Abstract
Hexokinase from Leishmania mexicana was purified to homogeneity from a glycosome-enriched fraction obtained after a differential centrifugation of promastigote form. The kinetic properties of the pure enzyme were determined and the Km values for glucose (Km = 66 μM) and ATP (Km = 303 μM) were comparable to those from hexokinase of Trypanosoma cruzi. L. mexicana hexokinase was able to use fructose (Km = 142 μM), which reflects the condition found in the insect host. In contrast with hexokinases from other trypanosomatids, the enzyme exhibited a moderate sensitivity to inhibition by glucose 6-phosphate. This inhibition was competitive with respect to both ATP and glucose, indicating that an allosteric site for glucose 6-phosphate does not exist in this enzyme. The enzyme was also inhibited by inorganic pyrophosphate, the inhibition being higher than that observed for T. cruzi enzyme. As expected, the enzyme was localized, by immunofluorescence analysis, in glycosomes and is present in both promastigotes and true amastigotes obtained from hamster lesion. Hexokinase specific activity increased with the aging of promastigote culture, and this increment was related to glucose consumption. However, the level of the hexokinase protein remains constant as determined by Western blotting. Several hypotheses are discussed to explain this result.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parasites of the genus Leishmania belong to the family of trypanosomatidae and are the causative agents of leishmaniasis. This disease has a spectrum of clinical manifestations ranging from cutaneous lesion to visceral infection. In these parasites, the glycolytic metabolism is an important path for the generation of ATP in which the initial step is the irreversible ATP-dependent phosphorylation of glucose catalyzed by hexokinase (EC 2.7.1.1). This enzyme is sequestered, like other enzymes of glycolysis, in the glycosome, an organelle unique to these organisms (Hannaert et al. 2003). Hexokinase, in trypanosomatids, has been pointed out as drug target due to its difference with its homologous enzyme from the human host (Opperdoes and Michels 2001). In Trypanosoma cruzi, the causative agent of Chagas disease, this enzyme has been shown to be sensible to inorganic pyrophosphate (PPi; Cáceres et al. 2003), and as a consequence, biphosphonates act as potent inhibitors of this enzyme (Hudock et al. 2006). Moreover, biphosphonates have been signaled as possible therapy in Chagas disease since their action is very selective (Hudock et al. 2006).
Hexokinase has been well-characterized kinetically in T. cruzi and Trypanosoma brucei. However, in Leishmania many characteristics of this enzyme that allow designing a rational strategy of selective inhibition are unknown. A molecular characterization through the coding sequence in several species of Leishmania including Leishmania mexicana has been recently performed and, like T. cruzi and T. brucei, this enzyme from Leishmania species are 50-kDa type hexokinases (Umasankar et al. 2005). In this work, we have purified to homogeneity hexokinase from L. mexicana in order to study its kinetic properties. Additionally, as part of an effort to better understand the role of hexokinase on the control of glucose metabolism in this parasite, we studied the expression of this enzyme throughout the growth curve in relation to the glucose consumption. Finally, we performed a subcellular distribution and fluorescence microscopy analyses to corroborate the localization of this enzyme in glycosome in both promastigote and amastigote forms.
Materials and methods
Growth of L. mexicana
Promastigotes of the L. mexicana strain AZV (Pérez et al. 1979) were cultivated at 28°C in Schneider medium supplemented with 20% fetal bovine serum. Hamster footpad lesions were used as sources of amastigotes for isolation and for maintenance of the parasites. An amastigotes-enriched preparation was obtained from the lesion as described elsewhere (Calcagno et al. 2002).
Subcellular fractionating of promastigotes of L. mexicana
Parasites were harvested in exponential phase by centrifugation and washed twice in buffer A (20 mM Tris–HCl, pH 7.2; 5 mM EDTA; 10 mM KH2PO4; 20 mM KCl; and 225 mM sucrose) and once in buffer B (25 mM Tris–HCl, pH 7.6; 1 mM EDTA; and 250 mM sucrose containing a cocktail of protease inhibitors: 10 μM leupeptin, 50 μg/ml trypsin inhibitor, 1 mM benzamidine, 50 μM phenylmethylsulfonyl fluoride, 100 μM tosyl-l-lysine chloromethyl ketone, 0.2 μM pepstatin, 1 μM E-64, 1 μM chemostatin, 1 μM bestatin). Cells were then broken by abrasion with silicon carbide (mesh 200). The resulting suspension was centrifuged at 150 × g for 3 min to eliminate the silicon carbide. Then the homogenate was submitted to differential centrifugation. First, the homogenate was centrifuged at 3,000 × g for 10 min to remove unbroken cells and nuclei, and then the supernatant was centrifuged at 5,000×g for 10 min to obtain the large granular fraction. With the following centrifugation at 33,000×g for 20 min, a small granular fraction was obtained. The supernatant, after this step, was centrifuged at 105,000 × g for 1.5 h to obtain the microsomal fraction. The supernatant after this last step corresponds to the cytosolic fraction. All centrifugation steps were performed at 4°C.
Purification of hexokinase
The small granular fraction (glycosome-enriched fraction) obtained from the differential centrifugation was used to purify hexokinase. This fraction was solubilized in buffer C (10 mM Tris–HCl, pH 7.5; 0.1% Triton X-100; and 150 mM NaCl) and centrifuged at 33,000 × g for 20 min. The supernatant was loaded on a sepharose CL-6B column (2.5 × 75 cm) equilibrated with 25 mM Tris, pH 7.5; 1 mM MgCl2; and 150 mM NaCl. After elution, the fractions displaying hexokinase activity were pooled, dialyzed, and loaded on a column of DEAE-52 cellulose (Whatman) equilibrated with 25 mM Tris–HCl, pH 7.5, and 1 mM MgCl2. Hexokinase was eluted in the wash step. The enzyme was stored at 4°C in the presence of 5% glycerol and 1 mM dithiothreitol.
Enzyme assays and kinetic analysis
Hexokinase activity was measured spectrophotometrically using a coupled assay with glucose 6-phosphate dehydrogenase. The velocity of the reaction was followed by the apparition of NADPH at 340 nm. The assay contained 300 mM Tris–HCl, pH 8.5; 25 mM NaCl; 2 mM MgCl2; 2 mM ATP; 3 mM glucose; 0.8 mM NADP+; and 1 U glucose 6-phosphate dehydrogenase from Leuconostoc mesenteroides (Sigma, USA). When fructose was used as substrate, the activity was measured following the ADP production as described elsewhere (Cáceres et al. 2003). The reaction was started with enzyme. For the measurement of kinetic parameters, the substrate concentrations were varied from 0.017 to 0.2 mM for glucose and fructose and 0.05 to 1 mM for ATP. Inhibition studies were performed varying the concentration of fructose (0–0.6 mM), ADP (0–1 mM), glucose 6-phosphate (0–1.5 mM), and inorganic pyrophosphate (PPi; 0–0.3 mM). The effect of the pH was studied using Tris–HCl buffer for the pH range from 7.5 to 10 and TES buffer for the pH range from 6 to 8.5. The divalent cation requirement was assayed by varying the concentration of MgCl2, MnCl2, CuCl2, ZnCl2, CoCl2, or NiCl2. One unit of hexokinase activity is defined as the amount of the enzyme that catalyzes the production of 1 μmol of glucose-6-phosphate or ADP per minute. When cell suspension was used to measure hexokinase, parasites were resuspended in buffer C.
The activity of other enzymes was assayed as described previously: pyruvate kinase, EC 2.7.1.40 (Adroher et al. 1990); phosphoenolpyruvate carboxykinase, EC 4.1.1.4.9 (Urbina 1987); and succinate dehydrogenase, EC 1.3.9.1 (Köhler and Bachmann 1980).
Glucose, ammonium, and protein determinations
Glucose and ammonium concentration in the growth medium was measured by an enzymatic method (Bergmeyer et al. 1974) using hexokinase and glutamate dehydrogenase (NAD+), respectively. Proteins were quantitatively assayed by the Lowry’s method as described by Schacterle and Pollack (1973) using bovine serum albumin as the standard.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis and Western blotting
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was performed according to Laemmli (1970). For Western blotting experiments, proteins were transferred to polyvinylidene difluoride membranes. The membrane was blocked with phosphate-buffered saline (PBS) containing 5% casein and incubated with rabbit anti-hexokinase antibody raised against the T. cruzi enzyme for 1 h at room temperature. After three washings with PBS, the membrane was incubated with peroxidase-conjugated secondary antibody and the labeled proteins were subsequently revealed by adding diaminobenzidine and H2O2.
Immunofluorescence
Promastigotes and the amastigote-enriched preparation were fixed with 4% (v/v) formaldehyde and allowed to adhere to poly-(l-lysine)-coated slides. Cells were then permeabilized with 0.2% (v/v) Triton X-100 and washed with PBS. Cells were incubated with PBS containing 3% bovine serum albumin and 50 mM ammonium chloride for 30 min, and washed again with PBS. Then, the cells were incubated with anti-T. cruzi hexokinase, dilution 1:40 for 1 h, rinsed with PBS and incubated for 1 h with goat anti-rabbit IgG conjugated with Cy3. The samples were examined using an Olympus fluorescence microscope.
Results
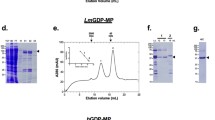
Hexokinase is expected to be localized mainly into the glycosome of all the trypanosomatids (Hannaert et al. 2003). For that reason, we performed a differential centrifugation to obtain a glycosome-enriched fraction as initial material to start the purification of this enzyme. After differential centrifugation of the crude extract of the parasite, the hexokinase activity as well the activity of some marker enzymes was measured in each fraction. As shown in Fig. 1, the activity of hexokinase was found in the small granular fraction associated to the enzyme phosphoenolpyruvate carboxykinase that also has been previously shown to be located in the glycosome (Mottram and Coombs 1985). After solubilization with Triton X-100 and NaCl 150 mM, the small granular fraction was submitted to filtration chromatography (Sepharose CL-6B) and anion exchange chromatography (DEAE-cellulose), as indicated in the “Materials and methods,” to purify hexokinase. In the DEAE-cellulose step, the enzyme was recovered in the flowthrough of the column due to its alkaline isoelectric point (Umasankar et al. 2005). The results of the purification procedure are summarized in Table 1. The final preparation obtained was purified 107-fold as compared with the initial cell homogenate. The specific activity of the purified enzyme was 25.7 U/mg with ATP and glucose as substrates. The purified enzyme was homogenous by SDS-PAGE (Fig. 2) with a molecular mass of 53 kDa similar to nonvertebrate hexokinases and vertebrate hexokinase IV (Wilson 1995; Cárdenas et al. 1998; Cáceres et al. 2003).
Subcellular distribution of hexokinase and marker enzymes obtained by differential centrifugation of a homogenate of L. mexicana promastigotes. The different fractions are shown in the order of their isolation from left to right. N Nuclear, LG large granular, SG small granular, M microsomal, S final supernatant corresponding to the cytosolic fraction. The abscissa indicates the cumulative protein content
Purification of L. mexicana hexokinase as followed by SDS-PAGE. Lane 1 Molecular mass marker, lane 2 small granular fraction (glycosome-enriched fraction), lane 3 small granular fraction solubilized with 0.1%Triton X-100 and 150 mM NaCl, lane 4 sepharose CL-6B step, lane 5 DEAE-cellulose flowthrough
Hexokinase from L. mexicana displayed an optimum pH around 8.5. The kinetic constants of pure hexokinase were determined for glucose, fructose, and ATP according to Lineweaver-Burk and are summarized in Table 2. L. mexicana hexokinase exhibited in all cases apparent Michaelis–Menten kinetics. The Km for glucose and ATP were comparable to those reported for the enzyme from T. cruzi (Cáceres et al. 2003). However, contrary to the T. cruzi enzyme and similarly to other hexokinases (Cárdenas et al. 1998), L. mexicana hexokinase was able to use fructose. The Km value for fructose (0.142 mM) indicates that this enzyme has a low specificity toward glucose. This Km value is lower than those for fructose of nonspecific hexokinases from other sources (Colowick 1973). Magnesium was essential for the activity of L. mexicana hexokinase. The enzyme was unable to use any of the other divalent cations tested.
Hexokinases from other trypanosomatids like T. brucei (Willson et al. 2002) and T. cruzi (Cáceres et al. 2003) lack activity regulation by glucose 6-phosphate as the majority of 50-kDa type hexokinases. In the case of L. mexicana hexokinase, a moderate competitive inhibition by this product was obtained with a Ki of 0.43 and 0.7 mM for glucose and ATP, respectively (Table 2). The fact that this inhibition was competitive with respect to glucose indicates that this inhibition is not the result of binding to an allosteric site of the enzyme.
Interestingly, L. mexicana hexokinase, similar to T. cruzi hexokinase, was inhibited by PPi with a Ki of 35 μM with respect to ATP (Fig. 3). L. mexicana hexokinase was 14 times more sensitive to PPi than T. cruzi hexokinase (Ki = 500 μM; Cáceres et al. 2003), and in the case of L. mexicana, the inhibition was competitive. Hexokinase did not present activity with PPi as substrate.
Hexokinase would be an interesting protein for designing a rational strategy of selective inhibition only if it is expressed in amastigote form. Umasankar et al. (2005) reported that hexokinase is poorly expressed in this form as determined by reverse transcription-polymerase chain reaction analysis. To know whether hexokinase is present in amastigote form, we performed an immunofluorescence experiment in amastigote and promastigote forms using anti-T. cruzi hexokinase. This antibody recognized one band principally in a Western blot of promastigote extract corresponding to the molecular size of hexokinase (Fig. 4a). As shown in Fig 4b,c, glycosomes were clearly stained by anti-T. cruzi hexokinase in both promastigote and amastigote forms. The number of glycosome in amastigote form is fewer than in promastigotes, corroborating a previous report (Coombs et al. 1986). However, hexokinase is present indicating active glycolysis.
Immunofluorescence of L. mexicana promastigotes and amastigotes. a Western blot of promastigote extract showing the specificity of the anti-T. cruzi hexokinase antibody. Arrow shows the band recognized by the antibody. b, c Formaldehyde-fixed and Triton X-100 permeabilized cells stained with anti-T. cruzi hexokinase antibody. promastigotes from culture (b). True amastigotes derived from hamster lesion (c)
Hexokinase expression could be regulated in this parasite. It has been previously reported in a cutaneous leishmaniasis strain of L. infantum (Louassini et al. 1999) that the specific activity increases from the logarithmic phase to stationary phase. To study the hexokinase activity during promastigote growth in L. mexicana, parasite samples at different days were assayed for enzyme activity. Additionally, glucose and ammonium were measured in the medium. As Fig. 5a,c shows, hexokinase specific activity increases continuously during promastigote growth, reaching maximum values on late stationary phase, which is about threefold higher than the specific activity measured on day 1. In our culture conditions, L. mexicana promastigotes consumed both glucose and amino acids, with the latter measured by ammonium excretion (Fig. 5b). The increase in hexokinase activity is coincident with the consumption of glucose as the parasites progress to the stationary phase (Fig. 5b). However, Western blotting analysis using parasites obtained in different points of the curve did not show an increase in the level of hexokinase protein (Fig. 5d).
Hexokinase activity as a function of time during the growth of L. mexicana promastigote culture. a Growth curve of L. mexicana parasites. b Levels of glucose and ammonium in the medium during the growth curve. c Hexokinase specific activity during the growth curve. d Western blot of extract of parasite obtained in the indicated time. The antibody used was raised against T. cruzi hexokinase
Discussion
Hexokinase enzyme has been previously well-studied in T. brucei (Willson et al. 2002) and T. cruzi (Racagni and Machado De Doménech 1983; Cáceres et al. 2003). However, in Leishmania parasite, only limited characterizations have been performed principally related to its subcellular localization (Coombs et al. 1982; Hart and Opperdoes 1984; Mottram and Coombs 1985) and its specific activity in cellular extract (Cazzulo et al. 1985). In the present study, hexokinase was purified to homogeneity from promatigotes of L. mexicana and the kinetic properties of this enzyme were determined. Several interesting properties of this enzyme emerged from this study. (1) This enzyme has the ability to use fructose. In this respect, it differs from T. cruzi hexokinase, which is specific for glucose. This feature might be related to adaptation to different environments. Leishmania parasites, but not T. cruzi, have stages in their life cycle where fructose is available (Leishmania in the insect vector that can feed on nectar). (2) L. mexicana hexokinase exhibits a moderate sensitivity to inhibition (competitive vs ATP and glucose) by the product glucose-6-phosphate. The inhibition by glucose-6-phosphate has been the subject of many studies in the 100-kDa type hexokinases and in a few 50-kDa type enzyme such as hexokinase from Schistosoma mansoni (Tielens et al. 1994; Armstrong et al. 1996), since this inhibitor binds to a discrete allosteric site, which has been defined by structural studies (Mulichak et al. 1998). In these cases, the inhibition is in a noncompetitive manner with respect to glucose. Thus, the result of competitive inhibition with respect to glucose in L. mexicana hexokinase confirms that this allosteric site does not exist in this enzyme. This result also confirms that all trypanosomatid hexokinases lack the glucose-6-phosphate regulatory site. The absence of allosteric regulation has been predicted by sequence analysis by Umasankar et al. (2005). The competitive inhibition by glucose-6-phosphate of L. mexicana hexokinase, in spite of being moderate, may have a role in the regulation of glycolysis in Leishmania. The implication of this regulation by glucose-6-phosphate needs further investigation. (3) L. mexicana hexokinase was inhibited by PPi, the inhibition being higher than that observed for T. cruzi hexokinase and, in the case of L. mexicana enzyme, the inhibition was clearly competitive. The physiological significance of this inhibition is not clear. However, PPi is formed inside the glycosome by several pathways such as β-oxidation of fatty acids, biosynthesis of purine and pyrymidine (Michels et al. 2000). Test of inhibition of this enzyme using analogues of PPi and biphosphonate can be performed, like in T. cruzi hexokinase, to develop therapeutic agents for leishmaniasis (Hudock et al. 2006).
As expected, hexokinase activity was exclusive to the glycosome-enriched fraction. Moreover, fluorescence microscopy analysis shows that glycosomes can be well-stained using anti-hexokinase antibody. This staining was well-observed in both promastigote and amastigote forms. This result clearly indicates that hexokinase is present in amastigotes. It has been suggested that the main source of energy in this stage is fatty acid metabolism (Coombs et al. 1982). However, glycolysis remains functional in amastigotes.
In agreement with the observations of Louassini et al. (1999), we also detected an increase in activity with the aging of culture and it was related to the glucose consumption. However, the immunoblotting analysis shows that this increase is not due to the synthesis of extra enzyme. Several hypotheses arise from this result. Hexokinase can change its specific activity due to a change in the metabolic condition product of glucose availability. This change might be related either to protein–protein association or posttranslational modifications. Other hypothesis to be tested is that measured hexokinase activity would be added to the activity of other enzymes such as glucokinase. It has been shown by the genome analysis of Leishmania major and T. cruzi that these parasites have a glucokinase (Opperdoes and Szikora 2006). This enzyme could be overexpressing with the aging of the culture.
In summary, the characteristics of hexokinase here reported allow the comparison of this enzyme with other hexokinases from trypanosomatids. It shares properties with the T. cruzi enzyme although some differences are notorious (i.e., inhibition by glucose-6-phosphate). The study of the structures of these enzymes will permit a better comprehension of these differences. The strong inhibition by PPi for L. mexicana hexokinase here reported will allow looking for strategies of therapy using PPi analogs such as biphosphonates.
References
Adroher FJ, Osuna A, Lupianez JA (1990) Differential energetic metabolism during Trypanosoma cruzi differentiation. II. Hexokinase, phosphofructokinase and pyruvate kinase. Mol Cell Biochem 94:71–82
Armstrong RL, Wilson JE, Shoemaker CB (1996) Purification and characterization of the hexokinase from Schistosoma mansoni, expressed in Escherichia coli. Protein Expr Purif 8:374–380
Bergmeyer HU, Bernt E, Schmidt F, Stork H (1974) D-Glucose: determination with hexokinase and glucose-6-phosphate dehydrogenase. In: Bergmeyer HU (ed) Methods of enzymatic analysis, vol. 3. Academic, New York, pp 1196–1201
Cáceres AJ, Portillo R, Acosta H, Rosales D, Quiñones W, Avilan L, Salazar L, Dubourdieu M, Michels PA, Concepción JL (2003) Molecular and biochemical characterization of hexokinase from Trypanosoma cruzi. Mol Biochem Parasitol 126:251–262
Calcagno M, Avilan L, Colasante C, Berrueta L, Salmen S (2002) Interaction of different Leishmania mexicana morphotypes with plasminogen. Parasitol Res 88:972–978
Cárdenas ML, Cornish-Bowden A, Ureta T (1998) Evolution and regulatory role of the hexokinases. Biochim Biophys Acta 1401:242–264
Cazzulo JJ, Franke de Cazzulo BM, Engel JC, Cannata JJ (1985) End products and enzyme levels of aerobic glucose fermentation in trypanosomatids. Mol Biochem Parasitol 16:329–343
Colowick S (1973) The hexokinases. In: Boyer PB (ed) The enzymes, vol. 9. Academic, New York, pp 1–48
Coombs GH, Craft JA, Hart DT (1982) A comparative study of Leishmania mexicana amastigotes and promastigotes. Enzyme activities and subcellular locations. Mol Biochem Parasitol 5:199–211
Coombs GH, Tetley L, Moss VA, Vickerman K (1986) Three dimensional structure of the Leishmania amastigote as revealed by computer-aided reconstruction from serial sections. Parasitology 92:13–23
Hannaert V, Bringaud F, Opperdoes FR, Michels PA (2003) Evolution of energy metabolism and its compartmentation in Kinetoplastida. Kinetoplastid Biol Dis 2:11
Hart DT, Opperdoes FR (1984) The occurrence of glycosomes (microbodies) in the promastigote stage of four major Leishmania species. Mol Biochem Parasitol 13:159–172
Hudock MP, Sanz-Rodriguez CE, Song Y, Chan JM, Zhang Y, Odeh S, Kosztowski T, Leon-Rossell A, Concepcion JL, Yardley V, Croft SL, Urbina JA, Oldfield E (2006) Inhibition of Trypanosoma cruzi hexokinase by bisphosphonates. J Med Chem 49:215–223
Köhler P, Bachmann R (1980) Mechanisms of respiration and phosphorylation in Ascaris muscle mitochondria. Mol Biochem Parasitol 1:75–90
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Louassini M, Foulquie MR, Benitez R, Adroher FJ (1999) Activity of key enzymes in glucose catabolism during the growth and metacyclogenesis of Leishmania infantum. Parasitol Res 85:300–306
Michels P, Hannaert V, Bringaud F (2000) Metabolic aspects of glycosomes in trypanosomatidae—new data and views. Mol Biochem Parasitol 16:482–489
Mottram JC, Coombs GH (1985) Leishmania mexicana: subcellular distribution of enzymes in amastigotes and promastigotes. Exp Parasitol 59:265–274
Mulichak AM, Wilson JE, Padmanabhan K, Garavito RM (1998) The structure of mammalian hexokinase-1. Nat Struct Biol 5:555–560
Opperdoes FR, Michels PA (2001) Enzymes of carbohydrate metabolism as potential drug targets. Int J Parasitol 31:482–490
Opperdoes FR, Szikora JP (2006) In silico prediction of the glycosomal enzymes of Leishmania major and trypanosomes. Mol Biochem Parasitol 147:193–206
Pérez H, Labrador F, Torrealba JW (1979) Variations in the response of five strains of mice to Leishmania mexicana. Int J Parasitol 9:27–32
Racagni GE, Machado De Doménech EE (1983) Characterization of Trypanosoma cruzi hexokinase. Mol Biochem Parasitol 9:181–188
Schacterle GR, Pollack RL (1973) A simplified method for the quantitative assay of small amounts of protein in biologic material. Anal Biochem 51:654–655
Tielens AG, van den Heuvel JM, van Mazijk HJ, Wilson JE, Shoemaker CB (1994) The 50-kDa glucose 6-phosphate-sensitive hexokinase of Schistosoma mansoni. J Biol Chem 269:24736–24741
Umasankar PK, Jayakumar PC, Shouche YS, Patole MS (2005) Molecular characterization of the hexokinase gene from Leishmania major. J Parasitol 91:1504–1509
Urbina JA (1987) The phosphoenolpyruvate carboxykinase of Trypanosoma (Schizotrypanum) cruzi epimastigotes: molecular, kinetic, and regulatory properties. Arch Biochem Biophys 258:186–195
Wilson JE (1995) Hexokinases. Rev Physiol Biochem Pharmacol 126:65–198
Willson M, Sanejouand YH, Perie J, Hannaert V, Opperdoes F (2002) Sequencing, modeling, and selective inhibition of Trypanosoma brucei hexokinase. Chem Biol 9:839–847
Acknowledgment
This study was supported by project C-1333-05-03-B CDCHT-ULA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pabón, M.A., Cáceres, A.J., Gualdrón, M. et al. Purification and characterization of hexokinase from Leishmania mexicana . Parasitol Res 100, 803–810 (2007). https://doi.org/10.1007/s00436-006-0351-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-006-0351-4