Abstract
Induction of penetration gland emptying by cercariae of the bird schistosomes Trichobilharzia szidati and T. regenti employing linoleic acid, linolenic acid, praziquantel and calcium ionophore A23187 showed that both postacetabular and circumacetabular cells released their content at chosen stimulant concentrations. The gland secretions consisted of soluble and insoluble parts. The former one adhering to the ground seemed to have different saccharide composition from the glands of Schistosoma mansoni. It bound labelled saccharides, thus exhibiting lectin-like activity. Protein profiles of the latter one were identical after stimulation by all four stimulants in T. szidati. The soluble secretions contained several proteolytic enzymes; 31 kDa and 33 kDa cysteine proteases were identified in E/S products of T. szidati and T. regenti, respectively. The circumacetabular glands contained a significant amount of calcium. Immunohistochemistry revealed that the origin of E/S products after in vitro stimulation is in both penetration glands and tegumental structures. No crossreactivity was observed between the bird schistosomes and a serum raised against S. mansoni elastase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Active penetration of cercariae into host bodies is an obligatory part of the life cycle of many trematode species. Cercariae penetrate either their intermediate hosts—which can be invertebrates (e.g. for family Plagiorchiidae) or vertebrates (e.g. for Diplostomatidae) - or definitive hosts which can be poikilotherm (e.g. for Sanguinicolidae) or homoiotherm (for Schistosomatidae) vertebrates. The process of penetration is poorly known in most species. Penetrating larvae employ specific secretions which enable them disruption of host surface epithelia and underlying tissues. These are usually products of specialized cells, the so-called penetration glands. Their arrangement is well known in schistosomatids. They are composed of five pairs of large secretory cells divided into two groups according to their position towards the ventral sucker, ultrastructure and composition. Three pairs have been designated as postacetabular and two as preacetabular or circumacetabular (Stirewalt and Kruidenier 1961; Horák et al. 2002).
The glands are able to empty upon a stimulus gained at the contact with a host. The stimuli have been studied in a few schistosome species. In Schistosoma mansoni and S. haematobium, the emptying of penetration glands is induced by unsaturated fatty acids such as linoleic and linolenic acids (e.g. MacInnis 1969; Shiff et al. 1972; Austin et al. 1972, 1974). In in vitro experiments, lipids from human skin surface fractions stimulated predominantly emptying of circumacetabular glands, whereas hydrophilic extracts stimulated mainly the secretion of postacetabular glands in S. mansoni. Glucosylceramides and phosphatidylcholine stimulated protease secretion by cercariae without provoking penetration behaviour (Haas et al. 1997). In a bovid schistosome, Orientobilharzia turkestanica, free fatty acids of the skin act as exclusive chemical penetration stimuli. A few experiments have also been done with a bird schistosome, Trichobilharzia ocellata; the strain used for the experiments by Haas and van de Roemer (1998), is identical to our laboratory strain of Trichobilharzia szidati used in this study, based on sequence homology of the ITS region of ribosomal DNA; the taxonomical status of T. ocellata has recently been solved and reconsidered by Rudolfová et al. (2005). For T. ocellata in that study, a similar response to fatty acids as in the case of S. mansoni (Haas and van de Roemer 1998) has been observed.
Some compounds are known to induce gland emptying by schistosomes in vitro. Besides the natural stimulants—unsaturated fatty acids (e.g. Haas and Schmitt 1982), they involve, e.g., praziquantel (Matsumura et al. 1990), phorbol esters (Matsumura et al. 1991), calcium ionophores (Matsumura et al. 1991; Hara et al. 1993), heavy metals (Hara et al. 1993), lectins (Coles et al. 1988). Secretion caused by these compounds usually proceeds more rapidly and some characteristic attributes are missing compared to in vivo process (Matsumura et al. 1990). Some of these compounds even disrupt cercarial surface or cause narcotization, and therefore the gland emptying seems to be stimulated nonspecifically in some of these cases (Haas et al. 1997).
The content of schistosome penetration glands has been studied by several authors but its composition is still poorly known. Most of the studies have been done on proteolytic enzymes. Several cercarial proteases have been described but the picture is still not complete and the data presented by different authors are often controversial. Cercarial elastase is well characterized and recently has been widely accepted as the main histolytic protease located in circumacetabular gland cells of S. mansoni and S. haematobium (e.g. Pierrot et al. 1996; Salter et al. 2002). Besides, several attempts to characterize cercarial serine proteases in S. mansoni have been made. They are able to cleave proteins of connective tissues (e.g. McKerrow et al. 1985; Chavez-Olortegui et al. 1992), complement factors and probably also cercarial surface proteins during glycocalyx shedding (Marikovsky et al. 1988). Also the data on the presence of cysteine proteases in penetration glands of S. mansoni are controversial. Dalton et al. (1997) showed by means of immunohistochemistry that cathepsins L and B are present in postacetabular glands. However, this observation was not supported by Skelly and Shoemaker (2001). Bahgat and Ruppel (2002) reported on a comparison of S. mansoni elastase and a serine protease from Trichobilharzia ocellata. Their physicochemical properties were similar, both occurred as a doublet around 28 kDa in zymographic gels. Bahgat et al. (2001) also published data on reaction of antibodies against elastase of S. mansoni with preacetabular penetration glands of T. ocellata.
Much less data exist on other compounds from cercarial penetration glands. S. mansoni is known to possess an extremely high concentration of calcium in preacetabular glands (Dresden and Edlin 1975) present in the form of carbonate (Dresden and Asch 1977) or in a ionic form (Dorsey and Stirewalt 1977). It was suggested to play a role in regulation of gland protease activity (Dresden and Edlin 1975; McKerrow et al. 1985), disaggregation of host skin proteoglycans (Landsperger et al. 1982), prevention of cercarial surface damage (Modha et al. 1998) etc., but still its true function during penetration has not been proved.
Lectin-like proteins have been found in postacetabular glands of the schistosome Trichobilharzia szidati (Horák et al. 1997) and penetration glands of the diplostomatid fluke D. pseudospathaceum (Mikeš and Horák 2001), the latter possessing cysteine protease activity (Mikeš and Man 2003). They are able to bind carbohydrate chains similar to glycosaminoglycans of connective tissue but their function has yet not been discovered. The secretions of S. mansoni postacetabular glands contain a thick carbohydrate-rich “glue-like” substance which most likely helps the cercariae attach to the skin (e.g. Stirewalt 1974). It is composed of acid and neutral mucopolysaccharides reacting with labelled lectins and antibodies against highly glycosylated keyhole limpet hemocyanin (Linder 1985, 1986).
The main goal of this study was to compare the effect of four potential stimulants of penetration gland emptying, two natural and two artificial, on the cercariae of a schistosome species Trichobilharzia szidati parasitizing the intestinal wall of birds. In some of the experiments, cercariae of a nasal neuropathogenic bird schistosome Trichobilharzia regenti have been used for comparison. The purpose was to develop a simple method for quantitative collection of penetration gland content for routine use. Attempts have been made to find out whether the two gland types empty separately, so that the protein profiles of the E/S products differ after incubation with particular stimulants and also, whether the obtained products are composed mainly of gland content or contaminated by other proteins, e.g. of tegument origin. Moreover, the E/S products and the glands themselves have been partially characterized as for the chromophilicity and the presence of proteolytic enzymes, lectins, mucous substances and calcium.
Materials and methods
Parasites and reagents
Cercariae of our laboratory strains of Trichobilharzia regenti Horák et al., 1998 and Trichobilharzia szidati Neuhaus, 1952 emerged from host snails Radix peregra and Lymnaea stagnalis, respectively, in 100 ml beakers under 40 W illumination. The larvae were first concentrated in a small volume of water employing their positive phototaxis and then transferred into clean tap water.
Lyophilized cercariae of Schistosoma mansoni and rabbit antiserum (BR 67) against S. mansoni cercarial elastase were obtained from Prof. M. Doenhoff, University of Wales, Bangor, UK. E/S products of S. mansoni cercariae produced in RPMI medium during 30 min in vitro incubation were supplied by Dr. A.P. Mountford, University of York, UK. DCG-04, a biotinylated analogue of the Clan CA cysteine protease-selective inhibitor E-64 was a gift of Dr. Caffrey and Dr. Greenbaum, Sandler Center for Basic Research in Parasitic Diseases, UCSF, USA. Biotinylated glycosaminoglycans were received from Prof. M. Tichá, Department of Biochemistry, Charles University Prague. Other chemicals were purchased from Sigma, unless otherwise noted.
Dynamics of artificially stimulated gland secretion by T. szidati and T. regenti
In order to estimate optimal conditions for stimulation of penetration gland emptying (based on concentration of substances to be used and observation of cercarial behavior and survival under stimulating conditions), defined volume of cercarial suspension was dropped onto a microscopic slide and the stimulating reagents were added: praziquantel, linoleic acid, linolenic acid or calcium ionophore A23187 at final concentrations shown in Tables 1, 2 and 3. The ionophore was also tested with added 2 mM CaCl2 and/or 20 mM EGTA. The slides were stored in moist chambers and behaviour of cercariae was observed under the microscope for 45 min at 2-min intervals. The number of cercariae on each slide ranged from 10 to 40. For either species, every stimulant and each concentration, the experiment was performed at least three times.
Staining of cercarial glands and secretions
The main aim of these experiments was to find dyes staining differentially the two types of cercarial penetration glands and thus enabling to find out which stimulant induces emptying of a particular type of gland. Cercariae of both species were dropped onto microscopic slides and praziquantel and linoleic acid or linolenic acid were added to make 0.1 μg/ml final concentration. After a 15-min incubation the cercariae attached to the slide by dint of the released insoluble gland products. Then, 1% aqueous solutions of 11 dyes (aldehyde-fuchsin, azocarmine, acid fuchsin, kongo-red, methylene blue, methyl-violet, nile blue, orcein, light green yellowish, thionine and trypan blue) were added. 1% of alcian blue was diluted in 3% glacial acetic acid and 1% alizarine solution was neutralized by sodium hydroxide. All 13 dyes mentioned above and 1% aqueous solution of lithium carmine were applied to cercariae fixed in 70% ethanol. During the 15-min staining, all slides were placed in a moist chamber, then washed by water and were embedded in buffered glycerin. In addition, some cercariae were fixed in 4% formaldehyde and penetration glands were stained by apomorphine using the method of Bruckner (1974). Unfixed cercariae dried on microscopic slides were examined for calcium deposits using the potassium oxalate method of Mussini et al. (1972). Samples were examined under the microscope.
Binding of labelled saccharides and lectins to cercarial gland products
To find out whether the insoluble gland products contain lectins or saccharide compounds, the cercariae of both species dropped onto microscopic slides were induced to produce gland secretions by addition of 0.1 μg/ml praziquantel. Adhered products were incubated for 30 min with the following saccharide probes: fluorescein-labelled laminarin, biotinylated hyaluronate, biotinylated chondroitinsulfate, biotinylated heparin (all 0.1 mg/ml) and heparin-coated Sepharose microparticles. Incubation with biotinylated probes was followed by washing in 20 mM Tris-buffered saline pH 7.8 (TBS) for 3×3 min and then by incubation with fluorescein-labelled avidin (5 μg/ml) for 30 min. In controls, non-labelled saccharides were used to inhibit the binding of the labelled ones (2 mg/ml laminarin, 1 mg/ml heparin or 1 mg/ml de-N-sulfated N-acetylated heparin). These were used for 10-min preincubation and also in the first incubation step together with the labelled compounds. Finally, all slides were washed for 3 min in TBS and covered with thin cover slides.
A set of 7 fluorescein-labelled lectins (Vector Labs, Burlingame CA, USA) were used for characterization of sacccharide composition of the products. Adhered secretions on slides were first blocked by 2% BSA for 30 min. Then 20 μg/ml lectins were applied for 30 min followed by a 5-min washing in TBS. Lectins with appropriate saccharide inhibitors (100 mM) served as controls (see Table 5). All reactions were evaluated under the fluorescence microscope.
Preparation of cercarial protein samples
For preparation of cercarial protein extracts, the concentrated cercariae were cooled to 0°C, centrifuged, transferred to a cold buffer (according to further use) and sonicated. Lyophilized S. mansoni cercariae were rehydrated in TBS and sonicated. Homogenates were centrifuged at 16,000x g for 20 min, supernatants were collected, recentrifuged and the second supernatants were used immediately or stored at −80°C.
Soluble cercarial E/S products were obtained from fresh cercariae (max. 1 h) transferred into 50 ml of water in Falcon tubes. Secretion was stimulated by addition of the respective stimuli: praziquantel (0.1 μg/ml), linoleic acid (0.1 μg/ml), linolenic acid (0.1 μg/ml) or calcium ionophore A23187 (1 μg/ml) for 30 min. Cercarial debris was then removed using paper-filtration and the products were concentrated by ultrafiltration (Centricon Plus tubes MWCO 10 kDa, Millipore) at 4°C to the final volume of 100 μl. Protein concentration was determined by bicinchoninic acid method (BCA-1, Sigma). To avoid autoproteolysis of the samples for the purpose of electrophoretic profiles of the E/S products, a mixture of protease inhibitors 50 μM leupeptin, 1 μM N-tosyl-lysine chloromethyl ketone (TLCK) and 10 μM trans-epoxysuccinyl-L-leucyl-amido(4-guanidino)butane (E-64) was added or the sample was denaturated by different concentrations of sodium dodecyl sulfate (SDS).
Antibody production against E/S products of cercariae of T. szidati
Two Balb/c mice were immunized subcutaneously with four doses of heat-inactivated (70°C for 30 min) cercarial E/S products obtained by praziquantel induction (35 μg of total protein each). First injection contained 25% of complete Freund’s adjuvant and 25% of incomplete adjuvant. The other three contained 50% of incomplete adjuvant. The interval between the first and second injections was 14 days, the other two were 7 days. Sera were collected 7 days after the last injection. Control sera were taken from the same individuals two weeks prior to immunization.
Electrophoretic procedures
Electrophoretic and proteolytic profiles of cercarial E/S products obtained by induction with different compounds were compared between the two species studied. Samples were run under denaturating (SDS) conditions employing the MiniProtean-3 apparatus (Bio-Rad). Reducing buffer, when used, contained 15 mM 2-mercaptoethanol. Gels were stained by Coomassie Brilliant Blue (CBB) or silver stained. For Western and ligand blotting, the proteins were electroblotted to nitrocellulose membranes.
Tests for proteolytic activity
For preliminary detection of proteolytic activity, the E/S products of both species were dropped onto 10% polyacrylamide gels copolymerized with 0.1% gelatin and TBS. Each 5 μl sample contained 0.5 μg of total protein. Inhibition tests were performed with a set of protease inhibitors added individually or in various combinations: 4 μg/ml aprotinin, 20 μM E-64, 2μM pepstatin, 2 mM phenyl-methyl-sulfonyl-fluoride (PMSF), 0.2 mM TLCK, 0.2 mM N-p-tosyl-L-phenylalanine chloromethyl ketone (TPCK), 80 mM ethylenediaminetetraacetic acid (EDTA). The gels were incubated overnight at 37°C in a moist chamber, then washed for 5 min in water and stained in CBB.
To see direct proteolytic effect of cercarial gland products, the cercariae were dropped onto an exposed and developed photographic film and praziquantel was added at the concentration of 0.1 μg/ml. After a 30 min incubation in a moist chamber, the lysis of gelatin film emulsion was evaluated under the microscope.
Zymographic analysis of protease activity was performed using SDS-PAGE. The gels were copolymerized with 0.1% gelatin. The samples were not boiled but mixed with nonreducing sample buffer for 30 min prior to loading. Following electrophoresis, the gels were washed 3×10 min in TBS containing 1% Triton X-100 to remove SDS. The final wash was in TBS alone for 5 min. The overnight incubation was performed at 37°C in TBS. Gels were finally stained in CBB.
Fluorometric assays for cysteine protease activity
Attention was focused on cysteine protease activity in homogenates of both species. It was measured using synthetic benzyloxycarbonyl-phenylalanine-arginine-7-amido-4-methylcoumarine substrate (Z-Phe-Arg-AMC) (Bachem, Switzerland), routinely used to measure the activities of cathepsins L and B (Barrett and Kirschke 1981). Assays were carried out in triplicate, in 96-well black plates (Nunc, Denmark). Release of AMC was measured at excitation and emission wavelengths of 350 and 460 nm, respectively, in Spex Fluoromax 3 (Jobin Yvon Horiba, France) at room temperature. For activation of cysteine proteases, solution of 5 mM dithiothreitol (DTT) was added to the sample. The reaction was carried out in 200 μl/well of 0.1 M citrate-phosphate buffer pH 5.5 containing 300 mM NaCl, 200 μM peptide-AMC substrate and 2 μg of cercarial homogenate (final values). Inhibition of protease activity was tested with 10 μM broad-spectrum cysteine protease irreversible inhibitor E-64 and 10 μM N–(L-3-trans-propylcarbamoyloxirane-2carbonyl)-isoleucyl-prolyl-OH (CA-074, selective inhibitor of cathepsin B) (Towatari et al. 1991) added to the sample 20 min prior to the substrate. Equal volumes of buffer instead of cercarial extracts served as controls.
Western and ligand blotting
Transblotted E/S products of T. szidati on nitrocellulose membranes were blocked for 2 h at RT using 5% nonfat milk in TBS containing 0.05% Tween-20 (T-TBS). Then, the preimmune or immune sera against the E/S products were applied for 1 h (dilution 1:50, 1:100, 1:500 and 1:800). Other membranes with transblotted cercarial homogenates from both species were examined using rabbit monospecific polyclonal antibodies against Schistosoma mansoni cercarial elastase (1:100). Membrane strips with S. mansoni proteins served as positive controls. The membranes were washed 3×5 min in T-TBS and then overlayed with peroxidase labelled swine antirabbit secondary antibodies (1:1,000, Sevac Prague) and washed again. Reaction was developed in 0.1 M Tris–HCl buffer pH 7.6 containing 0.6 mM 3,3′-diaminobenzidine and 0.01% hydrogen peroxide. Alternatively, some membranes were developed using the Opti-4CN Substrate Kit (Bio-Rad).
For ligand blotting, a biotinylated analogue of the cysteine protease inhibitor E-64 (DCG-04—Greenbaum et al. 2000) was applied to cercarial homogenates and E/S products of both species. It binds covalently to the active site of cysteine proteases and thus enables their detection on blots using avidin conjugates. The method was performed as described previously for cercariae of Diplostomum pseudospathaceum (Mikeš and Man 2003). Homogenates and E/S products containing 2 μg of total protein were incubated with 5 μM DCG-04. Samples preincubated with E-64 (10 μM) for 20 min and samples without DCG-04 were used as controls of reaction specificity.
Immunohistochemistry
Cercariae of both species were fixed in Bouin’s solution overnight at 4°C, then washed several times in 70% alcohol to remove picric acid, then in phosphate buffered saline (PBS) pH 7.0, and were finally embedded into JB-4TM Plus resin (Polysciences) according to manufacturer’s instructions and sectioned (2 μm). Sections were blocked by 5% bovine serumalbumin (BSA) in T-TBS for 1 h at RT. To discover the localization of cercarial E/S products, sera from immunized mice (see above) were applied to the sections (diluted 1:50 in T-TBS). To detect crossreactivity of antibodies against S. mansoni cercarial elastase with bird schistosome penetration glands, the rabbit anti-elastase serum was applied at final dilution 1:100. Moreover, reaction of cercarial glands was tested with the antibodies against the highly glycosylated keyhole limpet (Megathura crenulata) hemocyanin (H-0892, Sigma) as it could serve as an evidence for the presence of polysaccharides (Linder 1986). These antibodies were diluted 1:100. Sections were incubated with primary antibodies for 1 h, then washed 3×5 min in T-TBS and overlayed by corresponding secondary FITC-labelled antibodies diluted 1:500 for 45 min. Following the final 3×5 min wash the sections were mounted into buffered glycerin and examined under the fluorescence microscope.
Results
Dynamics of gland secretion
During the action of the four stimulants, these main categories of cercarial behaviour were recognized and further evaluated: (1) attempts to attach to the slide accompanied by crawling movement and production of “kissing marks”, (2) rapid emptying of penetration glands by settled larvae connected often with forceful shaking, spasms and release of the tail, (3) retardation of activity and (4) cease of activity, death, vacuolization of tegument. The time records in Tables 1, 2 and 3 have approximative character as the behaviour of cercariae was not uniform and slightly differed among individuals. Therefore the data were not statistically treated and served mainly as a starting point for designing methods for collection of either soluble or insoluble secretions.
Table 1 shows that cercariae of T. regenti were more resistant than T. szidati with respect to the action of praziquantel used at lower concentrations. At higher concentrations the differences between the two species were not so distinct, but still clearly visible. The concentration of 0.1 μg/ml was chosen as optimal for quantitative collection of E/S products. In the case of calcium ionophore A23187, the difference between the two species was even more obvious (Table 2) . T. regenti did not react to lower concentrations and higher concentrations or prolonged time had to be used for stimulation of gland emptying. For T. szidati, the concentration of 1 μg/ml was chosen as optimal for quantitative collection of E/S products. In both species, a different pattern of behaviour was observed using the ionophore, compared to praziquantel. The most conspicuous was the first phase of rapid swimming. Addition of CaCl2 to the ionophore had no obvious effect on cercarial behaviour. Conversely, addition of a chelator of calcium ions ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA) obviously inhibited the effect of the ionophore on cercarial behaviour (not shown).
Using the two unsaturated linoleic and linolenic fatty acids, known as natural stimulants of cercarial penetration, another pattern of behaviour was observed (Table 3). After stimulant addition, the cercariae attempted to attach immediately to the slides and gland secretion was started, followed by retardation of activity. After some minutes the cercariae started to crawl over the surface producing characteristic four-line “kissing marks” (marks of the products leaking from the gland openings on the head organ) (see Fig. 1a). Finally, their activity retarded and ceased. Concentration of 0.1 μg/ml was chosen for quantitative collection of E/S products.
Cercarial penetration gland products of T. szidati. a Four lines of gland products released from the four duct openings on the apex of the head organ. b Dark deposits of calcium detected by potassium oxalate in circumacetabular glands. c Lysis of gelatin emulsion on a photographic film by the released gland products after stimulation with praziquantel
Staining of cercariae and their gland secretions
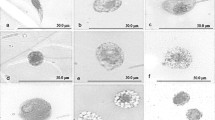
From the set of 15 dyes used, 14 stained either the glands or their products. The only exception was alcian blue (used in histology for detection of acid mucopolysacccharides). Aldehyde fuchsin stained uniformly the whole body, the glands could not be recognized. Lithium carmine staining the postacetabular glands, alizarin staining the circumacetabular glands and apomorphine staining differentially both types of glands (green postacetabular and brownish circumacetabular) were recognized as the selective dyes. The results are shown in Table 4 and Figs. 2a–c. However, the ease of the methods for lithium carmine and alizarin and their differential staining of both glands and products predetermined their maximal usefulness in such experiments. The results showed that the insoluble gland products are stained by both lithium carmine and alizarin, thus demonstrating that secretions of both gland types are released simultaneously upon stimulation by praziquantel. Detection of calcium deposits showed the presence of a significant amount of this element in circumacetabular gland cells manifested by the creation of calcium oxalate crystals (Fig. 1b).
Staining of penetration glands and insoluble gland products in cercariae of T. szidati. a Postacetabular glands and their products stained by lithium carmine after stimulation by praziquantel. b Circumacetabular glands and their products stained by alizarin after stimulation by praziquantel. c Staining of postacetabular (black) and circumacetabular (dark grey) glands by apomorphine
Detection of lectin-like activities and glycosubstances in the insoluble gland products
From the set of four saccharide compounds known to bind to cercarial lectins (Horák et al. 1997; Mikeš and Horák 2001), FITC-labelled laminarin and heparin covalently attached to Sepharose beads showed clearly positive reaction with adhered gland products which could be inhibited by corresponding non-labelled substances (Fig. 3a–c). Binding of biotinylated glycosaminoglycans was detected by FITC-labelled avidin. As FITC-avidin alone reacted strongly with the products, even after blocking with BSA or non-labelled avidin, results obtained with the biotinylated compounds could not be interpreted.
The same phenomenon was observed during characterization of glycosubstances in the adhered products by FITC-labelled lectins. All of the lectins shown in Table 5 reacted with the products, but in controls their binding could not be inhibited by corresponding specific saccharide inhibitors.
Collection of E/S products after stimulation
The yield of total protein obtained after stimulation of T. szidati cercariae by the four compounds usually ranged between 100–150 μg per 10,000 cercariae approximately. No significant differences were observed among particular stimulants. Cercariae of T. regenti were not used as their sensitivity to praziquantel and calcium ionophore was questionable.
Electrophoretic profiles of soluble E/S products of T. szidati
Protein profiles of the soluble gland products comprised of several bands over a whole spectrum of molecular sizes. No significant differences were observed among profiles obtained after stimulation with different compounds if the stimulation experiments, collection and treatment of the products were run simultaneously according to identical protocol. The most rich profiles were obtained when the collected products were immediatelly denatured by SDS (Fig. 4). Usage of different protease inhibitors or their mixtures and prolonged storage or repeated freezing/thawing have been giving inconsistent results, thus indicating rapid autodegradation of the samples caused by multiple endogenous proteolytic enzymes (not shown).
Electrophoretic protein profiles of E/S products after stimulation of T. szidati cercariae by four stimulants of penetration gland emptying. Ten percentage of polyacrylamide gel, silver stained, 20 μl of E/S products per lane (corresponds to 10 μg of protein measured immediately after gland stimulation, before ultrafiltration and addition of SDS). Lanes 1–4 treated by 0.4% SDS, lanes 1′–4′ treated by 0.04% SDS, lanes 1*–4* non-treated after collection. Loss of protein due to autoproteolysis can be seen with decreasing concentration of SDS. Lane 1 after stimulation by linoleic acid, lane 2 by linolenic acid, lane 3 by praziquantel, lane 4 by calcium ionophore CA23187
Dot and film tests for proteolytic activity in soluble E/S products of T. szidati
Soluble E/S products clearly exhibited gelatinolytic activity. Inhibition studies showed that among the protease inhibitors used, only EDTA significantly lowered this activity at the concentration of 20 mM (Fig. 5). Combinations of inhibitors were not efficient unless containing EDTA. The secreted cercarial products exhibited also a distinct gelatinolysis of the photographic film emulsion (Fig. 1c).
Zymographic analysis of proteolytic activity in soluble E/S products of T. szidati
Zymographic profiles of E/S products obtained after stimulation of cercariae by the four compounds revealed several bands of gelatin lysis. No differences were recorded between particular stimulants (Fig. 6). Proteolytic activities of individual bands were strongly affected by the mode of sample treatment and storage. Especially, the bands <50 kDa were very sensitive. Thus the picture was not always the same when comparing successive experiments. Fig. 6 shows the most complete type of zymogram obtained repeatedly and the impact of SDS concentration on proteolytic activities of particular bands.
Zymographic profiles of E/S products after stimulation of T. szidati cercariae by four stimulants of penetration gland emptying. 10% polyacrylamide-gelatin gel, coomassie blue stained, 20 μl of E/S products per lane (corresponds to 10 μg of protein measured immediately after gland stimulation, before ultrafiltration and addition of SDS). Lanes 1–4 treated by 0.4% SDS, lanes 1′–4′ treated by 0.04% SDS, lanes 1*-4* non-treated after collection. Loss of protease activity caused by SDS treatment can be seen with increasing concentration of SDS. Lane 1 after stimulation by linoleic acid, lane 2 by linolenic acid, lane 3 by praziquantel, lane 4 by calcium ionophore CA23187. Arrowhead shows a putative 31 kDa cysteine protease
Fluorometry
A cysteine peptidase activity was revealed in cercarial extracts of both T. regenti and T. szidati using the fluorogenic peptide substrate Z-Phe-Arg-AMC. The activity was comparable in both the species, reaching in T. szidati up to 79% of that recorded in T. regenti. A 96% inhibition of the activity in both the species was reached using 10 μM E-64. The less potent inhibitor was 10 μM CA-074 (90% in T. regenti and 88% in T. szidati) (Fig. 7). As CA-074 is a selective inhibitor of cathepsin B, whereas E-64 is an inhibitor of both cathepsins L and B, it was documented that cathepsin B-like activity predominates over cathepsin L-like activity in T. szidati cercarial extracts.
Fluorographic detection of cysteine protease activity in cercarial extracts of T. szidati and T. regenti with Z-phenylalanine-arginine-7-amido-4-methylcoumarine as a substrate. FR activity in samples without inhibitors. E-64 inhibition of the activity by broad-spectrum cysteine protease inhibitor E-64. CA-074 inhibition of the activity by cathepsin B-specific inhibitor CA-074
Blotting
Sera from mice immunized with E/S products of T. szidati reacted with the complete spectrum of proteins on the membrane resulting in diffuse coloration of the membrane strip (not shown). No clear bands could be distinguished. Rabbit antibodies against S. mansoni cercarial elastase exhibited a strong reaction with the 28 kDa S. mansoni elastase but did not react with proteins of T. szidati and T. regenti cercariae (Fig. 8).
Western blot of proteins of T. szidati, T. regenti and S. mansoni cercariae with rabbit antiserum against the 28 kDa cercarial elastase of S. mansoni. Twenty microgram of cercarial protein extract loaded per lane. Lanes 1–3 immune serum, lanes 4–6 control serum. 1 and 4 T. szidati, 2 and 5 T. regenti, 3 and 6 S. mansoni. No reaction was observed with the proteins of bird schistosomes
Binding of DCG-04 resulted in a clearly visible 31 kDa band in the case of T. szidati and a 33 kDa band of T. regenti cercarial extracts, thus indicating the presence of a cysteine protease. Controls without DCG-04 and with E-64 preincubated sample showed no reaction in these regions (Fig. 9). The same DCG-04 binding pattern was observed in cercarial E/S products after stimulation with praziquantel (not shown).
Binding of a cysteine protease-specific probe DCG-04 on the blot of cercarial proteins of T. regenti and T. szidati. 2 μg of protein per lane, 5 μM DCG-04. Lanes 1–3 T. regenti, lanes 4–6 T. szidati. 1 and 3 positive reaction with DCG-04. 2 and 5 reaction with DCG-04 blocked by cysteine protease inhibitor E-64. 3 and 6 controls of non-specific avidin-Px binding without DCG-04. Arrowheads show the detected cysteine proteases of T. regenti (33 kDa, lane 1) and T. szidati (31 kDa, lane 4)
Immunohistochemistry
Antibodies raised against T. szidati E/S products reacted with both types of penetration glands of T. szidati cercariae. Even a stronger reaction was observed with the surface of cercariae (Fig. 10a, b). A cross reaction was also observed with both the glands and the surface of T. regenti cercariae (not shown). The results demonstrated that the cercarial E/S products derived not only from penetration glands but also from tegumental antigens or glycocalyx. No binding of antibodies against S. mansoni elastase and against keyhole limpet hemocyanin occurred in the cercarial glands of both Trichobilharzia species (not shown).
Discussion
All stimulants used in this study have been shown as potent inducers of penetration gland emptying in the case of both Trichobilharzia species. The different sensitivity of the two bird schistosomes to praziquantel is an interesting phenomenon, which could have an impact on the treatment of the disease caused by neuropathogenic T. regenti in birds, if the lower sensitivity is retained in schistosomula or adult flukes. Preliminary laboratory results with treatment of T. regenti infections indicate that praziquantel is ineffective (Blažová et al. 2004). This could be of great importance as cercariae of bird schistosomes cause swimmer’s itch in humans (for review see Horák et al. 2002) and currently attempts proceed to eradicate the flukes from the environment in important recreational areas. Among other things, the chemotherapeutical treatment of infected wild ducks is also considered (Müller et al. 1993). The conspicuous difference in response to the calcium ionophore can be correlated with the lower sensitivity of T. regenti to praziquantel. Although the details of the mode of action of praziquantel are not known, it is supposed to cause calcium influx into the tissues of trematodes (Day et al. 1992). The low sensitivity to these two compounds was also the main reason for T. regenti not being used in some experiments.
The in vitro response of cercariae to unsaturated fatty acids seems to correspond to the natural behaviour upon contact with host skin (for review see Haas 2003). After the initial attachment to the ground and rapid gland emptying, the cercariae started to crawl over the surface, probably in search of a site for penetration—the crawling movement was observed in vivo in the case of cercariae of S. mansoni (Stirewalt and Kruidenier 1961).
The optimal concentrations of the stimulants used for quantitative collection of the E/S products and the period of their action were estimated regarding the amount of proteins obtained and the condition of cercariae, from the “on-slide” experiments, in effort to minimize gland product contamination by proteins from the rest of the body. In case of all four stimulants, various deformations of cercarial bodies and surface were observed after prolonged incubations, mainly in the anterior part surrounding the gland openings. This indicated that the gland products could be involved in tegument transformation during cercarial penetration and development of schistosomula as also suggested for S. mansoni (Marikovsky et al. 1990).
Although differential gland emptying in schistosomes has been described by some authors (Haas et al. 1997), based on our results, we believe that, both types of glands empty simultaneously in the two species investigated. In our experiments, the insoluble gland products adhered to the slides were stained by both the dyes specific for the particular gland types. We are aware that in vitro, the system could work in a different way than in vivo, and also the concentrations of stimulants had been unnaturally high. But there is a suggestion based on a hypothesis that the two gland types have evolved as two separate compartments containing some compounds which may become active when interfused during the release. This could be an advantage for storage of high amounts of biologically active molecules which might be able to erode cercarial structures in an active state (e.g. proteases). Thus we can also hypothesize on an additional putative role of a high amount of calcium in the circumacetabular gland cells. This might serve as a cross-linker of the mucopolysaccharides produced by the postacetabular cells, producing the sticky glue-like substance enabling tight cercarial attachment. As the released substance is thick and relatively wash-resistant, it would be more advantageous to produce a washy product which will “polymerize” outside the body. But this hypothesis requires further testing. We believe that the presence of compounds from both gland types in the insoluble products supports our hypothesis of two complementary compartments. Another support can be found in detailed description of the organization of S. mansoni gland ducts by Dorsey and Stirewalt (1971): about the level of acetabulum, the three postacetabular ducts on each side of cercarial body form a bundle. In front of the intestinal caeca, two preacetabular ducts join the bundle, which, at this point, contains five ducts. Within the head organ, the two bundles split into four. Each of the two outer bundles contains two post- and one circumacetabular duct. The two inner bundles possess one post- and one circumacetabular duct. There are four clearly visible openings at the apex of the cercariae covered by tegument elaborated into folds with ciliated bulbous rims melting when gland secretion starts (also see Fig. 1a for four-line marks of gland products released from the duct openings of Trichobilharzia). Thus, it is likely that the products from both types of ducts can leak out all at once.
The character of the insoluble products in the two Trichobilharzia species seems to be different from that of S. mansoni, based on the non-reactivity with alcian blue and antibodies against keyhole limpet hemocyanin. This could reflect a different saccharide composition of the substance. Unfortunately, binding of labelled lectins to the products in order to characterize their saccharide composition did not produce interpretable results.
The presence of lectin-like activity in postacetabular penetration glands of T. szidati has been formerly described by Horák et al. (1997). Here we confirmed the presence of this activity in the adhered gland products in both the investigated Trichobilharzia species. The binding of laminarin and heparin corresponds with the published results. Strong non-inhibitable binding of lectins and avidin to the gland products shows that besides specific binding interactions, non-specific binding can also occur, probably mediated by electrostatic or hydrophobic interactions.
The electrophoretic profiles of the E/S products after stimulation of cercariae by four different compounds revealed that, in our system, the gland emptying resulted in identical protein spectra obtained for further analysis. This also indicated that products of both gland types are probably present in the soluble material. Therefore, for further quantitative collection of the products, praziquantel was chosen as the cheapest alternative. Owing to its solubility and relative miscibility with water solutions, the desired concentrations could be easily reached even in larger volumes. The disadvantage of the two fatty acids was the formation of emulsions, so that uniform concentration could hardly be reached in larger volumes. Calcium ionophore A23187 was the less effective stimulant and also the most expensive one, therefore its use cannot be recommended. The edification obtained from these experiments is that routine quantitative collection of the E/S products from stimulated cercariae is problematic and strictly requires constant approach and rigid abidement of the method, otherwise it could be a source of incomparable results.
Preliminary screening using the dot-gel zymographic method revealed that multiple proteolytic enzymes are present in the E/S products of stimulated T. szidati cercariae, some of them being probably calcium-dependent. However, the full inhibition of proteolytic activity in the samples by high concentrations of EDTA alone seems to be rather an artefact which we have not been able to explain.
As in the case of whole protein profiles, the proteolytic zymographic profiles of the E/S products exhibited identical patterns independent of the kind of stimulant used thus indicating that no selective gland induction occurs among the four stimulants used. The zymographic method confirmed its limited applicability in protease detection as the proteases in cercarial samples were very sensitive to treatment by SDS and to the general procedure. The two bands of gelatin lysis between 25–30 kDa probably correspond to the proteolytic activity detected by Bahgat and Ruppel (2002) in zymographic gels. It has been believed by the authors to be an elastase, although no tests have been performed with elastin as a substrate and the activity of Trichobilharzia protease was rather trypsin-like, contrary to S. mansoni elastase used for comparison, which is chymotrypsin-like (Salter et al. 2000). From S. mansoni it is known, that a trypsin-like serine protease activity in cercarial homogenates is caused by snail contamination (Salter et al. 2000). The band of lysis around 31 kDa seems to correspond to the cysteine protease of T. szidati detected by ligand blotting. It could as well be the cathepsin B-like protease detected in cercarial homogenates by fluorometry.
The reaction on Western blots of sera raised against the E/S products after stimulation of cercariae by praziquantel did not disclose any significant protein band which could be further characterized. This was probably caused by the complexity of the sample used for immunization and proteolytic auto-degradation of the sample (which could not be treated by protease inhibitors or SDS prior to immunization) or by the presence of tegumental proteins. Also, the reaction of the sera with sections of cercariae clearly evidenced that, besides gland proteins, a significant amount of tegumental antigens was present in the E/S products. These are probably even more antigenic as the cercarial glycocalyx is highly glycosylated (e.g. Horák 1995). These results support the observations that the penetration gland products of schistosomes may be involved in glycocalyx shedding during penetration into a host, thus enabling the immune evasion by transforming cercaria/schistosomulum (e.g. Marikovsky et al 1990; Horák et al. 1998). The Western blots with the antibodies against S. mansoni elastase did not exhibit any cross-reaction with proteins from T. szidati and T. regenti. This indicates that in the two bird schistosomes, a homologue of S. mansoni elastase is not present and the serine protease activity around 28 kDa detected by Bahgat and Ruppel (2002) is most probably a product of a different gene or contamination of the sample by snail proteases. No reaction of the anti-elastase serum with sections of T. szidati is in contrast to the result of Bahgat et al. (2001).
In conclusion, we can evaluate the methods used here for collection of schistosome penetration gland products as a usable tool for the studies of molecules involved in the penetration process, e.g., proteolytic enzymes and lectins. However, it has been shown, that the proteins in E/S products after stimulation originated not only in penetration glands but also in cercarial tegument. Also, some limitations have been revealed during our experiments, the main one being a questionable reproducibility of the results among different laboratories, connected with an extreme sensitivity of samples to the mode of treatment.
References
Austin FG, Stirewalt MA, Danzinger RE (1972) Stimulatory effect of rat skin lipid fractions on cercarial penetration behavior. Exp Parasitol 31:217–224
Austin FG, Frappaolo P, Gilbert B, Landis W, da Rosa MN, Stirewalt MA (1974) Further studies of Schistosoma mansoni cercarial stimulation by crude egg lecithin and other lipids. Parasitology 69:455–463
Bahgat M, Ruppel A (2002) Biochemical comparison of the serine protease (elastase) activities in cercarial secretions from Trichobilharzia ocellata and Schistosoma mansoni. Parasitol Res 88:495–500
Bahgat M, Francklow K, Doenhoff MJ, Li YL, Ramzy RMR, Kirsten C, Ruppel A (2001) Infection induces antibodies against the cercarial secretions, but not against the cercarial elastases of Schistosoma mansoni, Schistosoma haematobium, Schistosoma japonicum and Trichobilharzia ocellata. Parasite Immunol 23:557–565
Barrett AJ, Kirschke H (1981) Cathepsin B, cathepsin H and cathepsin L. Methods Enzymol 80:535–561
Blažová K, Kolářová L, Horák P, Frýzková M (2004) Development of Trichobilharzia regenti in ducks: new routes of transmission and treatment with praziquantel (in Czech). Czech and Slovak Parasitological Days, Ostravice, Czech Republic p 2, 17– 21 May 2004 (Abstract Book)
Bruckner DA (1974) Differentiation of pre- and postacetabular glands of schistosome cercariae using apomorphine as a stain. J Parasitol 60:752–756
Chavez-Olortegui C, Resende M, Tavares CAP (1992) Purification and characterization of a 47 kDa protease from Schistosoma mansoni cercarial secretion. Parasitology 105:211–218
Coles GC, Jansson HB, Zuckerman BM (1988) Lectin studies of surface carbohydrates and induction of gland secretion in the free-living stages of Schistosoma mansoni. J Chem Ecol 14:691–700
Dalton JP, Clough KA, Jones MK, Brindley PJ (1997) The cysteine proteinases of Schistosoma mansoni cercariae. Parasitology 114:105–112
Day TA, Bennett JL, Pax RA (1992) Praziquantel: the enigmatic antiparasitic. Parasitol Today 8:342–344
Dorsey CH, Stirewalt MA (1971) Schistosoma mansoni: fine structure of cercarial acetabular glands. Exp Parasitol 30:199–214
Dorsey CH, Stirewalt MA (1977) Schistosoma mansoni: localization of calcium-detecting reagents in electron-lucent areas of specific preacetabular gland granules. Z Parasitenkd 54:165–173
Dresden MH, Asch HL (1977) Calcium carbonate content of the preacetabular glands of Schistosoma mansoni cercariae. J Parasitol 63:163–165
Dresden MH, Edlin EM (1975) Schistosoma mansoni: calcium content of cercariae and its effects on protease activity in vitro. J Parasitol61:398–402
Greenbaum D, Medzihradszky KF, Burlingame A, Bogyo M (2000) Epoxide electrophiles as activity-dependent cysteine protease profiling and discovery tools. Chem Biol 7:569–581
Haas W (2003) Parasitic worms: strategies of host finding, recognition and invasion. Zoology 106:349–364
Haas W, Schmitt R (1982) Characterization of chemical stimuli for the penetration of Schistosoma mansoni cercariae. I. Effective substances, hosts specificity. Z Parasitenkd 66:293–307
Haas W, van de Roemer A (1998) Invasion of the vertebrate skin by cercariae of Trichobilharzia ocellata: penetration processes and stimulating host signals. Parasitol Res 84:787–795
Haas W, Diekhoff D, Koch K, Schmalfuss G, Loy C (1997) Schistosoma mansoni cercariae: stimulation of acetabular gland secretion is adapted to the chemical composition of mammalian skin. J Parasitol 83:1079–1085
Hara I, Hara S, Fusco AC, Shibuya T (1993) Role of calcium ion in Schistosoma mansoni cercarial tail loss induced by unsaturated fatty acids. Am Soc Parasitol 79:504–509
Horák P (1995) Developmentally regulated expression of surface carbohydrate residues on larval stages of the avian schistosome Trichobilharzia szidati. Folia Parasitol 42:255–265
Horák P, Grubhoffer L, Mikeš L, Tichá M (1997) Lectins of Trichobilharzia szidati cercariae. Parasite 1:27–35
Horák P, Kovář L, Kolářová L, Nebesářová J (1998) Cercaria-schistosomulum surface transformation of Trichobilharzia szidati and its putative immunological impact. Parasitology 116:139–147
Horák P, Kolářová L, Adema CM (2002) Biology of the schistosome genus Trichobilharzia. Adv Parasitol 52:155–233
Landsperger WJ, Stirewalt MA, Dresden MH (1982) Purification and properties of a proteolytic enzyme from the cercariae of the human trematode parasite Schistosoma mansoni. Biochem J 201:137–144
Linder E (1985) Schistosoma mansoni: visualization with fluorescent lectins of secretions and surface carbohydrates of living cercariae. Exp Parasitol 59:307–312
Linder E (1986) Fluorochrome-labelled lectins reveal secreted glycoconjugates of schistosome larvae. Parasitol Today 2:219–221
MacInnis AJ (1969) Identification of chemicals triggering cercarial penetration responses of Schistosoma mansoni. Nature 224:1221–1222
Marikovsky M, Arnon R, Fishelson Z (1988) Proteases secreted by transforming schistosomula of Schistosoma mansoni promote resistance to killing by complement. J Immunol 141:273–278
Marikovsky M, Arnon R, Fishelson Z (1990) Schistosoma mansoni: localization of the 28 kDa secreted protease in cercaria. Parasite Immunol 12:389–401
Matsumura K, Shimada M, Sato K, Aoki Y (1990) Praziquantel-induced secretion of proteolytic enzyme from Schistosoma mansoni cercariae. J Parasitol 76:436–438
Matsumura K, Mitsui Y, Sato K, Sakamoto M, Aoki Y (1991) Schistosoma mansoni: possible involvement of protein kinase C in linoleic acid-induced proteolytic enzyme release from cercariae. Exp Parasitol 72:311–320
McKerrow JH, Pino-Heiss S, Linquist R, Werb Z (1985) Purification and characterization of an elastinolytic proteinase secreted by cercariae of Schistosoma mansoni. J Biol Chem 260:3703–3707
Mikeš L, Horák P (2001) A protein with lectin activity in penetration glands of Diplostomum pseudospathaceum cercariae. Int J Parasitol 31:245–252
Mikeš L, Man P (2003) Purification and characterization of a saccharide-binding protein from penetration glands of Diplostomum pseudospathaceum—a bifunctional molecule with cysteine protease activity. Parasitology 127:69–77
Modha J, Redman CA, Thornhill JA, Kusel JR (1998) Schistosomes: unanswered questions on the basic biology of the host-parasite relationship. Parasitol Today 14:396–401
Müller V, Kimmig P, Frank W (1993) The effect of praziquantel on Trichobilharzia (Digenea, Schistosomatidae), a causative agent of swimmer’s dermatitis in man (in German). Appl Parasitol 34:187–201
Mussini I, Magreth A, Salviati G (1972) On the criteria for oxalate in sarcoplasmatic reticulum fragments. J Ultrastruct Res 38:459–465
Pierrot C, Godin C, Liu JL, Capron A, Khalife J (1996) Schistosoma mansoni elastase: an immune target regulated during the parasite life-cycle. Parasitology 113:519–526
Rudolfová J, Hampl V, Bayssade-Dufour C, Lockyer AE, Littlewood DTJ, Horák P (2005) Validity reassessment of Trichobilharzia species using Lymnaea stagnalis as the intermediate host. Parasitol Res 95:79–89
Salter JP, Lim KC, Hansell E, Hsieh I, McKerrow JH (2000) Schistosome invasion of human skin and degradation of dermal elastin are mediated by a single serine protease. J Biol Chem 275:38667–38673
Salter JP, Choe Y, Albrecht H, Franklin C, Lim KC, Craik CS, McKerrow JH (2002) Cercarial elastase is encoded by a functionally conserved gene family across multiple species of schistosomes. J Biol Chem 277:24618–24624
Shiff CJ, Cmelik SHW, Ley HE, Kriel RL (1972) The influence of human skin lipids on the cercarial penetration responses of Schistosoma haematobium and Schistosoma mansoni. J Parasitol 58:476–480
Skelly PJ, Shoemaker CB (2001) Schistosoma mansoni proteases Sm31 (cathepsin B) and Sm32 (legumain) are expressed in the cecum and protonephridia of cercariae. J Parasitol 87:1218–1221
Stirewalt MA (1974) Schistosoma mansoni: cercaria to schistosomulum. Adv Parasitol 12:115–182
Stirewalt MA, Kruidenier FJ (1961) Activity of the acetabular secretory apparatus of cercariae of Schistosoma mansoni under experimental conditions. Exp Parasitol 11:191–211
Towatari T, Nikawa T, Murata M, Yokoo C, Tamai M, Hanada K, Katunuma N (1991) Novel epoxysuccinyl peptides. A selective inhibitor of cathepsin B, in vivo. FEBS Lett 280:311–315
Acknowledgements
We wish to thank Prof. M. Doenhoff, University of Wales, Bangor, UK and Dr. A. Mountford, University of York, UK, for provision of S. mansoni cercariae, E/S products and antielastase antibodies. Our grateful thanks belong also to Dr. C. Caffrey and Dr. D. Greenbaum, Sandler Center for Basic Research in Parasitic Diseases, UCSF, USA, for the supply of DCG-04 cysteine protease probe, and Prof. M. Tichá, Department of Biochemistry, Charles University Prague, for biotinylated glycosaminoglycans. The project was supported by the grants of the Grant Agency of the Czech Republic no. 524/04/P082 and 524/03/1263, Grant Agency of the Charles University no. 263/2004/B/Bio, the Wellcome Trust Collaborative Research Initiative Grant no. 072255 and the grant of the Ministry of Education of the Czech Republic no. 0021620828. The experiments presented in this paper comply with current laws of the Czech Republic.
Author information
Authors and Affiliations
Corresponding author
Additional information
The project was supported by the following grants: Grant Agency of the Czech Republic no. 524/04/P082 and 524/03/1263, Grant Agency of the Charles University no. 263/2004/B/Bio, the Wellcome Trust Collaborative Research Initiative Grant no. 072255 and the grant of the Ministry of Education of the Czech Republic no. 0021620828.
Rights and permissions
About this article
Cite this article
Mikeš, L., Zìdková, L., Kašný, M. et al. In vitro stimulation of penetration gland emptying by Trichobilharzia szidati and T. regenti (Schistosomatidae) cercariae. Quantitative collection and partial characterization of the products. Parasitol Res 96, 230–241 (2005). https://doi.org/10.1007/s00436-005-1347-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-005-1347-1