Abstract
The effects of a live Cryptobia salmositica (Kinetoplastida) vaccine on the humoral and cellular immune response, and changes in the peripheral leukocyte populations of Salmo salar were investigated. The vaccine produced detectable parasitemia in the blood which peaked at 5 weeks post-vaccination (w.p.v). Antibodies were detectable at 4 w.p.v. and the antibody titer increased as parasitemia declined. Respiratory burst activity in vaccinated fish was significantly higher than in control fish; the highest activity occurred with rising parasitemia and lower activity with declining parasitemia. There was a significant increase in the proportion of granulocytes (to total leukocytes) at 4 w.p.v. At 6 w.p.v., the proportion of lymphocytes and monocytes increased significantly and remained elevated. These results demonstrate innate (respiratory burst activity and an increase in the proportion of granulocytes corresponding to rising parasitemia) and adaptive (antibody production and increases in the proportion of monocytes and lymphocytes corresponding to declining parasitemia) immune responses to the live vaccine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cryptobia salmositica is considered a lethal pathogen of salmon kept under semi-natural and intensive culture facilities (Bower and Thompson 1987). The high densities in which fish are reared are associated with inherent physiologic stress due to crowding, and also allow for easier transmission of infectious diseases. These are factors that have caused sporadic epizootics resulting in high mortalities in British Columbia and Washington State (Woo 2003). Continuous subculturing of the parasite results in its attenuation (Woo and Li 1990), and the strain has been shown to be an effective live vaccine in a variety of salmonid species (Woo 2003). A single dose of the live vaccine protected rainbow trout (Oncorhynchus mykiss) for at least 24 months, via stimulation of protective complement-fixing antibody production and enhancing phagocytosis and cell-mediated cytotoxicity (Li and Woo 1995). The kinetics of the humoral immune response has been well characterized over the course of vaccination with C. salmositica (e.g. Sitja-Bobadilla and Woo 1994; Ardelli and Woo 2002; Chin et al. 2004), but the cellular immune response, specifically circulating leukocytes, is less well understood.
Cell-mediated immune responses against C. salmositica have been shown using delayed-type hypersensitivity reactions (Thomas and Woo 1990; Feng and Woo 1996), inhibition of macrophage migration (Thomas and Woo 1990), mixed lymphocyte reactions (Ardelli and Woo 2002), and respiratory burst activity of head kidney macrophages (Mehta and Woo 2002).
The objectives of this study are to investigate the respiratory burst activity of peripheral phagocytes (nitroblue tetrazolium slide assay), changes in the composition of circulating leukocyte populations, and the humoral (antibody) immune response after vaccination. Characterization of these immune responses will provide better insight into the kinetics of the immune response after vaccination, and also provide fundamental information that will shed some light on the cellular basis behind the immune response against blood parasites. Since Atlantic salmon (Salmo salar) have detectable and quantifiable parasitemias after inoculation with the live vaccine (Chin et al. 2004), the immune responses will be described in relation to parasitemias.
Materials and methods
C. salmositica
The C. salmositica (T4, cloned sub-strain) used in this study was initially isolated from the leech vector, Piscicola salmositica, found on spawning coho salmon (Oncorhynchus kisutch Walbaum) in streams on Vancouver Island. It was cloned and its morphology characterized (Woo 1978). Long-term, serial in vitro culture of C. salmositica resulted in attenuation of the parasite. This strain does not cause disease, but protects fish from disease. The strain was maintained at 10°C in minimum essential medium supplemented with 25% heat-inactivated fetal bovine serum (Gibco Life Technologies, Grand Island, N.Y.) and 25 mM HEPES buffer (Gibco). The pH of the culture medium was adjusted to 7.2–7.3 (Woo and Li 1990), and sterilized using a bottle-top filter with a 0.22 μm cellulose-acetate membrane (Corning, Corning, N.Y.). Inocula used for vaccination was produced by obtaining high numbers of parasites from culture and diluting to the required numbers using phosphate buffered saline (PBS).
Fish and experimental design
Thirteen Atlantic salmon (18 months old; Atlantic Salmon Broodstock Development Program, St. Andrew’s, New Brunswick.) were divided into two groups. The “V” group consisted of eight fish and the “N” group of five fish, with mean±SE weights of 304.49±7.68 and 332.2±16.99 g, respectively. Each fish was tagged with a passive integrated transponder (PIT tag) to identify it individually. The two groups were housed separately in 125 l cylindrical tanks and maintained with aerated, constantly flowing, recirculated, filtered, UV-treated well water (10°C) with an equatorial photoperiod. They were fed daily ad libitum with a 42% protein commercial feed (5PT, Martin’s Feed Mills, Elmira, Ontario).
Each fish in the V group was injected intraperitoneally (i.p.) with approximately 200,000 attenuated C. salmositica (live vaccine) in 0.2 ml PBS. Fish in the N group received 0.2 ml PBS i.p. Blood was obtained (0.15 ml) in heparinized syringes weekly by caudal venipuncture starting at 3 weeks pre-vaccination to 9 weeks post-vaccination (w.p.v.). It was used to determine the percentage of activated peripheral phagocytes in both groups. Parasitemia, antibody titer, and leukocyte profiles were also examined in the V group. Blood was centrifuged (10,000 g for 2 min) and the plasma collected to determine the antibody titer against C. salmositica by ELISA.
Determination of parasitemia and differential leukocyte profiles
Low parasitemias were detected using the hematocrit centrifuge technique (Woo and Wehnert 1983). If there were more than 30 parasites per hematocrit tube, parasitemias were quantified using a hemocytometer (Archer 1965). Blood smears were made from each fish, and were stained with a modified Wright-Geimsa stain (Diff-quik) according to the manufacturer’s instructions. The first 200 leukocytes were counted and identified under a light microscope (1,000×) as lymphocytes, granulocytes, monocytes, or thrombocytes (Ellis 1977; Yasutake and Wales 1983; Rowley 1990). Each leukocyte type was expressed as a percentage of total leukocytes.
Enzyme-linked immunosorbent assay to detect C. salmositica antibodies from serum
The method of Sitja-Bobadilla and Woo (1994) was used with slight modifications to determine antibody response. Briefly, the wells of a high-binding 96-well flat-bottomed polystyrene micotiter plate (Corning) were coated with 50 μl of parasite antigen [C. salmositica (vaccine strain) whole cell lysate; 25 μg protein ml−1] and left to incubate overnight at 4°C. After incubation, the wells were washed five times with washing buffer (PBS w/ 0.05% Tween-20). All subsequent washings were performed in this manner. Vacant sites in the wells were blocked using 200 μl of blocking buffer (5% skim milk powder in PBS) and incubated at room temperature (RT) for 30 minutes with gentle shaking, and then washed. A total of 50 μl of diluted (1:10) sample serum was added and incubated at RT for 1 h. After incubation, the plates were washed and 50 μl of a 1:1,000 dilution (in dilution buffer) of rabbit anti-salmon antibody (Buchmann and Pederson 1994) were added. Plates were incubated (1 h at RT), washed, and 50 μl of a 1:10,000 dilution of peroxidase-labeled goat anti-rabbit antibody (Sigma, St. Louis, Mich.) was added. After incubation (1 h at RT), the plates were washed, and 50 μl of o-phenylediamine dihydrochloride (OPD) (Sigma-Aldrich) was added to each well and left to incubate for 10 min before the reaction was stopped with 50 μl of 3 M HCl. Plates were shaken and absorbance (optical density) was read at 492 nm using a microplate reader (Vmax, Molecular Devices, Menlo Park, Md.).
Nitroblue tetrazolium slide assay to detect activated peripheral phagocytes
The method of Anderson et al. (1992) was used with modifications. Briefly, a drop of freshly collected blood (25 μl) was placed onto a glass slide and incubated inside a humid chamber (Petri dish with a moist paper towel) for 40 min at RT to allow phagocytes (neutrophils and monocytes) to adhere to the slide. The slide was then gently rinsed in a bowl containing PBS to remove non-adherent cells. Excess liquid was drained off by placing the slide on its edge on a paper towel. A total of 20 μl of nitroblue tetrazolium chloride (NBT; 0.2% w/v in PBS; Sigma) was added to the adherent cells and a coverslip was placed on top. This was then incubated at RT for 15 min before examination of the cells under a light microscope (400× objective). The first 100 phagocytes (morphology similar to neutrophils and monocytes) were counted. Cells with a blue reticulated stain in their cytoplasm, produced by the reduction of NBT by O2−, were considered “activated”. These cells were counted and expressed as a percentage of activated phagocytes (%AP) in the total cell count.
Statistical analysis
Treatment groups and families were compared using one-way analysis of variance (ANOVA). Repeated-measures ANOVA was used to determine significant changes of antibody titer, %AP, and differential leukocyte percentages within the vaccinated group. If the variances were not equal among groups, a Kruskal-Wallis test was used. If significant differences were found (P<0.05), Tukey’s test was used for pair-wise comparison for ANOVA and Dunn’s test for the Kruskal-Wallis test.
Results
Parasitemia and humoral responses following vaccination
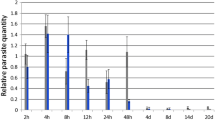
Using the hemotocrit centrifuge technique, parasites were detected in all fish in the V group at 3 w.p.v., but parasitemias were too low to count using a hemocytometer until 4 w.p.v. Peak parasitemia occurred at 5 w.p.v. (3.8±1.1×105 parasites ml−1 blood) and by 9 w.p.v., all fish had parasitemias too low to quantify using a hemocytometer (Fig. 1). A significant rise in antibody titer against C. salmositica was detected at 4 w.p.v. (Fig. 1). The titer continued to increase significantly as the parasitemia peaked and then declined to low numbers. The highest titer was detected at 8 w.p.v. (0.52±0.02).
Percentage of activated peripheral phagocytes in relation to parasitemia
The predominant peripheral phagocytes observed were neutrophilic granulocytes (neutrophils). The %AP in the V group following vaccination increased while the N group consistently had a very low %AP (<2%) during the experiment. The V group had significantly higher %AP than the N group from 3 to 7 w.p.v. (Fig. 2) and %AP peaked at 4 and 5 w.p.v. (23.1±6.2% and 23.4±5.8%, respectively), which corresponded to peak parasitemia and rising antibody titer. After 5 w.p.v., the %AP declined, as did the parasitemia, and returned to values comparable to the N group by 8 w.p.v.
Mean percentage of activated peripheral phagocytes (±SE) in Atlantic salmon (S. salar) over the course of vaccination with the live C. salmositica vaccine (approx. 200,000 per fish) in vaccinated (n=8) and Naive (n=5) groups. An asterisk denotes a significant difference (P<0.05) in %AP between vaccinated and naive groups
Differential leukocyte profiles in relation to parasitemia
The differential leukocyte profiles in relation to parasitemia are summarized in Table 1. There was a significant decrease (P=0.023) in the proportion of lymphocytes from 68.8±2.8% at 3 w.p.v. to 57.2±4.7% at 4 w.p.v, however, this was followed by increases in subsequent weeks. The percentages of lymphocytes were significantly higher (P<0.036) from 6 to 8 w.p.v. (low parasitemias), than at 4 w.p.v. (high parasitemia). There was a significant increase in the proportion of granulocytes (7.21±2.42%) at 4 w.p.v. from preceding weeks (−2, −1, 0, 1 w.p.v.) (P<0.025), which corresponded to rising parasitemia. This declined significantly compared to pre-vaccination levels by 6 w.p.v as parasitemia decreased (P<0.020). The proportion of monocytes increased significantly (P<0.05) at 6 w.p.v. (4.66±1.46%) and remained significantly elevated (P<0.025) at 7 (5.16±1.16%) and 8 w.p.v. (4.93±0.73%). This occurred as the parasitemia declined. The highest proportion of thrombocytes was found between −1 and 4 w.p.v. (>27%), and declines were significant (P<0.035) at 7 and 8 w.p.v. (14.8±1.6% and 17.0±1.8%, respectively) when parasitemias were low with increasing proportions of lymphocytes and monocytes.
Discussion
The NBT slide assay was first used to determine phagocytic dysfunction in diseases such as chronic granulomatous disease and sickle cell anemia in humans (Levinsky et al. 1983; Hernandez et al. 1983). This technique was modified by Anderson et al. (1992) to use as a general indicator of health status in fish, as NBT activity was positively correlated with phagocytosis and the killing activity of neutrophils and macrophages. This assay has subsequently been used to assess the efficacy of vaccines (Anderson et al. 1992; Chen et al. 1996, 1998; Munoz et al. 1999), immunostimulants (Jeney and Anderson 1993; Logambal et al. 2000), and the effect of environmental pollution on the immune system in fish (Stasiak and Baumann 1996). This is the first study on the effect of a live vaccine on the respiratory burst activity of circulating phagocytes in fish. The NBT slide assay showed a significant cellular response in vaccinated fish, with the respiratory burst activity (%AP) increasing with higher parasitemia and then decreasing as parasitemia declined.
Similarly to peripheral phagocytes, enhanced respiratory burst activity of head kidney macrophages was demonstrated in vaccinated and infected O. mykiss with C. salmositica lysate (Mehta and Woo 2002). Unlike in the present study, the respiratory burst activity of macrophages remained elevated (adaptive response) for the duration of the experiment (12 w.p.v.), even at low parasitemias. This would indicate that the NBT slide assay is sensitive in detecting innate cellular immunity in blood where the predominant phagocytes are neutrophils. These cells are assumed to have a more innate (non-specific) role in the immune system (Secombes 1994a). Also, macrophages are highly concentrated in the head kidney, and would probably provide sustained respiratory burst activity since the head kidney is a major site of antigen uptake and processing (secondary lymphoid organ). Additionally, the production of some lymphokines (e.g. macrophage activating factor, MAF) from leukocytes initiates the activation of macrophages (Secombes 1994b).
The live C. salmositica vaccine induced a characteristic parasitemia profile and humoral response in Atlantic salmon (Ardelli and Woo 2002; Chin et al. 2004). Antibodies were detected by 4 w.p.v., and the titer continued to increase as parasitemia decreased. Significant changes in leukocyte composition were detected, which corresponded to changes in parasitemia, %AP, and antibody titer. The increases in the proportion of lymphocytes and monocytes after 5 w.p.v. would explain increased antibody titers. Previous studies have shown changes in leukocyte composition after experimental infection with pathogens or vaccination. Muona and Virtanen (1993) showed an increase of neutrophils in Atlantic salmon during the first week of Vibrio anguillarum infection. After 4 weeks, a significant increase in lymphocytes was detected which was related to the acquisition of adaptive immunity. Pathiratne and Rajapakshe (1998) found that Asian cichlids affected by epizootic ulcerative syndrome (EUS) had significantly more neutrophils in early infection, and this was associated with local inflammation and damage due to severe EUS lesions. Brown trout infected with the fungal pathogen Saprolegnia declina had significantly higher proportions of granulocytes but reduced lymphocytes. This was related to immunodepression (Alvarez et al. 1988). Lonnstrom et al. (2001) found an increase in the proportions of lymphocytes and neutrophils in European whitefish (Coregonus lavaretus) vaccinated against vibriosis and furunculosis.
In the present study, peripheral phagocytes were activated by C. salmositica. The degree to which peripheral phagocyte respiratory burst activity controls C. salmositica infection requires further investigation using a pathogenic C. salmositica strain. The NBT slide assay and differential leukocyte counts were useful in determining increased cellular immune response and changes in leukocyte composition in response to a live C. salmositica vaccine. The increase in the proportion of phagocytes coincided with increased percentages of activated phagocytes and parasitemia. The proportions of granulocytes were significantly higher at 4 and 5 w.p.v. (rising parasitemia, high %AP, low antibody titer), and monocytes significantly increased from 6 to 8 w.p.v. (declining parasitemia, declining %AP, high antibody titer). Granulocytes are generally considered effector cells of the innate immune response (Secombes 1994a), while monocytes, which also function non-specifically, are important as accessory cells for initiating the adaptive immune response (Clem et al. 1985; van Muiswinkel 1995). Thus, the increase in the proportion of granulocytes would indicate earlier activation of innate immunity by the live C. salmositica vaccine while the increase in the proportion of monocytes later would signify activation of the adaptive immune response.
References
Anderson DP, Moritamo T, Grooth R (1992) Neutrophil, glass-adherent, nitroblue tetrazolium assay gives early indication of immunization effectiveness in rainbow trout. Vet Immunol Immunopathol 30:419–429
Alvarez F, Razquin B, Villena A, Lopez Fierro P, Zapata A (1988) Alterations in the peripheral lymphoid organs and differential leukocyte counts in Saprolegnia-infected brown trout, Salmo trutta. Vet Immunol Immunopathol 18:181–193
Archer RK (1965) Haematological techniques for use on animals. Blackwell, Oxford
Ardelli BF, Woo PTK (2002) Experimental Cryptobia salmositica (Kinetoplastida) infections in Atlantic salmon, Salmo salar L: cell-mediated and humoral immune responses against the pathogenic and vaccine strains of the parasite. J Fish Dis 25:265–274
Bower SM, Thompson AB (1987) Hatching of the Pacific salmon leech (Piscicola salmositica) from cocoons exposed to various treatments. Aquaculture 66:1–8
Buchmann K, Pederson K (1994) A study of teleost phylogeny using specific antisera. J Fish Biol 45:901–903
Chen SC, Yoshida T, Adams A, Thompson KD, Richards RH (1996) Immune response of rainbow trout to extracellular products of Mycobacterium spp. J Aqua Anim Health 8:216–222
Chen SC, Yoshida T, Adams A, Thompson KD, Richards RH (1998) Non-specific immune response of Nile tilapia, Oreochromis nilotica, to the extracellular products of Mycobacterium spp. and to various adjuvants. J Fish Dis 21:39–46
Chin A, Glebe BD, Woo PTK (2004) Humoral response and susceptibility of five full-sib families of Atlantic salmon (Salmo salar L.) to the haemoflagellate, Cryptobia salmositica Katz 1951. J Fish Dis 27:471–481
Clem LW, Sizemore RC, Ellsaesser CF, Miller NW (1985) Monocytes as accessory cells in fish immune responses. Dev Comp Immunol 9:803–809
Ellis AE (1977) The leucocytes of fish: a review. J Fish Biol 11:453–491
Feng S, Woo PTK (1996) Biological characterization of a monoclonal antibody against a surface membrane antigen on Cryptobia salmositica Katz 1951. Parasitol Res 82:604–611
Hernandez DE, Gonzalez N, Rios R, Merchan L, Wuani H (1983) Phagocytosis in patients with sickle cell disease. J. Clin Lab Immunol 12:137–140
Jeney G, Anderson DP (1993) Glucan injection or bath exposure given alone or in combination with a bacterin enhance the non-specific defence mechanisms in rainbow trout (Oncorhynchus mykiss). Aquaculture 116:315–329
Levinsky RJ, Harvey BAM, Rodeck CH, Soothill JF (1983) Phorbol myristate acetate stimulated NBT test: a simple method suitable for antenatal diagnosis of chronic granulomatous disease. Clin Exp Immunol 54:595–598
Li S, Woo PTK (1995) Efficacy of a live Cryptobia salmositica vaccine, and the mechanism of protection in vaccinated Oncorhynchus mykiss (Walbaum) against cryptobiosis. Vet Immunol Immunopathol 48:343–353
Logambal SM, Venkatalakshmi S, Dinakaran, MR (2000) Immunostimulatory effect of leaf extract of Ocimum sanctum Linn. in Oreochromis mossambicus (Peters). Hydrobiologia 430:113–120
Lonnstrom LG, Rahkonen R, Lunden T, Pasternack M, Koskela J, Grondahl, A (2001) Protection, immune response and side effects in whitefish (Coregonus lavaretus L.) vaccinated against vibriosis and furunculosis. Aquaculture 200:271–284
Mehta M, Woo PTK (2002) Acquired cell-mediated protection in rainbow trout, Oncorhynchus mykiss, against the haemoflagellate, Cryptobia salmositica. Parasitol Res 88:956–962
Muiswinkel WB van (1995) The piscine immune system: innate and acquired immunity. In: Woo PTK (ed) Fish diseases and disorders, vol 1. Protozoan and metazoan infections. CAB International, Wallingford, pp 729–750
Munoz P, Sitja-Bobadilla A, Alvarez-Pellitero P (2000) Cellular and humoral immune response of European sea bass (Dicentrarchus labrax L.) (Teleostei: Serranidae) immunized with Sphaerospora dicentrachi (Myxosporea: Bivalvulida). Parasitology 120:465–477
Muona M, Virtanen E (1993) Effect of dimethylglycine and trimethylglycine (betaine) on the response of Atlantic salmon (Salmo salar L.) smolts to experimental Vibrio anguillarum infection. Fish Shell Immunol 6:439–449
Pathiratne A, Rajapakshe W (1998) Hematological changes associated with epizootic ulcerative syndrome in the Asian cichlid fish Etroplus suratensis. Asian Fish Sci 3/4:203–211
Rowley A (1990) Collection, separation, and identification of fish leukocytes. In: Stolen JS, Fletcher TC, Anderson DP, Roberson BS, van Muiswinkel WB (eds) Techniques in fish immunology FITC 1. SOS, Fairhaven, pp 113–136
Secombes CJ (1994a) Cellular defences in fish: an update. In: Pike AW, Lewis JW (eds) Parasitic diseases of fish. Samara, Dyfed, pp 209–224
Secombes CJ (1994b) Macrophage activation in fish. In: Stolen JS, Fletcher TC (eds) Modulators of fish immune responses, vol 1. SOS, Fairhaven, pp 49–58
Sitja-Bobadilla A, Woo PTK (1994) An enzyme-linked assay (ELISA) for the detection of antibodies against the pathogenic haemoflagellate, Cryptobia salmositica Katz, and protection against cryptobiosis in juvenile rainbow trout, Oncorhynchus mykiss (Walbaum), inoculated with a live vaccine. J Fish Dis 17:399–408
Stasiak SA, Baumann PC (1996) Neutrophil activity as a potential bioindicator for contaminant analysis. Fish Shell Immunol 6:537–539
Thomas PT, Woo PTK (1990) In vivo and in vitro cell-mediated immune responses of Oncorhynchus mykiss against Cryptobia salmositica (Sarcomastigophora: Kinetoplastida). J Fish Dis 15:443–447
Woo PTK (1978) The division process of Cryptobia salmositica in experimentally infected rainbow trout, (Salmo gairdneri). Can J Zool 47:36–48
Woo PTK (2003) Cryptobia (Trypanoplasma) salmositica and salmonid cryptobiosis. J Fish Dis 26:627–646
Woo PTK, Li S (1990) In vitro attenuation of Cryptobia salmositica and its use as a live vaccine against cryptobiosis in Oncorhynchus mykiss. J Parasitol 76:752–755
Woo PTK, Wehnert SD (1983) Direct transmission of a haemoflagellate, Cryptobia salmositica (Katz, 1951; Kinetoplastida: Bodonina) between rainbow trout under laboratory conditions. J Protozool 39:334–337
Yasutake WT, Wales JH (1983) Microscopic anatomy of salmonids: an atlas. U.S. Dept. of the Interior, Fish and Wildlife Service Resource Publication 150, Washington
Acknowledgements
This study was supported by grants from AquaNet, Canadian Network Centres of Excellence for Aquaculture (Animal Production Project no. 19) to P.T.K.W. We gratefully acknowledge B. Glebe for providing the Atlantic salmon and F.C. Guo for providing technical assistance. The research was conducted at the Aquatic Sciences Facility (Hagen Aqualab) of the University of Guelph.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chin, A., Woo, P.T.K. Innate cell-mediated immune response and peripheral leukocyte populations in Atlantic salmon, Salmo salar L., to a live Cryptobia salmositica vaccine. Parasitol Res 95, 299–304 (2005). https://doi.org/10.1007/s00436-004-1270-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-004-1270-x