Abstract
We studied the distribution and faecal shedding pattern of the first-stage larvae (L1) of Elaphostrongylus cervi (Nematoda: Protostrongylidae) in the red deer (Cervus elaphus) across Spain, where excretion was widespread. We evaluated the effects of individual, population and environmental factors on E. cervi L1 counts in 18 free-ranging red deer populations in South Central Spain. In this area, prevalence was 71.42±2.14% (n=448) and mean intensity (n=320) was 74.50±10.35. Aggregation of deer at water-holes was positively associated with E. cervi L1 prevalence, possibly due to spatial and temporal odds of infected gastropods, red deer and infective E. cervi L1 larvae being encountered. Prevalence increased with age, and there was also a trend towards males having higher intensities than females. A slightly decreasing age–intensity profile was identified for females, which may suggest a role of acquired immunity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Elaphostrongylus cervi Cameron 1931 (Nematoda: Metastrongyloidea) are parasites of the central nervous system and skeletal muscles of red deer, Cervus elaphus (Mason 1995; Lankester 2001). The parasite has a wide natural distribution in Eurasia but is also present in New Zealand. The presence of large spiny-tailed protostrongylid larvae similar to those of E. cervi and immature E. cervi adults has also recently been reported for Central and South Spain (Vicente and Gortazar 2001; Valcárcel and Romero 2002).
Red deer acquire infection by accidentally ingesting gastropod intermediate host containing infective third-stage (L3) larvae of the nematode. These are liberated in the deer gut and mature during migration. E. cervi reach the adult stage in the CNS (subarachnoid spaces) and subsequently migrate into the fascia and connective tissue around skeletal muscles, where they mature and live in reproductive pairs and groups (Handeland et al. 2000). Presumably, adult females lay eggs that by the haematogenous route reach the lungs, where they hatch as first-stage larvae (L1). These travel up the bronchial tree, are swallowed and are dispersed in the host faeces. In the environment, L1 penetrate the foot of terrestrial gastropods, where they develop to the infective L3 (Rezac et al. 1994). Although clinical signs due to E. cervi infection are uncommon in red deer and elk (Watson 1983, but see Handeland et al. 2000), neurological signs and deaths result from natural or experimental infection of other Cervidae (Gajadhar and Tessaro 1995) as well as in domestic goats (Handeland et al. 2000).
In Spain, several wild ungulates including the deer have expanded their range in the last decades (Gortazar et al. 2000). Changes in wildlife management have already raised concerns regarding the control of infectious and parasitic diseases (e.g. Vicente et al. 2004a). Since E. cervi has considerable pathological consequences for other wild and domestic ungulates (Gajadhar and Tessaro 1995), it is important for wildlife managers, livestock breeders and sanitary authorities to know its abundance and distribution, especially when concurrent wild ungulate species co-occur with valued species such as the Spanish Ibex (Capra pyrenaica). Prevalence and intensity of infection and the dynamics of transmission may vary according to the availability of hosts and to local environmental conditions (Njau et al. 1992). In contrast with other Elaphostrongylinae worm/deer systems (e.g. Wasel et al. 2003), little is known regarding the correlation of ecological and management risk factors with infection rates of E. cervi in red deer populations. In this context, our objectives were to extend the knowledge of the distribution of E. cervi across Spanish Iberian red deer populations and to evaluate individual, population and environmental risk factors on E. cervi infection through the evaluation of L1 faecal counts.
Material and methods
Study sites
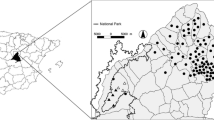
A total of 46 red deer populations (n=970 individuals) from throughout Spain were sampled to determine the general distribution of the parasite in the country (Figs. 1 and 2). This included samples from Northern Atlantic Spain (Cantabric and Pyrinean Mountains, respectively), Northern Central Spain (the Iberian mountains), South Central Spain (Montes de Toledo, Sierra Morena and Betic chains mountains) and finally in South Spain (Los Alcornocales Natural Park).
Map of Spain showing the sampling sites, sample size (on the right bottom corner of the hexagon) and E. cervi L1 prevalence (percentage of grey colour in relation to the whole hexagon represents numerical values) and mean intensity (figure inside the hexagon) from the study populations. Dots represent sampling sites where less then ten samples were obtained (grey colour when E. cervi L1 was found). Sampling sites are the number in brackets in the upper left corners (see Table 1). a Sampling sites where anthelminthic treatment was applied. The rectangle represents the core area from South Central Spain where risk factor analysis was performed (see Fig. 2)
Map of the core area from South Central Spain showing the sampling sites, sample size (on the right bottom corner of the hexagon) and E. cervi L1 prevalence (percentage of grey colour in relation to the whole hexagon represents numerical values) and mean intensity (figure inside the hexagon) from the study populations. Dots represent sampling sites where less then ten samples were obtained (grey colour when E. cervi L1 was found). Sampling sites are the number in brackets in the upper left corners (see Table 1) a Sampling sites where anthelminthic treatment was applied. Forests and scrublands are represented in grey
A variety of hunting estates are found across South Central Spain supporting what can be classified as semi-free-ranging cervids (large enclosures with relatively low densities of deer) and free-ranging cervids (not enclosed or intensively managed). We evaluated the effect of potential risk factors on E. cervi prevalence and intensity across 18 populations of red deer (Table 1) from Ciudad Real province and boundaries, South Central Spain (37°13′48″N to 39°31′43″N in latitude and from 06°34′06″W to 2°25′54″W in longitude, Figs. 1 and 2). The habitat in this area is Mediterranean and characterized by Quercus ilex forest and scrublands (dominated by Cystus spp, Pistacia spp, Rosmarinus spp, Erica spp and Phyllirea spp), with scattered pastures and small areas with crops. Seasonal streams cross many of the estates, but water is retained and available all-year-round in water-holes, in most cases artificially made along riverbeds. This area includes Montes de Toledo and Sierra Morena mountains. The Guadiana River valley consists of fragmented Mediterranean habitat and is a transition between Montes de Toledo and Sierra Morena (Fig. 2). The climate is Mediterranean. Annual rainfall is highly variable and ranges from 300 to 700 mm. The wet season typically extends from October to May when most of yearly rains are received. The dry season (from June to September) is when food and water resources become limited for ungulates. We deliberately chose sites where a priori a range of densities and management factors would be present. We rejected from the risk analysis hunting estates where anthelminthic treatment (usually orally on food) was supplied.
Sampling and diagnosis
Data were collected during the hunting season in a cross-sectional nationwide survey of wild red deer in Spain from year 2000 to 2004. Fresh faecal samples were collected from hunted animals directly from the rectum during field necropsy. Protostrongylid larvae were extracted in less than 24 h from 8 to 10 g of faeces using the Baermann beaker extraction method described by Forrester and Lankester (1997). Larvae were quantified in a Favatti counting chamber and expressed as number of L1 per gram of wet faeces (lpg). Larvae were identified to genus level according to their morphology and linear dimensions using the descriptions in Kutzer and Prosl (1975), English et al. (1985), Demiaszkiewicz (1986), Rezac (1990) Mason (1995), Vicente and Gortazar 2001 and Lankester (2001). The morphology and size of the dorsal-spined larvae were similar to the first-stage larvae of Elaphostrongylinae and not with those from other protostrongylids with spiny-tailed larvae. The absence of parasitic nodules characteristic of pulmonary protostrongylids at necropsy suggests that no adult worms (except by Dictyocaulus spp, which inhabits bronchi and bronchioles) had infected the lungs of the deer examined. In any case, both reindeer and European moose (host to E. rangiferi and E. alces, respectively) are present in Mediterranean Europe, and the morphology and size of the larvae found in this study, along with the high host specificity of the Elaphostrongylinae, suggest that deer populations studied are parasitized by E. cervi. Sex was recorded and age was determined by the tooth eruption pattern and incisor 1 sectioning (n=448; Klevezal and Kleinenberg 1967).
Estimating red deer densities, habitat variables and questionnaires
We visited 18 of the sampling sites in September 2002, immediately before the hunting season, in order to obtain field estimates of red deer relative abundances and habitat variables (Figs. 1 and 2). Red deer relative abundances (kilometric abundance index) were obtained by night-spotting along transect lines and employed as red deer abundance estimates (Whipple et al. 1994). At least one experienced observer participated in each transect.
Habitat and management variables considered in the study were chosen on the basis of their epidemiological plausibility and the local big game characteristics (Table 1). Environmental conditions for the 18 study areas were recorded every 200 m across our lineal transects (n=20 points per estate) to obtain mean values of habitat land uses and habitat structure of each estate. Through a personal interview with gamekeepers, we characterized management practices in each keeping area. We also obtained data on general estate-related features, watering sites (artificial water-hole or natural source), numbers and locations, feeding practices, anthelminthic treatment and presence of other big game species (Table 1). All the variables related to the year 2002. A listing of the variables included in the first step of the risk factor analysis is displayed in Table 1. Summary statistics of the most relevant management practices, land cover and habitat structure characteristics of the estates included in the risk factor study are given in Table 3.
Statistical analysis
The terms prevalence and intensity are used as in Margolis et al. (1982). Quantitative analysis of risk factors for E. cervi L1 prevalence and intensity were done using a two-stage analysis to create generalized linear mixed models (GLMMs). First, we correlated at the estate level (n=18) the pairwise association of the hypothesized risk factors and E. cervi L1 (%) prevalence and intensity (mean log10+1 E. cervi L1 count), respectively, by means of Spearman correlations as a screening step. Calves were excluded from this analysis to obtain comparable data (Table 2). Factors that were associated significantly with the outcome at P=0.01 (established by the Bonferroni adjustment test for multiple comparisons) were considered for further analysis (Table 1). For categorical variables, Kruskall–Wallis analysis was used for simple assessment of associations. We checked for collinearity in the associations between factors that passed the initial screening using chi-square and Spearman’s correlation for categorical and continuous variables, respectively. We only included in the final models the most significant variable within each set of collinear factors. These outcome variables were offered into GLMMs.
We constructed GLMMs with intensity and prevalence as response variables (Wilson and Grenfell 1997) that excluded calf age (n=448 after excluding 72 calves and 60 adult individual with unknown age). We designed a GLMM with the individual E. cervi L1 intensity (n=320) as a response variable to test the effect of sex (as categorical variable), age (years old, as continuous variable) and sampling month (from October to March, as a categorical variable). The estate (n=18) and sampling year (from 2000 to 2004) were considered as random factors. We considered a Poisson error distribution and a logarithmic link function (Wilson and Grenfell 1997). For the presence of E. cervi L1 (n=448), we designed a similar model where the outcome variable was defined as the individual’s status with respect to E. cervi L1 infection (as binomial variable; 0 absence, 1 presence). The number of red deer per watering-hole in the estate was also included. We considered a binomial error distribution and a logit link function. The resulting saturated up to two interactions models were reduced using a backward-selection approach until all variables remaining in the models were significant at P<0.05.
Results
The geographic distribution of the faecal prevalence and mean intensity of E. cervi L1 (excluding calves) in red deer populations across the study area are shown in Figs. 1 and 2. Mean prevalence and mean intensity from estates across South Central Spain with at least 10 sampled animals were lower at those where anthelminthic treatments were used (mean prevalence=39.10±13.80%, mean intensity=13.17±6.63, n=8) than at those without treatment (mean prevalence=73.65±13.74%, mean intensity=62.26±22.89, n=22, Z M−W=−2.747, P<0.01 and Z M−W=−2.99, P<0.05 for prevalence and intensity, respectively). For the study sites used in risk analysis, estate mean prevalence was 79.07±5.78% (ranging from 6.89% to 100%, n=18) and estate mean intensity was 66.90±12.14 (ranging from 2.76 to 181.93, Table 2). At the individual, mean prevalence was 71.42±2.14% (n=448) and mean intensity (n=320) was 74.50±10.35.
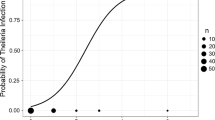
Of the variables taken into account in the risk factor analysis, the number of red deer per watering site associated to E. cervi prevalence L1 (r S=0.53, P=0.01, Table 3; Fig. 3). Prevalence was significantly lower for calves compared to elder individuals (χ 2 test, P<0.05; n=60 and n=516 for calves and elder individuals, respectively, Fig. 4). Regarding the animals included in the risk factor analysis, GLMM results for prevalence and intensity of E. cervi L1 are shown in Table 4. There was a clear and statistically significant positive age-prevalence pattern (Fig. 3), peaking approximately in 6-year-old animals and then maintaining high rates. The number of red deer per water-hole was significantly related to the probability to excrete E. cervi L1.
As regards intensity of E. cervi L1 excretion, sex and age interacted significantly. There was a trend towards males having higher intensities than females, but for males more than 2 years old, there was no evidence of a clear age–intensity pattern (estimated slope=0.005, SE=0.02); for females, a slightly decreasing age–intensity profile was identified (estimated slope=−0.1, SE=0.04) (see Fig. 4 and Table 4). The intensity of E. cervi L1 counts were significantly related to sampling month, with mean values peaking at October–November (Fig. 5).
Discussion
We found that E. cervi is widely distributed among Iberian red deer populations. The parasite occurs in all the sampled areas of Spain; thus, our study expands the known geographic distribution in Southwestern Europe. Finding of infected red deer in Cádiz province establishes the southern limit of E. cervi in the Mediterranean Europe. The prevalence figures are similar to those of natural wild red deer populations passing larvae elsewhere in enzootic situations across the geographical range of wild red deer. For example, Demiaszkiewicz (1985) reported that 64 to 91% of red deer were passing larvae upon examination of various hunting grounds in Poland, and English et al. (1985) found 83% in Scotland. As regards the risk factor study area, a wide range of population infection rates were found, even in closely located deer populations. For example, prevalence ranges from 40 to 100% in populations located in the Toledo Mountains area (Fig. 2).
Of factors related to the variability in E. cervi L1 excretion across individuals and populations, the main finding in this paper relates to the relationship between prevalence and the level of aggregation of animals at watering-holes. Epidemiological models and comparative data suggest in general a positive relationship between host population density and abundance of macroparasites (Arneberg 2001). Our study suggests that local host aggregation rather than large-scale host density is an important factor in E. cervi L1 transmission in our study area. Mediterranean environments are characterized by a drought period in summer, and this may critically affect the survival and spatial availability of larvae in the environment. In addition, summer weather forces gastropods to aestivate or concentrate in humid areas, usually the scarce water-holes that remain along riverbeds. Both the free-living first-stage larvae of meningeal worms and terrestrial gastropods require moisture for movement and survival. Therefore, water-holes are likely to concentrate the spatial and temporal odds of contact between infective E. cervi L1 larvae, gastropods and red deer. Due to this apparent dependence of the transmission dynamics of our studied parasite–host system on wet habitats, some parallelism may occur with other major water-borne helminth infections, such as fasciolosis (Njau et al. 1992). In these studies, prevalence and intensity of infection and the dynamics of transmission vary according to the availability of hosts and to local environmental conditions (Cringoli and Rinaldi, 2003).
An increasing age-prevalence pattern of E. cervi L1 faecal outputs was found. Older animals have a higher probability of having acquired infection than younger ones, and E. cervi is long-lived in its final host (Watson 1984). Thus faecal presence of E. cervi L1 is likely to reflect the way in which deer acquire the parasite across age. Prevalence increased most from calves to yearlings (Fig. 4), with peak prevalence reached in approximately 6-year-olds and maintained thereafter. Similar to our results, prevalence of infection was higher in adult deer than in calves on farms in New Zealand (Mason and Mcalumm 1976).
There was a trend towards males having higher intensities of E. cervi L1 than females, but no sex differences in prevalence. Whereas there was no evidence of a clear age–intensity pattern for males more than 2 years old, for females, a slightly decreasing age–intensity profile was identified (Fig. 4). In vertebrate species, it is often found that males tend to harbor more parasites than females (e.g. Alexander and Stimson 1988; Poulin 1996). In our case, the pattern could also reflect a higher female fecundity in male hosts than in females (Stear et al. 1997). This pattern may be due to ecological differences between sexes (Forbes 1993) or to differences in exposure to parasites because of their different feeding habits or habitat use (Wilson et al. 2001). Nevertheless, we could not test if exposure to this is affected by sex in our study area.
The majority of macroparasite systems show age–intensity curves with an initial increase after the age at which an animal becomes susceptible to infection (Wilson et al. 2001). As a result of accumulated experience to parasite antigens, the level of parasite infections may decline due to a protective immune response (Lloyd 1995). Prestwood and Nettles (1977) found in experimental infections of white-tailed deer signs of acquired immunity to reinfection with Paralephastrongylus andersoni, which was also suggested for the Paralephastrongylus tenuis/white-tailed deer system (Slomke et al. 1995), the reindeer/E. rangiferi system (Gaudernack et al. 1984) and the caribou/E. rangiferi system (Ball et al. 2001). P. tenuis is a long-lived parasite, and most white-tailed deer become infected in their first or second summer of life in Minnesota (Slomke et al. 1995). Interestingly, older male deer (more than 10 years old) showed higher intensities of E. cervi L1 excretion than females. The performance of animals may decline with age, but effects of senescence, however, may differ between the sexes because of differences in physiology and behaviour (Saino et al. 2003). Senescence occurs in general earlier in male deer than in females as result of the different reproductive investment (Carranza et al. 2004). We therefore suggest that higher L1 counts in old male deer may be due to senescence affecting immune function. In conclusion, sex by age differences in larvae excretion were evidenced despite the fact that sampling size variation across age classes and months probably affected the precision estimates for larvae excretion rates when comparing sex and ages.
The intensity of E. cervi L1 counts was significantly associated with sampling month, with peak values peaking during the October–November period (Fig. 5). The seasonal pattern of faecal excretion of E. cervi L1 in Iberian red deer has been monitored previously in South Central Spain (Vicente et al. 2004b). The lowest mean intensity of faecal L1 were found in summer, whereas no seasonal variation was found for prevalence across the year. Consistent with this, we found temporal variation only in the intensity of excretion and not in the prevalence of infection. The seasonal rhythm of E. cervi L1 discharge resulted from parasite adaptation to the seasonal Mediterranean climate and habitat constraints to improve the chance of parasite transmission, and positively associated with early coming rainfall (the next month) rather than with rainfall of the same month (Vicente et al. 2004b). Thus our results, with a peak of E. cervi L1 intensity of excretion in October–November, are consistent with the autumnal peak of rainfall expected for Mediterranean climate. In addition to climatic factors, individual factors could affect the temporal pattern of E. cervi L1 excretion (Gaudernack et al. 1984). Sampling size variation could probably influence results and statistical calculations as regards temporal variation in the intensity and in the prevalence of excretion. In particular, low December values could be biased by insufficient sampling during this month since this decrease in larvae production was not detected when the seasonal pattern of faecal excretion of E. cervi L1 in Iberian red deer was previously monitored in South Central Spain (Vicente et al. 2004b).
E. cervi, a sub-lethal parasite of red deer, was found to be widespread across Spanish populations. This points out to the fact that it may constitute a good model to evaluate host–parasite relationships under particular types of management. In particular, water-holes at Mediterranean habitats seem to have an important epidemiological role, which could extend to other infectious agents. In the future, more research on the effect of pathogens on this host species and transmission determinants should be carried out to contribute to the understanding of wildlife diseases in the field.
References
Alexander J, Stimson WH (1988) Sex hormones and the course of parasitic infection. Parasitol Today 4:189–193
Arneberg P (2001) An ecological law and its macroecological consequences as revealed by studies of relationships between host densities and parasite prevalence. Ecography 24:352–358
Ball MC, Lankester MW, Mahoney SP (2001) Factors affecting the distribution and transmission of Elaphostrongylus rangiferi (Protostrongylidae) in caribou (Rangifer tarandus caribou) of Newfoundland, Canada. Can J Zool 79:1265–1277
Carranza J, Alarcos S, Sanchez-Prieto CB, Valencia J, Mateos C (2004) Disposable-soma senescence mediated by sexual selection in an ungulate. Nature 432:215–218
Cringoli G, Rinaldi L (2003) Water as risk factor for helminthiasis in domestic ruminants in the central and southern Italy and zoonotic risk. Ann Ig 15:43–46
Demiaszkiewicz A (1985) Elaphostrongylosis. A new parasitic disease of Cervidae in Poland. Med Wet 41:616–618
Demiaszkiewicz A (1986) Laboratory diagnosis of Protostrongylid infection in Cervidae. Med Wet 41:660–663
English AW, Watt CF, Corrigall W (1985) Larvae of Elaphostrongylus cervi in the red deer of Scotland. Vet Rec 116:254–256
Forbes MRL (1993) Parasitism and host reproductive effort. Oikos 67:444–450
Forrester SG, Lankester MW (1997) Extracting Protostrongylid nematode from ungulate faeces. J Wildl Dis 33:511–516
Gajadhar AA, Tessaro SV (1995) Susceptibility of mule deer (Odocoileus hemionus) and two species of North American molluscs to Elaphostrongylus cervi. J Parasitol 81:593–596
Gaudernack G, Halvorsen O, Skorping A, Stokkan KA (1984) Humoral immunity and output of first-stage larvae of Elaphostrongylus rangiferi (Nematoda, Metastrongyloidea) by infected reindeer, Rangifer tarandus tarandus. J Helminthol 58:13–18
Gortazar C, Herrero J, Villafuerte R, Marco J (2000) Historical examination of the status of large mammals in Aragon, Spain. Mammalia 64:411–422
Handeland K, Gibbons LM, Skorping A (2000) Aspects of the life cycle and pathogenesis of Elaphostrongylus cervi in red deer (Cervus elaphus). J Parasitol 86:1061–1066
Jarvinen O, Vaisanen RA (1975) Estimating relative densities of breeding birds in the line transect method. Oikos 26:316–322
Klevezal GA, Kleinenberg SE (1967) Age determination of mammals from annual layers in teeth and bones. USSR Academy of Sciences. Translation by the Department of the Interior and National Science Foundation, US Department of Commerce, Springfield
Kutzer E, Prosl H (1975) Zur Kenntnis von Elaphostrongylus cervi Cameron 1931. 1. Morphologie und diagnose. Wien Tierärztl Monatsschr 62:258–266
Lankester MW (2001) Extrapulmonary lungworms of cervids. In: Samuel WM, Pybus MJ, Kocan AA (eds) Parasitic diseases of wild mammals, 2nd edn. Iowa State University Press, Ames, Iowa, pp 228–278
Lloyd S (1995) Environmental influences on hosts immunity. In: Grenfell BT, Dobson AP (eds) Ecology of infectious diseases in natural populations. Cambridge University Press, Cambridge, pp 327–361
Margolis L, Esch GW, Holmes JC, Kuris AM, Schad GA (1982) The use of ecological terms in parasitology. Report of an Ad Hoc Committee of the American Society of Parasitologists. J Parasitol 68:131–133
Mason P (1995) Elaphostrongylus cervi and its close relatives: a review of protostrongylids (Nematoda: Metastrongyloidea) with spiny-tailed larvae. Surveillance 22:19–24
Mason PC, Mcalumm HJF (1976) Dictyocaulus viviparus and Elaphostrongylus cervi in wapiti. N Z Vet J 24:23
Njau BC, Kasali OB, Scholtens RG, Akalework N (1992) The influence of watering practices on the transmission of Fasciola among sheep in the Ethiopian highlands. Vet Q 14:140–144
Poulin R (1996) Sexual inequalities in helminth infection: a cost of being male? Am Nat 147:287–295
Prestwood AK, Nettles VF (1977) Repeated low-level infection of white-tailed deer with Parelaphostrongylus andersoni. J Parasitol 63:974–978
Rezac P (1990) Diferenciální larev 1. Stadia hlístic Varestrongylus sagittatus a Elaphostrongylus cervi. Veterinárstvi 40:311–313
Rezac P, Palkovic L, Holasova E, Busta J (1994) Modes of entry of the first-stage larvae of Elaphostrongylus cervi (Nematoda: Protostrongylidae) into pulmonate snails Arianta arbustorum and Helix pomatia. Folia Parasitol (Praha) 41:209–214
Saino N, Ferrari RP, Romano M, Rubolini D, Moller AP (2003) Humoral immune response in relation to senescence, sex and sexual ornamentation in the barn swallow (Hirundo rustica). J Evol Biol 16:1127–1134
Slomke AM, Lankester MW, Peterson WJ (1995) Infrapopulation dynamics of Paralephastrongylus tenuis in white-tailed deer. J Wildl Dis 31:125–135
Stear MJ, Bairden K, Duncan JL, Holmes PH, Mckellar QA, Park M, Strain S, Murray M, Bishop SC, Gettinby G (1997) How hosts control worms. Nature 389:27
Valcárcel F, Romero CG (2002) First report of Elaphostrongylus cervi in Spanish red deer Cervus elaphus hispanicus. J Helminthol 76:91–93
Vicente J, Gortazar C (2001) High prevalence of large spiny-tailed protostrongylid larvae in Iberian red deer. Vet Parasitol 96:165–170
Vicente J, Segalés J, Höfle U, Balasch M, Plana-Durán J, Domingo M, Gortazar C (2004a) Epidemiological study on PCV2 infection in the European wild boar. Vet Res 35:1–11
Vicente J, Fierro Y, Gortazar C (2004b) Seasonal dynamics of the faecal excretion of Elaphostrongylus cervi (Nematoda: Metastrongyliodea) first stage larvae in Iberian red deer from South Spain. Parasitol Res 95:60–64
Wasel SM, Samuel WM, Crichton V (2003) Distribution and ecology of meningeal worm, Parelaphostrongylus tenuis, in northcentral North America. J Wildl Dis 39:338–346
Watson TG (1983) Some clinical and parasitological features of Elaphostrongylus cervi infections in Cervus elaphus. N Z J Zool 10:129
Watson TG (1984) Tissue worm in red deer: symptoms and control. Media Services, MAF, New Zealand, Wellington
Whipple JD, Rollins D, Schacht WH (1994) A field simulation for assessing accuracy of spotlight deer surveys. Wildl Soc Bull 22:667–673
Wilson K, Grenfell BT (1997) Generalised linear modelling for parasitologists. Parasitol Today 13:33–38
Wilson K, Bjornstad ON, Dobson AP, Merler S, Poglayen G, Randolph SE, Read AF, Skorping A (2001) Heterogeneities in macroparasite infections: patterns and processes. In: Hudson PJ, Rizzoli A, Grenfell BT, Heesterbeek H, Dobson AP (eds) The ecology of wildlife diseases. Oxford University Press, Oxford, pp 6–44
Acknowledgements
This study was supported by project AGL2001-3947, Ministerio de Ciencia y Tecnología and FEDER-EU proyect IFD97-0164. J. Vicente had a grant from JCCM. This is a result of the agreement between Yolanda Fierro and UCLM.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vicente, J., Fernández de Mera, I.G. & Gortazar, C. Epidemiology and risk factors analysis of elaphostrongylosis in red deer (Cervus elaphus) from Spain. Parasitol Res 98, 77–85 (2006). https://doi.org/10.1007/s00436-005-0001-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-005-0001-2