Abstract
Reservosomes are endocytic organelles from Trypanosoma cruzi epimastigotes that store proteins and lipids for future use. The lack of molecular markers for the compartments of this parasite makes it difficult to clarify all reservosome functions, as they present characteristics of pre-lysosomes, lysosomes and recycling compartments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Reservosomes are big compartments present at the postnuclear region of epimastigote forms of parasites belonging to the genus Trypanosoma, subgenus Schyzotrypanum. Each epimastigote presents several reservosomes, and, although reservosomes’ morphologies can vary according to growth conditions and parasite strain, they are usually spherical organelles surrounded by a unit membrane (Fig. 1). The reservosome matrix is more electrondense than the cytosol: cytochemical anaysis indicated that the reservosome matrix is mainly made of proteins and presents electronlucent inclusions of a lipid nature, as evidenced by the osmium-imidazol microscopy technique (Soares and De Souza 1988). The organelles were described as multivesicular bodies in the first study to describe them (De Souza et al. 1978), where exogenously added peroxidase was found inside big compartments with small vesicles inside. Reservosomes deserved their name because they store tracer macromolecules ingested by the parasite through an endocytic process (Soares and De Souza 1991). Subsequently, reservosomes were found to be the main functional site of cruzipain in epimastigotes (Souto-Padrón et al. 1990), pointing to a lysosome function, although no other lysosome marker has been identified in the organelle. Afterwards, Soares and coworkers (1992) demonstrated that both ingested cargo (gold-labeled transferrin) and the major lysosomal hydrolase (cruzipain) were found in the same reservosomes. Soares et al. used the DAMP technique to evaluate as 6.0 reservosome pH at the electron microscopy level, and compared reservosomes to mammalian late endosomes. The acidic environment characteristic of endocytic compartments is maintained by the action of a P-type H+-adenosine triphosphatase, as we have demonstrated recently (Vieira et al. 2005).
Longitudinal section of an epimastigote harboring five reservosomes at typical postnuclear localization. K kinetoplast, N nucleus, R reservosome. After Cunha-e-Silva et al. (2002). Bar represents 0.5 μm
Three reservosomes with eletronlucent inclusions of varied aspects: some are round, some look like planar bilayers (arrowheads), and others are very clear and combine round and planar surfaces (asterisks). A transport vesicle full of gold-labeled albumin can be observed at left (arrow). Bar represents 0.5 μm
Reservosome lipid inclusions may assume a crystalloid aspect and deform organelle membrane (arrowhead). Bar represents 0.5 μm
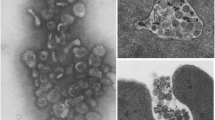
A membrane fraction obtained from the reservosome purified fraction. Bar represents 0.5 μm
In the 13 years since the study of Soares et al., no distinct lysosomes have been found in Trypanosoma cruzi epimastigotes, and reservosomes themselves have been referred to as lysosomes by several authors. During this time, the characterization of intracellular compartments incorporated the specific localization of Rab proteins, the small guanosine triphosphatases (GTPases) involved in vesicle recognition and fusion (Zerial and McBride 2001). Supposing that sequence homology between protozoan and mammalian Rabs would reflect function homology, three T. cruzi rab genes were identified, and their protein products were localized. The first gene, TcRab11 (Mendonça et al. 2000), was surprisingly found in reservosomes; TcRab11 is a small GTPase with high similarity to mammalian Rab11, a marker of recycling endosomes. As previous experiments have suggested that reservosomes are late endosomes, we were curious about the localization of TcRab7, the GTPase characteristic of mammalian late endosomes. Once more, the results were not those that were expected, as TcRab7 was found in the Golgi complex (2004), indicating that the assumption of sequence similarity correlating with function similarity is not adequate. Nevertheless, we have recently managed to demonstrate transferrin recycling from late endocytic compartments, possibly from reservosomes (unpublished results). All these data indicate that reservosomes need to be biochemically characterized; this is an issue that we have already started addressing by establishing a reproducible protocol for obtaining a purified reservosome fraction (Cunha-e-Silva et al. 2002). The first biochemical analyses have shown that reservosomes concentrate lipids, besides proteins: the total lipid/protein ratio in the purified fraction is twofold higher when compared to the total homogenate ratio. Reservosome electronlucent inclusions may assume a crystalloid format, suggesting that the large amount of lipids in the organelle may lead to heterogeneous arrangements (Figs. 2 and 3), creating differentiated lipid domains that may have a functional role. Starting from the total reservosome purified fraction, we also prepared a reservosome membrane fraction (Fig. 4), aiming to distinguish the reservosome’s molecules from those belonging to the soluble content, thus making the proteomic analyses and the establishment of a marker molecule easier. Protocols for lipid rafts identification and isolation were also applied to purified reservosomes, yielding a detergent-resistant fraction enriched in cholesterol and sphingolipids.
During metacyclogenesis, when epimastigotes differentiate to infective trypomastigote forms, reservosome content is massively degraded and the organelle disappears. Thus, it is very interesting to investigate what happens with the organelle during differentiation to trypomastigotes and how the organelle is formed in the reversed process, when epimastigotes originate from trypomastigotes in the insect digestive tract. Both issues have already been addressed in our lab (Soares et al. 1989; Sant’Anna et al. 2004). We have managed to establish a reproducible protocol for the metacyclogenesis reversal, obtaining epimastigotes directly from metacyclic trypomastigotes. In this process, new reservosomes seem to originate from the Golgi complex at the parasite’s anterior region (Fig. 5), and then migrate to the posterior region.
An epimastigote recently differentiated from metacyclic trypomastigote exhibiting several reservosomes distributed along the parasite body, from the anterior region. New reservosomes are very electrondense and poor in lipid inclusions. FP flagellar pocket, K kinetoplast, N nucleus, R reservosome. After Sant’Anna et al. (2004). Bar represents 0.5 μm
Immunocytochemical localization of cruzipain in an epimastigote recently differentiated from metacyclic trypomastigote. Parasites were formaldehyde-fixed, dehydrated, and embedded in hydrophilic resin. The enzyme was detected in the Golgi cisternae (arrows) and in reservosomes. GC Golgi complex, R reservosome. After Sant’Anna et al. (2004). Bar represents 0.5 μm
Transverse section of the posterior region of an epimastigote. Parasites were fixed in glutaraldehyde and tannic acid for 5 days before dehydration and epon embedding (Dallai and Afzelius 1990). This protocol enhances the observation of the cytoskeleton, showing a close relationship between endocytic compartments and subpellicular microtubules (arrow). R reservosome, V transport vesicle containing gold-labeled albumin. Bar represents 0.5 μm
New reservosomes are acidic and present cruzipain (Fig. 6). Associating biochemical, morphological, and molecular biology techniques, we are now investigating how cruzipain is addressed to new reservosomes. Both new reservosomes and cargo-containing vesicles must travel from the Golgi complex and flagellar pocket vicinity at the epimastigote anterior portion towards the typical reservosome position at the parasite posterior region. In this journey, the participation of the cytoskeleton is very likely, as suggested by the close apposition of endocytic compartments and subpellicular microtubules (Fig. 7). Using drugs that disrupt cytoskeleton polymerization dynamics in mammal cells, we could affect endocytosis, diminishing the amount of internalized cargo of varied sizes (transferrin or fluorbeads), or even blocking cargo arrival at reservosomes (unpublished results). The particular role of microtubules and microfilaments and the participation of motor proteins are now under investigation. It seems likely that reservosomes in an epimastigote do not constitute a uniform organelle population. Different reservosomes might have storing, recycling, or lysosome-typical functions according to their maturation and position within the parasite’s body.
References
Araripe JR, Cunha-e-Silva NL, Leal ST, de Souza W, Rondinelli E (2004) Trypanosoma cruzi: TcRAB7 protein is localized at the Golgi apparatus in epimastigotes. Biochem Biophys Res Commun 321:397–402
Cunha-e-Silva NL, Atella GC, Porto-Carreiro IA, Morgado-Diaz JA, Pereira MG, De Souza W (2002) Isolation and characterization of a reservosome fraction from Trypanosoma cruzi. FEMS Microbiol Lett 214:7–12
Dallai R, Afzelius BA (1990) Ultrastructural patterns of the flagellar axoneme in the non-motile part of the mole-cricket sperm. Biol Cell 70:19–26
De Souza W, Carvalho TU, Benchimol M (1978) Trypanosoma cruzi: ultraestructural, cytochemical and freeze-fracture studies of protein uptake. Exp Parasitol 45:101–115
Mendonça SM, Da Silva JLN, Cunha-e-Silva NL, De Souza W, Lopes UG (2000) Characterization of a Rab11 homologue in Trypanosoma cruzi. Gene 243:179–185
Sant’Anna C, de Souza W, Cunha-e-Silva N (2004) Biogenesis of the reservosomes of Trypanosoma cruzi. Microsc Microanal 10:637–646
Soares MJ, De Souza W (1988) Cytoplasmic organelles of trypanosomatids: a cytochemical and stereological study. J Submicrosc Cytol Pathol 20:349–361
Soares MJ, De Souza W (1991) Endocytosis of gold-labeled proteins and LDL by Trypanosoma cruzi. Parasitol Res 77:461–468
Soares MJ, Souto-Padrón T, Bonaldo MC, Goldenberg S, De Souza W (1989) A steriological study of the differentiation process in Trypanosoma cruzi. Parasitol Res 75:522–527
Soares MJ, Souto-Padrón T, De Souza W (1992) Identification of a large pre-lysosomal compartment in the pathogenic protozoon Trypanosoma cruzi. J Cell Sci 102:157–167
Souto-Padrón T, Campetella OE, Cazzulo JJ, De Souza W (1990) Cysteine proteinase in Trypanosoma cruzi: immunocytochemical localization and involvement in parasite–host interaction. J Cell Sci 96:485–490
Vieira M, Rohloff P, Luo S, Cunha-e-Silva NL, De Souza W, Docampo R (2005) Role for a P-type H+-ATPase in the acidification of the endocytic pathway of Trypanosoma cruzi. Biochem J 392:467–474
Zerial M, McBride H (2001) Rab proteins as membrane organizers. Nat Rev Mol Cell Biol 2:107–117
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cunha-e-Silva, N., Sant’Anna, C., Pereira, M.G. et al. Reservosomes: multipurpose organelles?. Parasitol Res 99, 325–327 (2006). https://doi.org/10.1007/s00436-006-0190-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-006-0190-3