Abstract
Our objective was to reveal whether host immune response in hamster opisthorchiasis post-praziquantel treatment could induce apoptotic cell death in inflammatory cells. We, therefore, investigated apoptosis-related gene expression in hamsters re-infected with Opisthorchis viverrini (OV) and re-treated with praziquantel. Hamsters were re-infected with OV metacercariae then re-treated with praziquantel. The expression of apoptosis-related genes (i.e. apoptosis gene Bcl-2 associated protein X [BAX], caspase 9, p53 and protein kinase B [PKB]) was detected by real-time reverse transcription-polymerase chain reaction. Histopathological analyses of liver tissues were performed by staining the sections with haematoxylin and eosin using light microscopy. The results show that BAX, Akt/PKB, p53 and caspase 9 expression levels were significantly increased on day 30 post-infection and at 6 h post-treatment and gradually decreased to a level near the uninfected control and at 24 h post-treatment, perhaps because of a decrease in inflammatory cells. Apoptotic cell death was observed at the nuclei of epithelial cells of the bile ducts and of T cells. Our results suggest that repeated infection with OV and re-treatment with praziquantel induces a host immune response that increases inflammatory cells, which in turn leads to increase, apoptosis-related gene expression in the short term post-treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The occurrence of cholangiocarcinoma (CCA) is highly endemic in Northeast Thailand and is the highest in the world. Several risk factors for CCA, such as radiation, parasite infection and chronic inflammation, have been reported (IARC 1994, 1997; WHO 2004). Opisthorchiasis-associated CCA was most often observed and remains the leading cause of death in this region (WHO 2004), in part because of local dietary habits, which increase the risk of Opisthorchis viverrini (OV) re-infection, which is related to the development of CCA.
Although recent reports show that praziquantel could reduce the risk for the pathogenesis of CCA (Pinlaor et al. 2006), frequent treatment with praziquantel may be detrimental to the host through the inflammation process. The opisthorchiasis-pathological changes include the aggregation of inflammatory cells surrounding the intra- and extra-hepatic bile ducts. Chronic infection with OV for many years is associated with several hepatobiliary diseases (Sripa 2003), which are in turn associated with the development of hepatobiliary cancer and CCA, albeit the precise genesis remains obscure.
Praziquantel is an effective anti-helminthic drug widely used for treatment of opisthorchiasis. The chemo-activity of the medication damages the tegument of worms leading to vacuolization, swelling and finally disruption and detachment of the tegument and parasite death. Although praziquantel is useful for treatment of trematodes, there is no report about its virulent side effects (Supanvanich et al. 1981).
Our preliminary findings indicate that inflammatory cells were more numerous surrounding the hepatic bile duct after praziquantel treatment; therefore, we hypothesized that after praziquantel re-treatment, residuals of parasite antigens enhance the stimulation of the host cellular immune response and the release of free radicals, leading to cell damage and apoptosis. This would be a distinct effect of praziquantel treatment in persons infected with OV, which we intended to investigate using a hamster model.
Materials and methods
Parasites and infection
OV metacercariae were obtained from naturally infected cyprinoid fish captured from a fresh water reservoir in an endemic area of Khon Kaen, Northeast Thailand. Fresh fish were minced and digested with pepsin–HCl, filtrated and then washed with normal saline solution until the solution was clear. Metacercariae were isolated and identified under a dissecting microscope. Finally, the metacercariae were divided into groups of 50 for infecting hamsters via intragastric intubation.
Male hamsters, 6 to 8 weeks old, from the Animal Unit, Faculty of Medicine, Khon Kaen University, were used in the experiments. The hamsters were divided into three groups (i.e. the OV-infected group, the uninfected group [normal control] and the OV-re-infected praziquantel treatment or re-treated group). Hamsters were killed on days 14, 30 and 90 post-infection. The liver samples were prepared according to conventional methods for light microscopic observation and real-time reverse transcription polymerase chain reaction (real-time RT-PCR). This protocol was approved by the Animal Ethics Committee of the Faculty of Medicine, Khon Kean University, Thailand (Ethical Clearance no. AEKKU0023105).
Real-time RT-PCR was used to investigate the apoptosis-related gene expression (i.e. BAX, Akt/PKB, p53 and caspase 9) during OV infection and pre- and post-praziquantel treatment. Light microscopy was used to investigate the histopathological changes and apoptotic cell death.
Praziquantel treatment
Praziquantel, purchased from the Medicpharma, Bangkok, Thailand, was used in this experiment. A dosage of 400 mg/kg was given orally to each hamster. Uninfected hamsters treated with praziquantel constituted the control. Three months after infection, OV-infected hamsters were treated with praziquantel and re-infected with OV within a week then re-treated with praziquantel on day 30 post-infection. Fours groups of hamsters were studied in this experiment: (1) untreated control at 90 days post-infection (dpi), (2) OV-re-infected praziquantel re-treatment at 6, (3) 12 and (4) 24 h post-treatment. All of the hamster groups were fed ad libitum and maintained at the Animal Unit, Faculty of Medicine, Khon Kaen University, Thailand.
Light microscopic observation
Liver samples were processed for light microscopic observation according to Boonmars et al. (2004), i.e. fixed with 10% formalin solution, processed in a conventional manner and stained with haematoxylin and eosin.
Primers for quantitative reverse transcription
The primer pairs for apoptosis-related gene expression (i.e. BAX, Akt/PKB, p53 and caspase 9) and endogenous controls (MG3PDH, mouse glyceraldehyde-3-phosphate dehydrogenase) were designed based on the published sequence (Tables 1 and 2).
RNA extraction
Ribonucleic acid (RNA) was extracted from whole liver at the hilar region (200 mg), obtained from the uninfected group, the infected control groups (0, 14, 30 and 90 dpi) and the treated groups (6, 12 and 24 h), were used for messenger RNA (mRNA) analysis. Total RNA was isolated using TRIZOL (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. The isolated RNA was treated with DNase (RQ1 RNase-Free DNase, Promega, Madison, WI) and Ribonuclease Inhibitor (Takara Shuzo, Kyoto, Japan) in buffer (400 mM Tris–HCl, 100 mM NaCl, 60 mM MgCl2 and 20 mM ditheothreitol, pH 7.5). The treated RNA was extracted and precipitated with phenol/chloroform and ethanol and dissolved in RNase-free water (100 μL). The total RNA was reverse transcribed into complementary deoxyribonucleic acid (cDNA) using Oligo (dT)15 primers (Amersham Phamacia Biotech, Piscataway, NJ) and Moloney Murine Leukaemia Virus (Invitrogen, USA). The cDNA was kept at −20°C until used.
SYBR Green real-time PCR assay
The real-time RT-PCR using the SYBR® Green method on an ABIprism 7500 sequence detector system (Applied Biosystems, Foster City, CA) was performed to analyse the relative quantification of mRNA expression. The real-time RT-PCR reaction contained 3 μL of 1:5 diluted single-strand cDNA, 1× PCR buffer (20 mM Tris–HCl pH 8.3, 20 mM KCl, 5 mM (NH4)2SO4), 10 mM deoxynucleotide phosphates, 5 pmole forward and reverse primer, 0.5× SYBR Green and 1 U of Hot start Taq DNA polymerase (MBI Fermentous, St. Leon-Rot, Germany). The PCR-cycling conditions were 95°C for 10 min, then 40 cycles of 95°C for 15 s, 60°C for 30 s and 72°C for 1 min, followed by 72°C for 10 min. At each cycle, the accumulated PCR products were detected by monitoring the increase in fluorescence of the reporter dye from double-strand DNA-binding SYBR Green. After PCR, a melting curve was constructed in the range of 60 to 99°C. All of the data were analysed using the Rotor Gene 5 software (Corbett, Australia). Relative expression of BAX, Akt/PKB, p53 and caspase 9 mRNA was calculated using the comparative Ct method as previously described (Gerard et al. 1998). All values were normalized to the G3PDH gene and reported as fold changes over background levels, detected in untreated control or before drug treatment as a calibrator.
Statistical analysis
The data of relative gene expression levels in each group were represented in three hamsters as mean ± SD. The statistical comparison between the normal control or untreated control and the parziquantel-treated groups was performed using a one-way analysis of variance, with Duncan tests (SPSS v.11.5, USA; and EXCEL Microsoft, USA). A p < 0.05 was required for statistical significance.
Results
Pathological changes in OV infection
Figure 1 demonstrates that inflammation surrounding the extra- and intra-hepatic bile ducts does not occur in the uninfected group (Fig. 1a), while inflammation surrounding the parasite was clearly observed on day 14 (Fig. 1b). The severity of inflammation gradually increased and reached a maximum on day 30 (Fig. 1c) demonstrated by the infiltration of mononuclear cells and eosinophils around the intra-hepatic bile ducts. The inflammatory reactions tended to decrease on day 90 post-infection. Lymphoid follicles as well as plasma infiltration were predominant and thickened, and dilated hepatic bile ducts were clearly observed (Fig. 1d).
Pathological changes in hamster opisthorchiasis post praziquantel treatment
Figure 2 shows the pathological changes in OV-re-infected livers followed by praziquantel re-treatment. The fibrosis, surrounding the hepatic bile duct of OV-re-infected and untreated control groups, was largest at 90 dpi with numerous inflammatory cells (Fig. 2a). At 6 h post-treatment, most of the parasites were killed, and the inflammatory cells increased in number surrounding the parasites and the hepatic bile ducts (Fig. 2b). The inflammatory cells especially eosinophils, mononuclear cells and neutrophils with lymphoid aggregation were predominant at 6 to 12 h post-treatment (Fig. 2b,c). At 24 h post-treatment, parasites could not be observed suggesting they were degraded (Fig. 2d). It is interesting to note that the apoptotic cell death (condense nucleus) were observed in all groups (Fig. 3).
Histopathological study of the livers of Opisthorchis viverrini-re-infected hamsters. Untreated control at 90 dpi (a), re-treated with praziquantel at 6 h post-treatment (b), 12 h post-treatment (c) and 24 h post-treatment (d); P parasite, Bd bile duct, asterisk, inflammatory cell. Original magnification = ×10
Relative real-time PCR of apoptosis-related gene expression
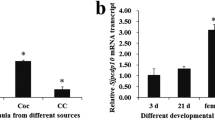
Figure 4 shows that apoptosis-related gene expression in uninfected control, OV-infected (14, 30 and 90 dpi) and OV-re-infected praziquantel re-treatment (6, 12 and 24 h) groups and the untreated control groups was observed in the liver. The BAX expression was observed in all groups but different in the expression level. The BAX gene expression level increased on 14 dpi and tended to decrease by 90 dpi and seemed to increase again at 6 and 12 h in the OV-re-infected praziquantel re-treatment groups albeit not significantly (Fig. 4a). The highest expression level was observed at 30 dpi. The caspase-9 gene expression profile was the same as that of BAX gene, but the highest expression level was observed 6 h post-treatment (Fig. 4b). The p53 and Akt/PKB gene expression profiles were similar to the BAX gene, but those increased by 14 dpi and tended to decrease by 90 dpi and significantly increased again at 6 and 12 h in the OV-re-infected praziquantel re-treatment group (Fig. 4c,d).
The relative real-time RT-PCR has shown that the BAX (a), caspase-9 (b), p53 (c) and PKB (d) expression level (folds) relative to MG3PDH. Uninfected control (1), OV-infected on 14 (2), 30 (3), 90 dpi (4), Untreated control at 90 dpi (5), OV-re-infected followed by praziquantel re-treatment at 6 h post-treatment (6), 12 h post-treatment (7) and 24 h post-treatment (8). Values are given as mean ± SD from three hamsters in each group. Values not sharing a common superscript letter differ significantly at P < 0.05`. Duncan procedure
Discussion
The disadvantages of using praziquantel for parasitic treatment have been obscured because of the well-known advantages of praziquantel as an anti-trematode agent (by destroying the parasite tegument; Apinhasmit and Sobhon 1996). It is indeed effective in reducing the pathology of parasitic infection such as shistosomiasis (Richards et al. 1989; Richter 2000) and opisthorchiasis (Pungpak et al. 1998). It also reduces free radicals and nitric oxide (NO) products induced by OV-infection (Pinlaor et al. 2004a, b). Recently, Pinlaor et al. (2006) reported that after praziquantel, no effect on cell damage through the pathological changes and inducible NO synthase-dependent DNA damage via nuclear factor-kappaB expression was observed for a long time. Notwithstanding praziquantel’s effectiveness in killing parasites, it may have uninvestigated, serious, short-duration post-treatment disadvantages. We sought to determine which key points may reveal the effect of praziquantel on cell damage.
It is well established that OV infection induces host immunologic activities, parasite-specific responses and/or parasite products and NO synthesis in human and animal models (Pinlaor et al. 2004a, b), which is why we observed the aggregation of inflammatory cells 30 dpi. In the case of praziquantel treatment, large numbers of inflammatory cells were observed 6 h post-treatment, when the broken tegument resulted in leakage of parasite waste products, which might trigger a host immune response. Enlargement of the hepatic bile duct was observed after prolonged infection in a time-dependent manner (Sripa 2003; Pinlaor et al. 2006). Apoptosis was observed in T cells and the nuclei of bile duct epithelial cells (Fig. 3). The most important point of our study is that increasing apoptosis-related gene expression level was indirectly induced by praziquantel treatment through the host immune response inflammatory cells.
The normally apoptotic mitochondrial pathway starts from increased BAX, which induces opening of the outer membrane pore of the mitochondria so that cytochrome c would be released and bind with Apaf1 and caspase 9 causing the cell death cascade. There are many kinds of apoptotic gene regulators (either/or inhibitors or anti) in each pathway (i.e. BCL-2, MDM2), and Akt/PKB is one that is well-known for inhibiting apoptosis through caspase 9 (Boonmars et al. 2004; Lodish et al. 2000).
Thus far, our present results suggest a possible way of increasing cell death through the apoptotic mitochondrial pathway and its inhibitor in opisthorchiasis and opisthorchiasis treated with praziquantel. Antigenic materials released from broken-down parasites increase the host immune response as demonstrated by the aggregation of inflammatory cells and increasing free radicals surrounding the infected area (Pinlaor et al. 2006). DNA damage and fragmentation would immediately increase the predisposing of cells to apoptosis, which would lead to increase expression of apoptosis-related gene expression (i.e. BAX, caspase 9, p53 and Akt/PKB) to nearly the same as the infected control group.
Our real-time RT-PCR result (Fig. 4) shows that after OV infection, the apoptotic genes (i.e. BAX, caspase9, p53) and anti-apoptotic gene (Akt/PKB) expression levels were significantly increased at day 30 because of increasing inflammatory cells surrounding the hepatic bile duct (Fig. 1). It is surprising to note that the expression level of apoptotic genes and the anti-apoptotic gene in the praziquantel-treated group at 6 h were similar to that of those treated 30 dpi because of increasing inflammatory cells. This present report may be the first to document these pathological changes after praziquantel treatment.
Data supporting the increased aggregation of inflammatory cells and free radicals inducing increased apoptosis-related genes have been reported (Kuwano and Hara 2000; Kim et al. 2002) with respect to T. spiralis infection in mouse muscle where the expression of apoptosis-related genes (BAX, caspase 9, Apaf-1, caspase 3 and Akt/PKB) occurred during aggregation of inflammatory cells in the early stages of infection. Subsequently, the levels were reduced to near the uninfected control after completed capsule formation with only a few inflammatory cells being observed (Boonmars et al. 2004; Wu et al. 2005a, b).
The immune system can result in excessive tissue re-modelling, loss of tissue architecture because of tissue destruction, protein and DNA alterations because of oxidative stress and, under some circumstances, increase risk of cancer development. Chronic inflammation is also a risk factor for cancer development included in CCA (Shacter and Weitzman 2002). Repeated healing may cause DNA or protein mutation leading to cancer development. People, especially in Northeast Thailand, are re-infected and re-treated many times, indicating repeated tissue repairs, which may bring about gene mutation and CCA development. This new finding indicates a caveat on repeated praziquantel treatments, as praziquantel may be associated with CCA development by inducing the host immune response. Further studies are needed to explore this hypothesis.
References
Apinhasmit W, Sobhon P (1996) Opisthorchis viverrini : effect of praziquantel on the adult tegument. Southeast Asian J Trop Med Public Health 27(2):304–311
Boonmars T, Wu Z, Nagano I, Takahashi Y (2004) Expression of apoptosis-related factors in muscles infected with Trichinella spiralis. Parasitology 128(Pt 3):323–332
Boonmars T, Wu Z, Nagano I, Takahashi Y (2005) Trichinella pseudospiralis infection is characterized by more continuous and diffuse myopathy than T. spiralis infection. Parasitol Res 97:13–20
Gerard CJ, Olsson K, Ramanathan R, Reading C, Hanania EG (1998) Improved quantitation of minimal residual disease in multiple myeloma using real-time polymerase chain reaction and plasmid-DNA complementarity determining region III standards. Cancer Res 58:3957–3964
IARC (1994) Infection with liver flukes (Opisthorchis viverrini, Opisthorchis felineus and Clonorchis sinensis). IARC Monogr Eval Carcinog Risks Hum 61:121–175
IARC (1997) Working group on the evaluation of carcinogenic risks to humans: Polychlorinated dibenzo-para-dioxins and polychlorinated dibenzofurans. IARC Monogr Eval Carcinog Risks Hum 69:1–631 Lyon, France, 4–11 February 1997
Kim SJ, Kim HG, Oh CD, Hwang SG, Song W-K, Yoo YJ, Kang SS, Chun JS (2002) p38 kinase-dependent and -independent inhibition of protein kinase C and -α regulates nitric oxide-induced apoptosis and dedifferentiation of articular chondrocytes. J Biol Chem 277:30375–30381
Kuwano K, Hara N (2000) Signal transduction pathways of apoptosis and inflammation induced by the tumor necrosis factor receptor family. Am J Respir Cell Mol Biol 22:147–149
Lodish HBA, Zipursky LS, Matsudaira P, Baltimore D, Darnell J (2000) Molecular cell biology. Freeman, New York
Richards F Jr, Sullivan J, Ruiz-Tiben E, Eberhard M, Bishop H (1989) Effect of praziquantel on the eggs of Schistosoma mansoni, with a note on the implications for managing central nervous system schistosomiasis. Ann Trop Med Parasitol 83(5):465–472
Richter J (2000) Evolution of schistosomiasis-induced pathology after therapy and interruption of exposure to schistosomes: a review of ultrasonographic studies. Acta Trop 77(1):111–131
Pinlaor S, Hiraku Y, Ma N, Yongvanit P, Semba R, Oikawa S, Murata M, Sripa B, Sithithaworn P, Kawanishi S (2004a) Mechanism of NO-mediated oxidative and nitrative DNA damage in hamsters infected with Opisthorchis viverrini: a model of inflammation-mediated carcinogenesis. Nitric Oxide 11(2):175–183
Pinlaor S, Ma N, Hiraku Y, Yongvanit P, Semba R, Oikawa S, Murata M, Sripa B, Sithithaworn P, Kawanishi S (2004b) Repeated infection with Opisthorchis viverrini induces accumulation of 8-nitroguanine and 8-oxo-7,8-dihydro-2-deoxyguanine in the bile duct of hamsters via inducible nitric oxide synthase. Carcinogenesis 25(8):1535–1542
Pinlaor S, Hiraku Y, Yongvanit P, Tada-Oikawa S, Ma N, Pinlaor P, Sithithaworn P, Sripa B, Murata M, Oikawa S et al (2006) iNOS-dependent DNA damage via NF-kappaB expression in hamsters infected with Opisthorchis viverrini and its suppression by the antihelminthic drug praziquantel. Int J Cancer 119(5):1067–1072
Pungpak S, Radomyos P, Radomyos BE, Schelp FP, Jongsuksuntigul P, Bunnag D (1998) Treatment of Opisthorchis viverrini and intestinal fluke infections with praziquantel. Southeast Asian J Trop Med Public Health 29(2):246–249
Shacter E, Weitzman SA (2002) Chronic inflammation and cancer. Oncology 16:217–232
Sripa B (2003) Pathobiology of opisthorchiasis: an update. Acta Trop 88(3):209–220
Supanvanich S, Supanvanich K, Pawabut P (1981) Field trial of praziquantel in human opisthorchiasis in Thailand. Southeast Asian J Trop Med Public Health 12(4):598–602
WHO (2004) Food -borne trematode infections in Asia. Report of the joint WHO/FAO.WHO Regional Office for the Western Pacific, RS 2002 GE/40 (VTN), pp 1–62
Wu Z, Nagano I, Boonmars T, Takahashi Y (2005a) Tumor necrosis factor receptor-mediated apoptosis in Trichinella spiralis-infected muscle cells. Parasitology 131(Pt 3):373–381
Wu Z, Nagano I, Boonmars T, Takahashi Y (2005b) A spectrum of functional genes mobilized after Trichinella spiralis infection in skeletal muscle. Parasitology 130(Pt 5):561–573
Acknowledgements
This study was supported in part by a grant from the Research Foundation of the Faculty of Medicine, Khon Kaen University, the Commission on Higher Education and the Thailand Research Fund. We thank the Department of Parasitology, Liver Fluke and Cholangiocarcinoma Research Center and the Animal Experimental Unit, Faculty of Medicine, Khon Kaen University, the Department of Parasitology, Gifu University School of Medicine, Japan and Dr. Watcharin loilom for their supportive contributions and Mr. Bryan Roderick Hamman for assistance with the English language preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boonmars, T., Srisawangwong, T., Srirach, P. et al. Apoptosis-related gene expressions in hamsters re-infected with Opisthorchis viverrini and re-treated with praziquantel. Parasitol Res 102, 57–62 (2007). https://doi.org/10.1007/s00436-007-0724-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-007-0724-3