium, the morphometric paramters of Steinernematidae IJs, specifically TBL, can also vary between individuals emerging at different times from the insect cadaver. Knowing this, extreme care was taken in our experiment when all emergent IJs of each species/host were mixed, thus forming four populations to be sampled.
Four statistical analyses were performed to compare from measurements of TBL and TBW from 50 specimens of each strain (population). Table 3 shows that there were significant differences in all groups (P = 0.0002). The larger the “resource” (host) offered, the larger the TBL and TBW of emergent IJs from both nematode species. Thus, following direct proportionality: The smaller the “resource” (host), the smaller (TBL and TBW) the emergent IJs.
H. riobravus IJs emerging from A. diaperinus had larger TBL (516 ± 21.18 μm) than S. carpocapsae IJs from the same host (510.2 ± 32.54 μm). However, S. carpocapsae IJs emerging from A. diaperinus had larger TBWs (27.6 ± 2.76 μm) than H. riobravus IJs from the same host (17.85 ± 1.89 μm).
Poinar (1990) reported that for larger species such as S. glaseri (Steiner) (IJ length = 864–1.448 μm), production was about 30.000 IJs per G. mellonella larva (mass = 0.25 g), and for smaller species such as H. bacteriophora (Poinar) (IJ length = 512–671 μm), production was around 400.000 IJs. In comparison terms, our study found a mean production of around 42.734 S. carpocapsae IJs (559.2 ± 28.98 μm length) from G. mellonella larva (mass = 0.17 g) and around 109.474 H. riobravus IJs {555.6 ± 31.04 μm length} from the same host (Tables 1 and 3). Our results are in agreement with Poinar (1990), suggesting that the smaller the nematode, the higher the numerical production when using G. mellonella larvae.
Both EPN species seem to invest more in IJ production than IJ size, as both morphological parameters significantly reduced with smaller host size. Furthermore, S. carpocapsae seems to have a higher tendency to prioritize IJ TBW rather than TBL; the opposite being the case with H. riobravus. This might be explained because of the fact that the Heterorhabditidae have an additional infection strategy to the Steinernematidae; this is the ability to penetrate the host’s cuticle by using an adaptive structure known as a “tooth.”
Final considerations
Under our experimental conditions, the two nematode species presented different behaviors in respect to the multiplication potential in G. mellonella larvae. This did not happen in A. diaperinus host larvae, where these potentials became equal.
Host species-specific characteristics, such as physiology, size, and body weight—this being treated as a “resource” for the parasite—can provoke considerable morphological changes in these populations. Furthermore, behavioral interaction between H. riobravus IJs from different hosts can be changed when stored in aqueous solutions. Therefore, larger studies are needed to determine at what point all these changes in IJs species populations are prejudicial to attempts at biologically controlling insect pest species in a natural environment.
References
Berry RE, Liu J, Reed G (1997) Comparison of endemic and exotic entomopathogenic nematode species for control of Colorado potato beetle (Coleoptera: Chrysomelidae). J Econ Entomol 90(6):1528–1533
de Doucet MMA, Miranda MB, Bertolotti MA (1998) Infectivity of entomogenous nematodes (Steinernematidae and Heterorhabditidae) to Pediculus humanus capitis De Geer (Anoplura: Pediculidae). Fund Appl Nematol 21:13–16
Gaugler R, Wang Y, Campbell JF (1994) Aggressive and evasive behaviors in Popillia japonica (Coleoptera: Scarabaeidae) larvae: defenses against entomopathogenic nematode attack. J Invertebr Pathol 64:193–199
Geden CJ, Axtell RC, Brooks WM (1985) Susceptibility of the lesser mealworm, Alphitobius diaperinus to the entomogenous nematodes Steinemema feltiae, S. glaseri (Steinemematidae) and Heterorhabditis heliothidis (Heterorhabditidae). J Entomol Sci 20(3):331–339
Hatab MA, Gaugler R, Ehlers R (1998) Influence of the methods of culture on lipids Steinernema Glaseri. J Parasitol 2(84):215–221
Kaya HK, Koppenhöfer AM (1996) Effects of microbial and other antagonistic organism and competition on entomopathogenic nematodes. Biocontrol Sci Techn 6:357–371
Kaya HK, Stock SP (1997) Techniques in insect nematology. In: Lacey LA (ed) Manual of techniques in insect pathology. Biological techniques series. Academic, London, pp 281–324
Koppenhöfer AM, Kaya HK (1995) Density-dependent effects on Steinernema glaseri (Nematoda: Steinernematidae) within an insect host. J Parasitol 80:797–799
Martin WR (1997) Using entomopathogenic nematodes to control insects during stand establishment. Hort Sci 32(2):196–200
Molyneux AS, Bedding RA, Akhurst RJ (1983) Susceptibility of the sheep blowfly Lucilia cuprina to various Heterorhabditis spp., Neoaplectana spp., and undescribed steinernematid (Nematoda). J invertebr Pathol 42:1–7
Morrison AH, Ritter KS (1986) Effect of host insect sterols on the development and sterol composition of Steinernema feltiae. Mol Biochem Parasit 19:135–142
Nguyen KB, Smart GC Jr (1995) Morphometrics of infective juveniles of Steinernema spp. and Heterorhabditis bacteriophora (Nemata: Rhabditida). J Nematol 27:206–212
Peters A (1996) The natural host range of Steinernema and Heterorhabditis spp. and their impact on insect populations. Biocontrol Sci Techn 6:389–402
Poinar GO Jr (1990) Taxonomy and biology of Steinernematidae and Heterorhabditidae. In: Gaugler R, Kaya HK (eds) Entomopathogenic nematodes in biological control. CRC, Boca Raton, FL, pp 23–61
UFV (2000) SAEG (Sistemas para Análises Estatísticas e Genéticas) versão 8.0—manual de instruções. CPD/UFV, Divisão de Pesquisa e Desenvolvimento, Viçosa, MG, p 142
Vasconcelos VO, Furlong J, Freitas GM, Aguillera MM, Dolinski C (2004) Steinernema glaseri Santa Rosa strain (Rhabditida: Steinernematidae) and Heterorhabditis bacteriophora CCA strain (Rhabditida: Heterorhaditidae) as biological control agents of Boophilus microplus (Acari: Ixodidae). Parasitol Res 94:201–206
Vaughan JA, Turner EC Jr (1984) Residual and topical toxicity of various insecticides to the lesser mealworm (Coleoptera: Tenebrionidae). J Econ Entomol 77:216–220
Salin C, Delettre YR, Vernon P (2003) Controlling the mealworm Alphitobius diaperinus (Coleoptera: Tenebrionidae) in broiler and turkey houses: field trials with a combined insecticide treatment: insect growth regulator and pyrethroid. J Econ Entomol 96(1):126–130
Selvan S, Campbell JF, Gaugler R (1993) Density-dependent effects on enthomopathogenic nematodes (Heterorhabditidae and Steinernematidae) within an insect host. J Invertebr Pathol 62:278–284
Svoboda JA, Thompson MJ, Robbins WE, Kaplanis JN (1978) Insect steroid metabolism. Lipids 13:742–753
Wang Y, Gaugler R, Cui L (1994) Variations in immune response of Popillia japonica and Achetus domesticus to Heterohabditis bacteriophora and Steinernema species. J Nematol 26:11–18
Zervos S, Johnson SC, Webster JM (1991) Effect of temperature and inoculum size on reproduction and development of Heterorhabditis heliothidis and Steinernema glaseri (Nematoda: Rhabditoidea) in Galleria mellonella. Can J Zool 69:1261–1264
Zioni S, Glazer L, Segal O (1992) Ufe cycle and reproductive potential of the entomopathogenic nematode Heterorhabditis bacteriophora strain HP88. J Nematol 24:352–358
Acknowledgments
We thank the researchers at Embrapa Diary Cattle—Dr. John Furlong and Dr. Márcia Prata—for their collaboration with this work and for making the Laboratory of Parasitology, in which our whole experiment was carried out, available to us. We also acknowledge the comments and suggestions made by the editor and the anonymous reviewers.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00436-008-0889-4
Rights and permissions
About this article
Cite this article
Rocha Costa, J.C., Pedroso Dias, R.J. & Frota Morenz, M.J. Determining the adaptation potential of entomopathogenic nematode multiplication of Heterorhabditis riobravus and Steinernema carpocapsae (Rhabditida: Heterorhabditidae, Steinernematidae) in larvae of Alphitobius diaperinus (Coleoptera: Tenebrionidae) and Galleria mellonella (Lepidoptera: Pyralidae). Parasitol Res 102, 139–144 (2007). https://doi.org/10.1007/s00436-007-0747-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-007-0747-9