Abstract
Intestinal parasitism was studied in children of Trujillo (Peru) to create a prevention and control program. Fecal samples of 489 children were examined. The general prevalence of intestinal parasitosis was found to be 68%. The most frequent pathogenic enteroparasites were Giardia lamblia (26.4%), Cyclospora cayetanensis (13%), Hymenolepis nana (2%), Hymenolepis diminuta (1.6%), and Cryptosporidium spp. (1%). All these parasites appeared both in diarrheic and nondiarrheic children, except Cryptosporidium, which invariably caused diarrhea. Multiple parasitism was frequent, 45.6% of the children presenting two, three, or four intestinal parasites. Cryptosporidium was the only parasite that was not associated with the others. Only five children were affected of cryptosporidiosis, presenting explosive diarrhea, nausea, and vomiting. Cryptosporidium species and genotypes involved in the infantile cryptosporidiosis were determined by polymerase chain reaction-restriction fragment length polymorphism. Four children were parasitized by Cryptosporidium hominis and only one by Cryptosporidium parvum. Our results confirm that anthroponotic transmission of Cryptosporidium is predominant in Peru.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intestinal parasitism among children can be considered indicative of the socioeconomic status and sanitation levels of a population. The prevalence of enteroparasitoses is linked to both poor hygiene and inadequate sources of drinking water. In children, intestinal parasites are associated with deficient growth, impaired cognition, and death (WHO 1996). In San Juan de Miraflores, Peru, Berkman et al. (2002) studied the way in which stunted growth, diarrhea, and parasitic infection during infancy affect cognition in late childhood. This work showed parasitic infection and stunting during infancy having a strong adverse effect on cognitive function in late childhood associated with reduction in IQ scores after adjustment for socioeconomic, schooling, and other significant factors.
Therefore, the establishment of prevention programs, control, and treatment of intestinal parasites is fundamental in developing countries. In Peru, infections from intestinal parasites are frequent in children. Previous epidemiological surveys conducted in Trujillo established a prevalence of 30% for Giardia in children under 10 years of age with acute diarrhea (Vargas et al. 1987). In Puno (Maco et al. 2002), intestinal parasitosis was found to be 91.2%.
Cryptosporidium was first studied in Peru by Soave and Armstrong (1986), reporting 8.1% prevalence in Lima, using a total of 111 diarrheic stool samples. Chang (1987) described a case associated with acute diarrhea in Oxapampa, while Huaynalaya et al. (1988) detected the parasite in a female child in Esperanza. Black et al. (1989), having analyzed fecal samples from 153 children in a community near Lima, included Cryptosporidium among the agents causing diarrhea. Sarabia-Arce et al. (1990) determined cryptosporidiosis in 10% (24 of 248) of the children admitted to the rehydration ward at Cayetano Heredia University Hospital, Lima. These researchers stated that, in children hospitalized for diarrhea, Cryptosporidium parvum occurs more frequently in malnourished subjects. In Sterling et al. (1991), reported a study of 211 Peruvian children and their mothers comparing antibody titers in mother’s milk and incidence of cryptosporidiosis. There was no significant difference in the incidence (0.17, 0.19, and 0.38, respectively) or duration of infection among children regardless of the level of maternal antibodies.
According to Berkman et al. (2002), Giardia intestinalis incidence in Lima ranged from 0 to 4.8 episodes per year during infancy, 86% of children having at least one G. intestinalis episode during the first 2 years of life. The C. parvum incidence ranged from 0.56 to 0.61, with 3% of stool samples testing positive for C. parvum. Cryptosporidium isolated from different regions have different antigens, virulence, infectivity, and sensitivity to drugs and disinfectants (Fayer and Ungar 1986; Fayer et al. 2000). Therefore, for the design of control programs, it is vital to know the species and genotypes of Cryptosporidium in each region.
Two distinct C. parvum genotypes, genotype 1 (anthroponotic genotype) and genotype 2 (zoonotic genotype), have been recognized for some time to be responsible for human cryptosporidiosis. However, such species as Cryptosporidium felis and Cryptosporidium meleagridis, Cryptosporidium muris, and a dog genotype of C. parvum are also considered responsible for human cryptosporidiosis (Xiao et al. 2001; Palmer et al. 2003). Recently, Morgan et al. (2002) proposed a new species, Cryptosporidium hominis, to denote the human genotype. In Lima, C. hominis has been the most frequently detected species (Xiao et al. 2001; Cama et al. 2003).
The Spanish Agency for International Cooperation (Agencia Española de Cooperación Internacional) and the regional Agencia Andaluza de Cooperación Internacional have since January 2004 been financing a project of prevention, control, and treatment of intestinal parasites in Trujillo, to lower the prevalence of parasitic infections among children.
For this, it is essential to study the prevalence of intestinal parasites as well as the molecular epidemiology of some of these pathogens. Therefore, in the present work, we have analyzed the most frequent types of intestinal parasitism in children from three districts of Trujillo (Peru) as well as the species and genotypes of Cryptosporidium involved in infantile cryptosporidiosis, one of the gravest afflictions in undernourished children in developing countries (Fayer et al. 2000).
Materials and methods
Study site and sampling
Trujillo (Peru), capital of the department Libertad, has more than one million inhabitants, concentrated in city center with marginal populations on the periphery lacking a sanitary infrastructure.
Temperatures are constant throughout the year, at about 22°C, and relative humidity stays at roughly 70%, being close to the Pacific Ocean. Rainfall is sparse and restricted to winter.
The present study on intestinal parasites in children was conducted in three rural districts on the periphery of the city of Trujillo: El Porvenir, Buenos Aires, and La Esperanza, with very low socioeconomic and sanitary levels. El Porvenir has 18,757 inhabitants, Buenos Aires 8,656, and La Esperanza 12,404. The three districts have similar living conditions, in that not all the dwellings have running water, and thus, inhabitants get water from public fountains or water trucks, and most of the houses lack toilets.
Prior to the sampling, the sanitation staff of the health center in each district called a meeting where the study methods were verbally explained to the parents of the children to be studied. A questionnaire was administered to the parents to return with a fecal sample. The questionnaire asked the age of the child, the disease, and details on the diarrhea and its symptoms. After the fecal samples were turned in, the children were weighed and measured to complete the questionnaire.
From January to December 2004, stool samples were collected from children between 1 month and 9 years old to study intestinal parasites in the schools of each district.
Analysis
Samples were transported to the laboratory and preserved in potassium dichromate at 2.5% and kept at 4°C until macroscopically and microscopically examined. Macroscopic inspection determined the consistency and mucosity as well as the blood and fat contents of the samples. After examination under a binocular microscope, and afterward a light microscope, using lugol in some cases, samples were stained with Ziehl-Neelsen, Giemsa, and Heidenheim and analyzed by the Kato–Katz method for nematode ova (WHO 1991). Positive fecal samples for Cryptosporidium were processed to purify the oocysts by potassium bromide discontinuous gradient (Entrala et al. 2000). The Teleman concentration was used when other parasites were detected.
Data were compared between diarrheic and nondiarrheic children using χ 2 test. Prevalence of intestinal parasites was studied having a P value <0.02.
Molecular characterization of Cryptosporidium species and genotypes
DNA extraction
All the positive fecal samples for Cryptosporidium were processed for DNA extraction. Briefly, 200 μl of fecal sample in water was suspended in 200 μl of lysis buffer supplied in the QIAamp DNA mini kit (QIAGEN, USA). Oocysts were digested using the technique of Robertson et al. (1993). Genomic DNA was isolated by a QIAamp DNA stool mini-kit protocol (QIAGEN) directly from the fecal sample (200 μl/sample). DNA samples were stored at −20°C until further use.
Polymerase chain reaction-restriction fragment length polymorphism analysis
Cryptosporidium species and genotypes were determined by nested polymerase chain reaction (PCR) of an SSU rRNA gene fragment and restriction fragment length polymorphism (RFLP) analysis as previously described (Xiao et al. 1999a,b) but using the endonucleases SspI and VspI.
For the primary PCR step, a PCR product about 1,325 bp long was amplified by using primers 5′-TTCTAGAGCTAATACATGCG-3′ and 5′-CCCATTTCCTTCGAAACAGGA-3′ (Invitrogen, Spain). Each PCR mixture (total volume, 50 μl) contained 5 μl of 10× PCR buffer (BioRad, Spain), 6 mM MgCl2, each deoxynucleoside triphosphate at a concentration of 200 μM (BioRad), each primer at a concentration of 100 nM, 1.5 U of Taq polymerase (BioRad, Spain), 0.5 μl of DNA template, and 0.1 μg/μl of bovine serum albumin (BSA; BioRad). Each PCR was performed in a thermocycler (MyCycler, BioRad), under the conditions described by Xiao et al. (1999a,b). For the secondary PCR step, a PCR product 819–837 bp long (depending on the species) was amplified by using 0.5 μl of the primary PCR product and primers 5′-GGAAGGGTTGTATTTATTAGATAAAG-3′ and 5′-AAGGAGTAAGGAACAACCTCCA-3′ (Invitrogen). The PCR mixture and cycling conditions were identical to the conditions used for the primary PCR step, except that 3 mM MgCl2 was used in the PCR and BSA was not added.
For the restriction fragment analysis, an aliquot of 20 μl secondary PCR products was digested in a 50-μl reaction mixture containing 20 U of SspI (Sigma, Spain) for species diagnosis, or 20 U of VspI (Sigma, Spain) for genotyping of C. parvum, and 5 μl of the appropriate restriction buffer at 37°C for 1 h, under conditions recommended by the supplier.
As a positive control, a sample of C. parvum genotype 2 was used from cattle belonging to Granja Puleva, Granada (Spain). The PCR products and a ladder of 1,000 bp (Sigma, USA) were electrophoresed in a 2.0% agarose gel and visualized by ethidium bromide staining.
Results
Epidemiological study
A total of 489 fecal samples were collected and analyzed: 225 from El Porvenir elementary school (which had 550 students), 178 from La Esperanza (which had 500 students), and 86 from Buenos Aires (which had 100 students). The median age of the children was 5.3 years (interquartile range=4.1). One or more intestinal parasitic infections were identified in 333 (68%) of the children, 152 (45.6%) of them having multiple parasitic infection by two, three, or four parasites. The protozoa detected (Table 1) were Giardia lamblia, Entamoeba coli, Cyclospora cayetanensis, Iodamoeba buschlii, Endolimax nana, Chilomastix mesnili, and Cryptosporidium spp. Blastocystis hominis was frequent. The helminths were Hymenolepis nana and Hymenolepis diminuta. In the three districts studied, the parasite prevalence proved similar: 26.4% G. lamblia, 13% C. cayetanensis, 2% H. nana, 1.6% H. diminuta, and 1% Cryptosporidium spp.
Of the 489 children sampled, 147 had diarrhea (defined as a change in the normal pattern of bowel movements, with at least three loose stools daily).
Table 1 shows the general prevalence of each parasite and the prevalence for both populations, children with and without diarrhea. Of all the intestinal parasites found in the fecal samples, only Cryptosporidium appeared exclusively in diarrheic children. In the case of G. lamblia, a slight difference is also appreciable between the diarrheic children (34%) and nondiarrheic ones (23%).
Multiple parasitic infections were common, with 333 presenting intestinal parasitosis, 152 (45.6%) of these with two, three, or four intestinal parasites. The most frequent cases of multiple parasitism were G. lamblia and B. hominis, with a frequent association of the two pathogens, G. lamblia and C. cayetanensis. All the fecal samples in which these latter two species appeared in association belonged to children affected by acute diarrhea with nausea and vomiting.
Cryptosporidium was not associated with other parasites in any of the five cases diagnosed.
Molecular characterization of Cryptosporidium spp.
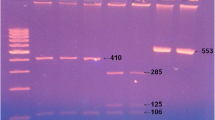
Of the 489 children analyzed, only 5 presented Cryptosporidium spp., and all had acute diarrhea. Species diagnosis was made by digesting the secondary PCR product of 831–837 bp with SspI. All the samples generated three visible bands of 448, 247, and 106 bp (Fig. 1). For C. parvum to be differentiated from C. hominis, the secondary PCR product was digested with VspI. C. parvum (C. parvum bovine genotype) produced a visible band of 628 bp, while the human genotype, C. hominis, produced a band of 556 bp (Fig. 2). Thus, it could be clearly distinguished that four children had been parasitized by C. hominis and only one by C. parvum (the latter being indistinguishable from the control isolate from Granada, Spain), which was the bovine genotype of C. parvum.
Molecular diagnosis of Cryptosporidium spp. by a nested polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) based on SSU rRNA gene sequences, using SspI in the digestion. Lane 1, molecular weight marker (1,000 bp); lanes 2–6, Cryptosporidium parvum from children; lane 7, C. parvum from bovine control
Genotype diagnosis of Cryptosporidium by a nested PCR-RFLP based on SSU rRNA gene sequences, using VspI in the digestion. Lane 1, molecular weight marker (1,000 bp); lanes 2–4 human genotype of C. parvum (Cryptosporidium hominis) from children; lane 5, bovine genotype of C. parvum from a child; lane 6, bovine genotype of C. parvum from bovine control
Discussion
This study determines the intestinal parasites that affect children from three districts of Trujillo (Peru) as well as the Cryptosporidium species and genotypes responsible for infantile cryptosporidiosis, one of the gravest afflictions in malnourished children in developing countries (Fayer et al. 2000).
The prevalence of intestinal parasites in children in this study was very high (68%) though lower than the 91.2% found in another study in Puno, Peru (Maco et al. 2002). The prevalence of pathogenic enteroparasites was also different. The Puno study reported 6.6% H. nana, 5.5% Entamoeba histolytica, 3.3% G. lamblia, 2.2% Taenia sp., 2.2% Ascaris lumbricoides, 1.1% Trichuris trichura, and 1.1% Enterobius vermicularis. Our study found 26.4% G. lamblia, 13% C. cayetanensis, 4% H. nana, 1.6% H. diminuta, and 1% Cryptosporidium spp. Low intestinal cestodosis was found possibly because the basic foods of these children are vegetables and carbohydrates, while meat, scarcely eaten, is chicken, the cheapest and apparently the only animal consumed in these communities.
The high prevalence of G. lamblia (26.4%) was striking, this continuing to register one of the highest infection rates among children in Trujillo since 1987 (Vargas et al. 1987). C. cayetanensis had the second highest prevalence, at 13%, this being very high with respect to previous studies in Lima (Ortega et al. 1994) or Pampas (Madico et al. 1997), where prevalence among children was 6 and 1%, respectively. Cryptosporidium spp. were detected in 13.3% of HIV-positive patients in Lima (Cama et al. 2003) and 11% in diarrheic children in Pampas de San Juan de Miraflores (Xiao et al. 2001). In our study, only five cases (1%) were diagnosed, all in diarrheic children. By contrast, such enteropathogens as G. lamblia, C. cayetanensis, H. nana, and H. diminuta appeared in diarrheic and nondiarrheic children (Table 1) with similar prevalence. It has been demonstrated that the Giardia cysts isolated from the feces of asymptomatic children and used to infect gerbils were more infective than those from symptomatic children (Astiazaran et al. 2000). Our results underscore the importance of asymptomatic children as carriers in communities with poor hygiene where there is poor control over water or food.
Multiple parasitism was frequent, with 45.6% of the parasitized children presenting two, three, or four parasites. Notably, in nine cases, G. lamblia and C. cayetanensis were associated; in four cases, these two were associated with B. hominis; and in two cases, G. lamblia, C. cayetanensis, E. coli, and I. buschlii were all found together (Table 2). In all cases, the children presented acute diarrhea.
Infantile cryptosporidiosis is especially grave in children who are immune depressed by malnutrition, as there is currently no completely effective treatment against Cryptosporidium. Therefore, it is fundamental to establish prevention and control programs against this parasite. In this regard, it is essential to know the species and genotypes of Cryptosporidium in each region, as isolates from different regions have different antigens, virulence, infectivity, and sensitivity to drugs and disinfectants (Fayer and Ungar 1986; Fayer et al. 2000).
In the present study, five fecal samples from children with diarrhea contained Cryptosporidium spp. unassociated with other parasites. By molecular characterization, we could clearly distinguish that four children were parasitized by C. hominis and only one by C. parvum. These data suggest the possibility of two distinct Cryptosporidium populations cycling in children in Trujillo. The most frequent population appears to involve an anthroponotic transmission cycle, fundamentally in humans. The other population appears to involve zoonotic transmission from cattle to humans with subsequent human-to-human and human-to-cattle transmission. The predominance of C. hominis in children may be due to the fact that bovine cryptosporidiosis is low because cattle are not corralled but rather are pastured in the fields. It is known that the crowding of the animals in the corrals favors the transmission of the parasite (Xiao et al. 2004). Similarly, other studies in Peru have also identified C. hominis as the predominant species (Xiao et al. 2001; Cama et al. 2003). Transmission of C. hominis in children can be attributed to their habit of defecating on the ground in the absence of toilets in houses and schools and, thus, to contamination of water and food with human waste.
The present work demonstrates the high prevalence of intestinal parasites in the infantile population considered. Based on the results of this study, control and prevention programs are being started against the most frequent intestinal parasites, and all the affected children have been treated.
References
Astiazaran H, Espinosa M, Castanon G, Chavez-Munguia B, Martinez-Palomo A (2000) Giardia lamblia: effect of infection with symptomatic and asymptomatic isolates on the growth of gerbils (Meriones unguiculatus). Exp Parasitol 95:128–135
Berkman DS, Lescano AG, Gilman RH, Lopez LS, Black MM (2002) Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood: a follow-up study. Lancet 358:564–571
Black RE, Lopez de Romana G, Brown KH, Bravo N, Bazalar OG, Kanashiro HC (1989) Incidence and etiology of infantile diarrhea and major routes of transmission in Huascar, Perú. Am J Epidemiol 129:785–799
Cama VA, Bern C, Sulaiman IM, Gilman RH, Ticona E, Vivar A, Hawai V, Vargas D, Zhou L, Xiao L (2003) Cryptosporidium species and genotypes in HIV-positive patients in Lima, Peru. J Eukaryot Microbiol 50:531–533
Chang JO (1987) Cryptosporidiosis asociada a diarrea aguda en Oxapampa, provincia de Oxapampa (Perú). Bol Chil Parasitol 42:39–42
Entrala E, Molina JM, Rosales MJ, Sánchez-Moreno M, Mascaró C (2000) Cryptosporidium parvum: oocysts purification using potassium bromide discontinuous gradient. Vet Parasitol 92:223–226
Fayer R, Ungar BLP (1986) Cryptosporidium spp. and cryptosporidiosis. Microbiol Rev 50:458–483
Fayer R, Morgan U, Upton SJ (2000) Epidemiology of Cryptosporidium: transmission, detection and identification. Int J Parasitol 30:1305–1322
Huaynalaya E, Miranda H, Mendoza J (1988). Cryptosporidium sp.: frecuencia en niños con diarrea atendidos en el Centro de Salud Madre de Cristo del Distrito de la Esperanza, Trujillo. Resúmenes I Congreso Internacional del Norte de Perú, p 16
Ortega YR, Gilman RH, Sterling C (1994) A new coccidian parasite (Apicomplexa: Eimeridae) from humans. J Parasitol 80:625–629
Maco V, Marcos LA, Terashima A, Samalvides F, Gotuzzo E (2002) Distribution of entero-parasitic infections in the Peruvian highland: study carried out in six rural communities of the department of Puno, Peru. Rev Gastroenterol Peru 22:304–309
Madico G, Mc Donald J, Gilman RH, Cabrera L, Sterling CR (1997) Epidemiology and treatment of Cyclospora cayetanensis infection in Peruvian children. Clin Infect Dis 24:977–981
Morgan U, Fall A, Ward LA, Hijjawi N, Sulaiman I, Fayer R, Thomson RC, Olson M, Lal A, Xiao M (2002) Cryptosporidium hominis n. sp. (Apicomplexa: Cryptosporidiidae) from Homo sapiens. J Eukaryot Microbiol 49:433–440
Palmer CJ, Xiao L, Terashima A, Guerra H, Gotuzzo E, Saldias G, Bonilla JA, Zhou, L, Lindquist A, Upton SJ (2003) Cryptosporidium muris, a rodent pathogen, recovered from a human in Peru. Emerg Infect Dis 9:1174–1176
Robertson LJ, Campbell AT, Smith HV (1993) In vitro excystation of Cryptosporidium parvum. Parasitology 106:13–19
Sarabia-Arce S, Salazar-Lindo E, Gilman RH, Naranjo J, Miranda E (1990) Case-control study of Cryptosporidium parvum infection in Peruvian children hospitalized for diarrhea: possible association with malnutrition and nosocomial infection. Pediatr Infect Dis J 9:627–631
Soave R, Armstrong D (1986) Cryptosporidium and cryptosporidiosis. Rev Infect Dis 8:1012–1023
Sterling CR, Gilman RH, Sinclair NA, Cama V, Castillo R, Diaz F (1991) The role of breast milk in protecting urban Peruvian children against cryptosporidiosis. J Protozool 38:23S–25S
Vargas F, Jara C, Castillo R (1987) Protozoarios y helmintos intestinales en pobladores de Trujillo, Perú. Rebiol 6:3–14
WHO (1991) Basic laboratory methods in medical parasitology. World Health Organization, Geneva
WHO (1996) Fighting disease, fostering development. World Health Organization, Geneva
Xiao L, Escalante L, Yang C, Sulaiman I, Escalante A, Montali R, Fayer R, Altaf A (1999a) Phylogenetic análisis of Cryptosporidium parasites based on the small subunit rRNA gene locus. Appl Environ Microbiol 65:1578–1583
Xiao L, Morgan U, Limor J, Escalante A, Arrowood M, Shulaw W, Thompson R, Fayer R, Altaf A (1999b). Genetic diversity within Cryptosporidium parvum and related Cryptosporidium species. Appl Environ Microbiol 65:3386–3391
Xiao L, Bern C, Limor J, Sulaiman I, Roberts J, Checkley W, Cabrera L, Gilman R, Lal A (2001) Identification of 5 types of Cryptosporidium in children in Lima, Peru. J Infect Dis 183:492–497
Xiao L, Fayer R, Ryan U, Upton S (2004) Cryptosporidium taxonomy: recent advances and implications for public health. Clin Microbiol Rev 17:72–97
Acknowledgements
This work has received financial support from the Agencia Española de Cooperación Internacional and the Agencia Andaluza de Cooperación Internacional (AI29/04).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cordova Paz Soldan, O., Vargas Vásquez, F., Gonzalez Varas, A. et al. Intestinal parasitism in Peruvian children and molecular characterization of Cryptosporidium species. Parasitol Res 98, 576–581 (2006). https://doi.org/10.1007/s00436-005-0114-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-005-0114-7