Abstract
Recombinant fatty acid binding protein (rFABP) of Fasciola gigantica was expressed in Escherichia coli and used as vaccine in Freund’s adjuvant to evaluate the level of protection induced in buffalo (Bubalus bubalis) calves. Fifteen buffalo calves were distributed to three groups of five calves each. An antigen dose of 400 μg for each of the three immunizations at 3-week intervals, and a challenge dose of 600 metacercariae was administered per calf. Levels of anti-FABP antibodies increased rapidly by 2 weeks after the first immunization and were always significantly higher in the immunized-challenged group than in the infected control group. Immunization with FABP induced both humoral and cell-mediated immune response in these animals. Vaccination showed a moderate level of protection in terms of reduced fluke burden (35.8%) and liver damage as assayed by aspartate aminotransferase and sulfhydryl group levels as well as anti-fecundity effect of the vaccine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fasciola gigantica inflicts heavy economic loss on the livestock industry in the tropical world in general and an adverse impact on health, weight gain, feed conversion efficiency and reproduction of buffaloes (Bubalus bubalis) in particular (FAO 1994; Yadav et al. 1999; Mehra et al. 1999). India, being an agrarian society, has the largest buffalo population in the world, which constitutes a very significant component of the Indian livestock sector (Banerjee 1991; Agricultural Research Data Book 2002). Prevalence of F. gigantica infection in buffaloes, a reservoir host, is high in India and the situation is no better when we look at the statistics of this infection in other ruminants (Sanyal 2001). Control of this disease is achieved by regular use of anthelmintics, but vaccination is the long-term strategy for the intervention of this scourge. Of the various vaccine candidates under evaluation for the development of a commercially viable vaccine for fasciolosis, fatty acid binding protein (FABP) is a promising molecule (Tendler et al. 1996; Estuningsih et al. 1997; Ramajo et al. 2001). Trematode parasites lack a de novo pathway of lipid synthesis, utilizing host fatty acids and sterols for their lipid metabolism and appear to use FABPs as transport molecules (Meyer et al. 1970; Ockner 1990). FABP has been the target for disrupting this vital link in the survival of the parasite in various vaccination trials in sheep and cattle but the potential of this molecule has not been evaluated in buffaloes so far. Therefore, this study focuses on the evaluation of immunoprophylactic potential of recombinant fatty acid binding protein (rFABP) against F. gigantica infection in buffaloes.
Materials and methods
Buffalo calves
Fifteen buffalo calves, aged 8–10 months, were used in this immunoprophylactic study. Buffalo calves used in the present experimental studies were procured from the dairy farm of the Indian Veterinary Research Institute (stall-fed) and were screened for F. gigantica and other helminth infections coprologically and serologically prior to immunization studies. All the experimental animals were treated with albendazole (15 mg/kg body wt) and it was only after ensuring their helminth free status that they were used for the vaccination trial. These animals were housed indoors on a concrete floor and maintained under an intensive system of management. The animals were fed as per Kearl (1982) to meet their maintenance requirements during the course of the experiment. Buffalo calves were used for the experimental purposes as per the regulations laid down by the animal ethics committee.
F gigantica metacercariae
Fasciola gigantica metacercariae were harvested on polyethylene sheets from field-infected Lymnaea auricularia and stored at 4°C until use. Viable metacercariae less than 3 months old were used for challenge infection per os.
Expression of F gigantica rFABP
cDNA-encoding F. gigantica FABP was cloned in a prokaryotic expression vector, pPROEXHT-b (Invitrogen) and expressed in Escherichia coli (Sriveny et al. 2003). Protein expression was induced with 1 mM isopropyl-thio-D galactopyranoside for 7–8 h at 37°C in 1 l batches of LB medium. Two hundred and fifty millilitre culture aliquots were pelleted by centrifugation at 4,000×g for 10 min at room temperature (RT). The polyhistidine-tagged fusion protein was purified by nickel-chelating affinity chromatography using urea as a mild denaturant (Sriveny et al. 2002). Briefly, the cell extract was prepared by suspending 0.5 g (wet wt) bacterial pellet in 7 ml of lysis buffer (10 mM Tris, 100 mM NaH2PO4; 8 M Urea, pH 8.0) for 1 h at RT. Cell debris was pelleted and the lysate was incubated with 700 μl of Ni-NTA resin (Qiagen) for 1 h at RT in the presence of 10 mM imidazole and packed into the column. Unbound proteins were washed using wash buffer (10 mM Tris, 100 mM NaH2PO4; 8 M Urea, pH 8.0) and the fusion protein eluted in 3 ml elution buffer (10 mM Tris, 100 mM NaH2PO4, pH 4.5). The eluted rFABP was dialyzed against phosphate buffered saline (PBS) (pH 7.4) at 4°C, concentrated by lyophilization, and protein concentration was estimated as per Lowry et al. (1951). Homogeneity of the purified protein was determined by SDS-PAGE (Laemmli 1970).
Antigen formulation, administration and challenge
Buffalo calves were randomly assigned to three groups (I, II and III) of five animals each. Group I animals (immunized-challenged group) were immunized with 400 μg of F. gigantica rFABP subcutaneously in Freund’s complete adjuvant for primary immunization at 2–3 sites in the neck region on day 0 and subsequent doses of the same antigen concentration were given on days 21 and 42, as second and third immunizations with Freund’s incomplete adjuvant. Each of these calves were challenged with 600 viable metacercariae after 4 weeks of third immunization along with group II calves (infected control) while group III animals were kept as uninfected control. Blood was collected weekly during the immunization schedule and every 2 weeks from animals of all three groups after challenge infection. Sera was retrieved and stored at −20°C.
Enzyme linked immunosorbent assay
Sera samples were analysed by ELISA. Plates (Greiner, Germany) were coated with 100 μl of 2 μg/ml of rFABP antigen in 0.1 M carbonate/bicarbonate buffer (pH 9.6) per well. Following overnight incubation at 4°C, plates were washed five times with PBS containing 0.05% Tween-20. Wells were blocked with 3% skim milk in PBS (SM-PBS) for 1 h at 37°C. Subsequent to five additional washes in PBS-T, 100 μl/well of buffalo sera diluted 1:50 in 1% SM-PBS were incubated for 1 h at 37°C. Subsequently wells were washed five times and 100 μl per well of 1:8,000 diluted rabbit anti-bovine IgG-peroxidase conjugate was incubated for 1 h at 37°C. Finally, after additional five washes, colour was developed with 100 μl of orthophenylene diamine (1 mg/ml) in sodium citrate buffer, pH 4.6 for 30 min at RT. The reaction was stopped with 50 μl 3N HCl per well, and absorbance recorded as the mean OD492 of duplicate samples. Sera from four uninfected buffalo calves were used as negative control.
Western blot
The electrophoretic transfer of rFABP to nitro-cellulose paper was carried out as per Towbin et al. (1979). The protein was resolved on 15% SDS-PAGE and transferred to a nitro-cellulose membrane using transfer buffer (25 mM Tris, 192 mM glycine, and 20% methanol) for 60 min at 350 mA/100 V at 4°C. The membrane was washed with PBS-T (wash buffer) and blocked with 5% skim milk in PBS overnight at 4°C. Washing of the membrane was carried out four times for 20 min with PBS-T and assembled into the mini protean multiscreen apparatus (BioRad). Buffalo sera (immunized-challenged, uninfected and infected control) diluted 1:50 in 1% SM-PBS were probed for 1 h at RT. After five washes, NCP was incubated for 1 h at 37°C in rabbit anti-bovine IgG-alkaline phosphatase (1:4,000 dil. in 1% SM-PBS). The blots were finally washed and membrane was incubated in alkaline phosphate buffer (pH 9.5) containing nitro-blue tetrazolium and 5 bromo-4-chloro-3-indolyl phosphate for colour development. The reaction was stopped by washing the membrane in distilled water.
Lymphocyte transformation test
Blood was collected in 2.7% EDTA from immunized-challenged calves 2 weeks after each immunization and also from the negative control animals on these corresponding dates. Peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation using Histopaque 1.077, (Sigma) as per Backer and Knoblock (1982) and 3×106 cells/ml were suspended in RPMI 1640 containing 10% foetal calf serum. A 100-μl cell suspension was seeded in each well of the 96 well flat-bottomed microtitre plate and 10 μl of rFABP (2 μg) was added in quadruplicate to assess the lymphoproliferative response. ConA (10 μg/ml) was added as a positive control and unstimulated cells served as the cell control. The plates were incubated at 37°C in a CO2 incubator for 5 days for sensitization of the PBM cells by rFABP. The lymphoproliferation assay was performed by MTT dye reduction test (Bounous et al. 1992). The MTT dye was added to each well of the microtitre plate (100 μg final concentration) and the plates were incubated at 37°C for another 4 h in a CO2 incubator. A volume of 150 μl DMSO was added to each well to dissolve formazan crystals. Absorbance was read at 492 nm with reference reduction at 650 nm and stimulation index (SI) calculated as the ratio of OD of stimulated cells to that of unstimulated control cells.
Biochemical assays
Liver damage in all the immunized and infected control calves was assessed by measuring the level of aspartate aminotransferase (AST) activity in blood, collected every 14 days from the time of challenge infection to 90 days post infection (PI) in both immunized and infected control groups as per Reitman and Frankel (1957) using a commercial kit (Glaxo, India). AST activity was also measured in the serum of negative control animals collected simultaneously along with the blood samples of immunized and infected control animals.
Also the status of sulfhydryl groups in the livers of necropsied calves was measured as per Sedlack and Lindsay (1968). The total, protein-bound and non-protein bound sulfhydryl group level in liver samples collected from the buffalo calves (uninfected, infected and immunized-challenged groups) at necropsy (15 weeks PI) was estimated. The amount of sulfhydryl groups was calculated using molar extinction coefficient (13,600 M−1 cm−1) of GSH-DTNB conjugate at 412 nm and was expressed as micromoles of sulfhydryl groups/gram liver.
Fluke size and wet weight
All calves were euthanized in the 15th week (105 days) after challenge. Liver was cut into slices approximately 1 cm thick and squeezed in warm saline for fluke collection. Flukes from each necropsied animal in the respective groups were recorded for their wet weight, length and width. The size and wet weight of flukes recovered from each calf of the immunized group were compared with flukes recovered from the infected control group.
Bile egg count
Bile collected from each immunized and infected control animal was cleared by repeated sedimentation/decantation of the eggs. Egg counts were performed under a compound light microscope by measuring a definite volume (10 μl×5) of each sample to calculate the total eggs in the given volume of bile as per the standard protocol.
Statistical analysis
Data were statistically analysed using the Student’s t-test
Results
Humoral immune response in immunized and infected control calves was detected using rFABP antigen by ELISA and immunoblotting. Absorbance values of each animal in group I increased with each immunization. The highest absorbance values were found 3 weeks after the last immunization. However, buffalo calf no. 22 showed an elevated and highest absorbance value only after third immunization. No further increase in the antibody titre was detected in immunized calves on challenge infection; instead the mean absorbance value of these animals started decreasing at the post-challenge period, which remained at plateau up to 90 days post challenge. However, in the infected control calves absorbance values remained at baseline with no substantial increase at subsequent weeks of challenge (Fig. 1). In western blot too, the same response was observed in the immunized group, but in the infected control animals no detectable humoral response to this antigen was observed in this assay (Fig. 2). One out of five buffalo calves in the immunized group died during experimental study prior to the challenge infection. The cause of the death on postmortem was confirmed to be due to pneumonia and the death of the animal was not related to the immunization process.
Cell-mediated immune response to this antigen was measured by lympho-proliferative assay in three buffalo calves each from group I (pre and post immunization) and group III. Lymphocytes of immunized buffalo calves showed increased antigen-specific proliferation in vitro after each immunization and maximum SI was attained after the second immunization (1.58±0.23). However, a decreasing pattern of SI was observed thereafter. This increase in SI indicated that the immunized buffalo calves were sensitized with rFABP (Fig. 3).
AST activity and sulfhydryl group levels in vaccinated calves
Serum AST activity was lower in the immunized group than in the infected control group with a significant difference between the two groups at day 30 PI (Fig. 4). Increased AST level in the infected control group at 30 days PI is indicative of maximum liver damage at this stage of fluke maturation. These results suggest that calves in this group suffered from more hepatic damage than the immunized group and indirectly suggest that the immunized group had a lower fluke burden than the infected control animals.
The total sulfhydryl group level in the infected control (group II) calves (8.5 μmol±0.72) was lower than in the negative control animals (group III) (13.9 μmol±1.4). However, immunized calves (group I) (14.6 μmol±2.4) showed a slightly higher level than group III. The protein-bound sulfhydryl-group level in group I (12.1 μmol±2.42) and group III (11.05 μmol±3.37) was higher than group II (5.5 μmol±0.499) calves (Fig. 5). This indicates that immunization with rFABP restored the glutathione status even after challenge infection, whereas these values were significantly lower in the infected control group.
Fluke recovery
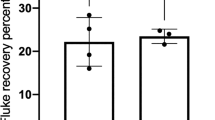
At necropsy, 41.2±5.86 and 26.4±3.68% of F. gigantica metacercariae were recovered as adult flukes from the hepatic parenchyma, bile duct and gall bladder of group II and I buffalo calves, respectively. The mean wet weight and size (length and width) of flukes recovered from group I calves was less than those recovered from the group II animals. A 13.5%, 12.5% and 9.8% reduction in wet weight, length and width respectively was observed in flukes recovered from group I calves. This reduction in the fluke length and wet weight was not found to be statistically significant but reduction in the fluke width in the immunized group was found statistically significant (P<0.01). The mean fluke recovery from group I and group II buffalo calves was 158.5±22.1 and 247±35.13 respectively (Table 1). The percentage fluke recovery of 26.4±3.68 and 41.2±5.86 from group I and group II calves respectively indicated that immunization with rFABP conferred 35.8% protection in immunized-challenged buffalo calves.
Bile egg count
An overall reduction in mean bile egg count (98.6%) was found in the group I calves when compared to the group II calves, depicting anti-fecundity effect of the immunization or delayed development of gonads in the flukes recovered from the immunized group.
Discussion
Fatty acid binding proteins are crucial for the lipid metabolism of these parasites and vaccination with parasite FABP could induce antibodies that would interfere with the transportation of fatty acids from the hosts to the flukes, thereby jeopardizing the survival of the parasite. Incidentally, FABP was the first defined antigen to be tested as a vaccine against fasciolosis, when Hillyer (1985) and Hillyer et al. (1987) found F. hepatica antigen fraction FhSmIII(M) inducing a reduction in worm burden by 69–78% in mice and 55% in cattle. However, there have been variations in the results obtained by vaccination with native and rFABP. Muro et al. (1997) tested the efficacy of F. hepatica rFABP as vaccine in rabbits but reduction in the worm burden was not significant (11–17%) and was less than that elicited by the native molecule. Similarly, a low but significant 31% reduction in fluke burden and 36% reduction in fluke wet weight were observed in cattle vaccinated with native FABP in FCA; however, with rFABP this reduction in fluke burden was 1% and 11% (in FCA and Quil A, respectively) (Estuningsih et al. 1997). Ramajo et al. (2001) found no reduction in the worm burden in rFABP and native FABP-vaccinated sheep but there was significant reduction in worm size and faecal egg counts, suggesting an anti-fecundity effect of the vaccine.
But in our vaccination trial in buffalo with rFABP in FCA, worm burden was reduced by 35.8%, which is in contrast to the results of Estuningsih et al. (1997) in cattle, with this antigen formulation. In this vaccination trial with rFABP, an overall protection of 35.8% was observed with a reduction of 13.4%, 12.6% and 9.8% in mean fluke wet weight, length and width respectively, when compared with flukes from infected control calves. It shows that vaccine formulation exerted an adverse effect on the migratory stages of the parasite, with overall growth stunted in many of the flukes. However, the protection level achieved in terms of reduction in fluke burden, fluke length and wet weight and anti-fecundity effect was not found to be statistically significant. This could be attributed to the small number of animals used in the immunization trial. Since the absorbance values defining the antibody level elicited by the four immunized calves were significantly high and when these absorbance values were corroborated with the fluke recovery (fluke burden and fluke size), there was a direct correlation with the protection level. However, this correlation could not be determined in the infected control calves as there was no substantial increase in antibody level up to 90 days PI in these animals. Effector components of immunity operative against this parasite in buffalo host were not evaluated in this study, though it appears that both humoral and cell-mediated immunity are effective in curbing the parasite’s development.
Serum AST level, an indicator of liver cell damage, was significantly high at 30 day PI in the infected control group but declined in the subsequent weeks PI. This correlates with the migratory stages of the flukes when maximum damage to the liver tissue occurs. However, AST level in vaccinated calves was at par with negative control animals, suggesting immunization having prevented liver damage by flukes.
Similarly, the decrease in the total and protein-bound sulfhydryl groups in infected control animals might have impaired the normal physiological functions of various enzymes involved in liver cell metabolism. However, higher levels of protein bound and total sulfhydryl groups in vaccinated calves suggest better antioxidant status of liver cells and indirectly indicate that liver cells were less prone to cell injury during infection. The level of reduced glutathione in the antigen presenting cells is known to modulate immunity through Th1/Th2 response (Peterson et al. 1998). Although we have estimated the sulfhydryl status in liver rather than antigen presenting cells, similar levels in the negative control and immunized group and resultant protection following challenge in immunized animals indicates that there might be some influence of sulfhydryl status on modulation of cell-mediated immune response.
A significant effect of this vaccine appears to be on the fecundity of the parasite. In this vaccination trial 98.6% reduction in the mean bile egg count was observed in the vaccinated group calves. This observation was made at necropsy 15-weeks post challenge. Data on the anti-fecundity effect of the vaccine appears incomplete in the present investigation, since the experiment was not extended beyond 15 weeks post challenge to have EPG assay conducted in both the groups. Also the anti-embryonation effect of the vaccine was not studied in this experiment. However, with the available data on the bile egg count in vaccinated and infected control animals, a high level of anti-fecundity effect was recorded. This effect of vaccination can have a profound effect on pasture contamination and disease transmission.
Though this level of protection induced by rFABP may not appear sufficient to protect these ruminants against the deleterious effects of this infection, yet it is highly protective in terms of reduction in liver pathological signs, fluke burden/size and anti-fecundity effect of this immunization. Further studies are warranted by using this antigen with other defined immunogenic molecules (cocktail vaccine), along with other commercially acceptable adjuvant formulations for boosting the protection level against this helminth.
References
Agricultural Research Data Book (2002) Indian Agricultural statistics Research Institute. New Delhi, p 28
Backer PE, Knoblock KF (1982) Bovine co-stimulator II: generation and maintenance of co-stimulator dependent bovine lymphoblastoid cell lines. Vet Immunol Immunopathol 2:467–469
Banerjee GC (1991) A textbook of animal husbandry, 7th edn. Mohan Primlani for Oxford and IBH pub, pp 112–114
Bounous DI, Campagndi RP, Brown J (1992) Comparison of MTT colorimetric assay and tritiated thymidine uptake for lymphocyte proliferation assay using chicken spleenocytes. Adv Dis 36:1022–1027
Estuningsih ES, Smooker PM, Wiedosari E, Widjajanti S, Vaiano A, Partotomo S, Spithill TW (1997) Evaluation of antigens of Fasciola gigantica as vaccine against tropical fasciolosis. Int J Parasitol 11:1419–1428
FAO (1994) Diseases of domestic animals caused by flukes. Food and Agriculture Organization of the United Nations, Rome, p 49
Hillyer GV (1985) Induction of immunity in mice to Fasciola hepatica with a Fasciola/Schistosoma cross-reactive defined antigen. Am J Trop Med Hyg 34:1127–1131
Hillyer GV, Haroun EM, Hermandez A, De Galanes MS (1987) Acquired resistance to Fasciola hepatica in cattle using a purified adult worm antigen. Am J Trop Med Hyg 37:363–369
Kearl LC (1982) Nutrient requirements of ruminants in developing countries. International Feedstuffs Institute, Utah Agricultural Experiment Station, Utah State University, Logan
Laemmli UK (1970) Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227:630–684
Lowry OH, Rosenberg NJ, Farr AM, Randell RJ (1951) Protein measurement with folin reagent. J Biol Chem 193:265–275
Mehra UR, Dass RS, Verma AK, Sharma RL, Yadav SC (1999) Effect of Fasciola gigantica infection on growth and nutrient utilization in buffalo calves. Vet Rec 145:699–702
Meyer F, Meyer H, Bueding E (1970) Lipid metabolism in the parasitic and free living flatworms, Schistosoma mansoni and Dugesia dorotocephala. Biochem Biophys Acta 210:257–266
Muro A, Ramajo V, Lopez J, Simon F, Hillyer GV (1997) Fasciola hepatica: vaccination of rabbits with recombinant and native antigens related to fatty acid binding proteins. Vet Parasitol 69:219–229
Ockner RK (1990) Historic overviews of the studies on fatty acid binding proteins. Mol Cell Biochem 98:3–9
Peterson JD, Herzenberg LA, Vasquez K, Waltenbaugh C (1998) Glutathione levels in the antigen presenting cells modulate Th1 versus Th2 response patterns. Proc Natl Acad Sci USA 95:3071–3076
Ramajo V, Oleaga A, Casanueva P, Hillyer GV, Muro A (2001) Vaccination of sheep against Fasciola hepatica with homologous fatty acid binding proteins. Vet Parasitol 97:35–46
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28:56–63
Sanyal PK (2001) Control of tropical fasciolosis in cattle and buffaloes in India at the backdrop of its integrated management. J Vet Parasitol 15:13–16
Sedlack J, Lindsay RH (1968) Estimation of total, protein bound, and nonprotein bound sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25:192–205
Sriveny D, Raina OK, Verma J, Singh BP, Yadav SC (2002) Purification of Fasciola gigantica recombinant fatty acid binding protein. J Vet Parasitol 16:39–41
Sriveny D, Raina OK, Verma J, Yadav SC (2003) Expression of Fasciola gigantica recombinant fatty acid binding protein. Ind J Biotech 2:549–557
Tendler M, Brito CA, Vilar MM, Serra-Freire N, Diogo CM, Almeida MS, Delbem AC, De Silva JF, Sanivo W, Garratt RC, Simpson AJ (1996) A Schistosoma mansoni fatty acid binding protein Sm14 is the potential basis of dual purpose anti-helminth vaccine. Proc Natl Acad Sci USA 93:269–273
Towbin H, Stachelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350
Yadav SC, Sharma RL, Kalicharan A, Mehra UR, Das RS, Verma AK (1999) Primary experimental infection of riverine buffaloes with Fasciola gigantica. Vet Parasitol 82:285–296
Acknowledgements
The authors are thankful to the authorities of the National Agricultural Technology Project (CGP) for financial assistance to undertake this study. The first author is also thankful to IVRI for providing JRF during his MVSc programme.
Author information
Authors and Affiliations
Corresponding author
Additional information
Declaration: All the experiments conducted on the buffalo calves were as per the guidelines laid down by the Animal Ethics Committee of Indian Veterinary Research Institute, Izatnagar, Bareilly, India.
Rights and permissions
About this article
Cite this article
Nambi, P.A., Yadav, S.C., Raina, O.K. et al. Vaccination of buffaloes with Fasciola gigantica recombinant fatty acid binding protein. Parasitol Res 97, 129–135 (2005). https://doi.org/10.1007/s00436-005-1397-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-005-1397-4