Abstract.
This study describes the possible role of Mg2+-dependent ecto-ATPase activity on the Trypanosoma cruzi–host cell interaction. Mg2+-dependent ecto-ATPase activity is observed on the cell body and flagellar membranes of the parasite and is about 20 times greater in trypomastigotes, as compared with epimastigotes. Suramin (a competitive antagonist of P2 receptors) and the impermeant agent 4,4′-diisothiocyanostylbene 2′,2′-disulfonic acid (DIDS), both inhibitors of ecto-ATPases, strongly inhibited ATPase activity and the adhesion and internalization of both evolutive forms by mouse resident macrophages. Suramin inhibited the growth of epimastigotes, suggesting a direct participation of ecto-ATPase activity in this process. To overcome the presence of suramin in the culture medium during the time of growth, Mg2+ ecto-ATPase activity was enhanced 4-fold, as compared with control parasites. The over-expression in enzyme activity was followed by a dramatic increase in the adhesion of epimastigotes to resident macrophages above the level observed for non-treated parasites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trypanosoma cruzi is the etiological agent of Chagas′ disease, a chronic debilitating disease highly prevalent in Latin America, with no immunoprophylactic agents available. The life cycle of T. cruzi takes place in vertebrate and invertebrate hosts. Transmission to vertebrates is carried out by insects belonging to the Reduviidae family, through feces contaminated with an infective stage of the parasite, called the metacyclic trypomastigote. In the vertebrate host, T. cruzi is an obligate intracellular parasite that, to complete its life cycle in different nucleated cell types, must gain access to the cytoplasm of the host cell. The invasion process can be divided into three phases: parasite attachment to the plasma membrane of the host cell, internalization via the formation of a host cell-derived parasitophorous vacuole and disruption of the vacuolar membrane so that the parasite reaches the cytoplasm and replicates into amastigote forms (De Souza 1984). Attachment of the parasite is an energy-dependent process that also requires the presence of specific surface molecules (Schenkman et al. 1991a, 1991b, 1991c). Besides being an energy-dependent process, internalization of T. cruzi is dependent on the presence of specific glycoproteins (Abuin et al. 1989; Ouaissi et al. 1990; Yoshida et al. 1990; Ortega-Barria and Pereira 1991), mucins (Acosta-Serrano et al, 2001), protein kinase activities (Vieira et al. 1994; Yoshida et al. 2000) and a transient increase in the Ca2+ concentration in the parasite and host cells (Moreno et al. 1994; Burleigh and Andrews 1998).
Internalization of T. cruzi also depends on enzymes whose active sites face the external medium rather than the cytoplasm (Souto-Padrón et al. 1990; Meirelles et al. 1992; Colli 1993; Schenkman and Eichinger 1993; Schenkman et al. 1994; Scharfstein et al. 2000). The activities of these enzymes, referred to as ecto-enzymes, can be measured using intact cells (Furuya et al. 1998; Meyer-Fernandes et al. 2000). Some examples are trans-sialidase (Colli 1993; Ming et al. 1993; Vieira et al. 1994; Burleigh and Andrews 1998; Yoshida et al. 2000), cysteine proteinase (Souto-Padrón et al. 1990; Meirelles et al. 1992; Colli 1993; Schenkman and Eichinger 1993; Moreno et al. 1994; Scharfstein et al. 2000) and the protein tyrosine phosphatase (Zhong et al. 1998) of T. cruzi. Different functions in host-cell infection have been attributed to these enzymes. More recently, considerable progress was achieved in the study of cell membrane ecto-ATPases (Plesner 1995; Dombrowski et al. 1998). They hydrolyze extracellular nucleoside tri-and/or diphosphates such as ATP which, in contrast to the established view, can be found in significant concentrations outside the cells (Dombrowski et al. 1998). Extracellular ATP mediates diverse effects by interacting with P2 receptors (purine, pyrimidine receptors) and several hypothesis about putative ecto-ATPase functions have been proposed, such as: (1) regulation of P2 receptors, neurotransmission and signal transduction (Dubyak and El-Moatassim 1993), (2) involvement in cellular adhesion and cancer metastasis (Aurivillius et al. 1990; Dzhandzhugazyan and Bock 1993; Dzhandzhugazyan et al. 1998), (3) modulation of apoptosis (Zheng et al. 1991) and (4) regulation of NO (Yagi et al. 1994) and the production of reactive oxygen radicals (Nakanishi et al. 1991). Filippini et al. (1990) showed that ATP can kill different cells, with the exception of those that express a high level of ATP-breakdown activity on their surfaces.
Ecto-ATPase activities were identified in different cells and tissues from different animals (Plesner 1995) and on the cell surface of protozoan parasites from the genera Toxoplasma (Nakaar et al. 1998), Leishmania (Meyer-Fernandes et al. 1997; Berrêdo-Pinho et al. 2001; Peres-Sampaio et al. 2001), Entamoeba (Barros et al. 2000), Trichomonas (Jesus et al. 2002) and Crithidia (Lemos et al. 2002). In some of these parasites, the ecto-ATPase activity is associated with virulence and the evasion of parasites from the host defense mechanisms (Barros et al. 2000; Berrêdo-Pinho et al. 2001).
In this paper, we present evidence for a correlation between the extracellular ATP hydrolysis catalyzed by an ecto-ATPase and the adhesion and internalization of T. cruzi by resident macrophages.
Materials and methods
Reagents
All reagents were purchased from E. Merck (Darmstadt, Germany) or Sigma Chemical Co. (St Louis, MO). [γ32P] ATP was prepared as described by Glynn and Chappell (1964). Deionized water (MilliQ system of resins; Millipore Corp., Belford, Mass.) was used in the preparation of all solutions.
Parasites
The Y strain of Trypanosoma cruzi was used throughout this study. It was isolated from an acute case of Chagas′ disease (Silva and Nussenzweig 1953) and exhibited an in vivo tropism for mouse macrophages. Epimastigote forms were maintained at 28 °C in LIT medium (Camargo 1964) supplemented with 10% fetal calf serum (FCS) and used on cultivation days 1–3. Parasites were collected by centrifugation 1,000 g for 10 min and washed once with 10 mM phosphate-buffered saline (PBS). For the isolation of trypomastigote forms, LLC-MK 2 cells were infected with tissue culture trypomastigotes. After 5–6 days, the supernatant was collected, centrifuged at 500 g for 5 min and allowed to stand at 37 °C for 30 min. During this period, the trypomastigotes in the pellet moved into the supernatant medium which was then collected and centrifuged at 1,000 g for 10 min. Cellular viability was assessed, before and after incubation, by motility and trypan blue dye exclusion (Dutra et al. 2001).
DIDS, suramin and ATP treatments
Epimastigotes were grown for 1–7 days in the absence or presence of 500 µM suramin. About 1×106 cell/ml were incubated per tube and the cellular growth was estimated daily by counting parasites in a Neubauer chamber.
Epimastigote and trypomastigote forms were submitted to treatment with 500 µM DIDS or suramin for 1 h before interaction with resident macrophages. Trypomastigote–host cell interaction was also analyzed in the presence of 50, 100 and 200 µM ATP. Cellular viability was assessed, before and after incubation, by motility and trypan blue exclusion (Dutra et al. 2001).
Macrophages
Peritoneal macrophages from normal 6- to 8-week-old male Swiss mice were collected in Hanks′ balanced salt solution and plated onto glass coverslips in 24-well tissue culture plates (Falcon; Becton Dickinson Labware, New Jersey, N.J.). The cells were allowed to adhere for 30 min at 37 °C in a 5% CO2 atmosphere, after which the non-adhering cells were removed and fresh culture medium (RPMI 1640 plus 10% FCS) was added. Adhered cells were then incubated overnight under the same conditions as above before the interaction assays.
Ecto-ATPase activity measurements
Intact parasites were incubated for 1 h at 30 °C in 500 µl of a mixture containing, unless otherwise specified, 116 mM NaCl, 5.4 mM KCl, 5.5 mM d-glucose, 50 mM Hepes-Tris buffer (pH 7.2), 5 mM ATP and 1.0×108 cells/ml in the absence or presence of 5 mM MgCl2. Mg2+-dependent ecto-ATPase activity was calculated from the total activity, measured in the presence of 5 mM MgCl2, minus the basal activity, measured in the absence of MgCl2. ATPase activity was determined by measuring the hydrolysis of [γ-32P]ATP (104 Bq/nmol ATP; Lemos et al. 2000). The experiments were started by the addition of living cells and terminated by the addition of 1 ml of a cold mixture containing 200 mg charcoal in 1 M HCl. The tubes were then centrifuged at 1,500 g for 10 min at 4 °C. Aliquots (500 µl) of the supernatants containing the released inorganic phosphate (32Pi) were transferred to scintillation vials containing 9 ml of scintillation fluid (2 g PPO in 1 l of toluene). ATPase activity was calculated by subtracting the non-specific ATP hydrolysis measured in the absence of cells. ATP hydrolysis was linear with time under the assay conditions used and was proportional to cell numbers. Using other nucleotides, the hydrolytic activities measured under the same conditions described above were assayed spectrophotometrically by measuring the release of Pi from the nucleotides (Lowry and Lopes 1946). The values obtained for ATPase activities measured using both methods (spectrophotometric, radioactive) were exactly the same.
Phosphatase measurements
In addition to the measurements of ecto-ATPase activity, ecto-p-nitrophenylphosphatase activity was determined in the same medium as that for ATP hydrolysis, except that ATP was replaced by 5 mM p-nitrophenylphosphate. The reaction was determined spectrophotometrically at 425 nm, using a molar extinction coefficient of ε=14.3×103 (Meyer-Fernandes et al. 1999).
Parasite–macrophage interaction
Parasites and macrophages were left in contact at 37 °C in a parasite–macrophage ratio of 20:1 in RPMI 1640 medium. A set of experiments was simultaneously carried out at 4 °C in order to discriminate the adhesion and internalization phases. Coverslips were collected after 30 min at 4 °C or 1 h at 37 °C, rinsed in PBS, fixed in Bouin′s fixative and stained with Giemsa. The percentages of infected macrophages or those containing adhered parasites were determined by counting 1,000–2,000 cells on duplicate or triplicate coverslips of each preparation; and each experiment was repeated at least three times. The adhesion indices were determined by multiplying the percentage of macrophages with adhered parasites by the mean number of parasites per cell. The endocytic indices were determined by multiplying the percentage of infected cells by the mean number of parasites per cell. In each experiment, the endocytic indices obtained were normalized by considering the value obtained for the control as 100. The results obtained in experiments in which the parasites were treated with suramin, DIDS or ATP were expressed as a percentage in relation to the controls.
Scanning electron microscopy
For scanning electron microscopy, macrophages were plated onto glass coverslips and cultivated overnight, as already described. After interaction with parasites, coverslips were washed twice with Ringer′s solution and fixed with a solution containing 2.5% glutaraldehyde and 4% formaldehyde in 100 mM cacodylate buffer (pH 7.2) for 1 h at room temperature. They were then washed with cacodylate buffer and post-fixed with 1% OsO4 in 100 mM cacodylate buffer for 10 min, dehydrated in ethanol, critical-point-dried in CO2, covered with a layer of gold and observed in a JEOL JSM-5310 scanning electron microscope.
Transmission electron microscopy
For cytochemical detection of Mg2+ ecto-ATPase activity, control and suramin-treated trypomastigotes were fixed in 1% glutaraldehyde in 100 mM cacodylate buffer (pH 7.2) with 100 mM sucrose for 10 min at room temperature. After that, cells were washed twice in 100 mM cacodylate buffer and twice in Tris-maleate buffer (pH 7.2). Wachstein and Meisel medium (Wachstein and Meisel 1957) with a little modification was used for the detection of Mg2+-activated ATPase. The medium had the following composition: 50 mM Tris-maleate buffer (pH 7.2), 2 mM adenosine-5′-triphosphate, 100 mM MgSO4, 100 mM sucrose and 3 mM CeCl3. This medium was filtered before use. Cells were incubated for 1 h at 37 °C, after which they were rinsed twice in 50 mM Tris-maleate buffer and twice in 100 mM cacodylate buffer (pH 7.2). Parasites were fixed again in 2.5% glutaraldehyde in 100 mM cacodylate buffer and post-fixed in 1% osmium tetroxide in 100 mM cacodylate buffer for 1 h at room temperature. Cells were then rinsed in 100 mM cacodylate buffer, dehydrated in acetone and embedded in Polybed 812. Thin sections obtained with a Reichert Ultracut S were observed unstained, using a ZEISS 900 transmission electron microscope operating at 80 kV.
Statistical analysis
All experiments were performed in triplicates, with similar results obtained in at least three separate cell suspensions. Statistical significance was determined by Student′s t-test. Significance was considered as P<0.05.
Results
Suramin inhibits Mg2+ ecto-ATPase activity
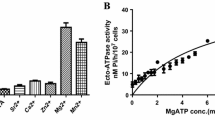
Living epimastigote and trypomastigote forms of Trypanosoma cruzi (108 cells) were able to hydrolyze ATP at a rate of 27.9±2.6 nmol Pi/h and 596.4±61.7 nmol Pi/h, respectively (Fig. 1). Mg2+-dependent ecto-ATPase activity on the surface of trypomastigote forms was about 20 times higher, when compared with epimastigote forms (Fig. 1). Incubation of parasites for 1 h in the presence of 500 µM suramin promoted a reduction of about 80% in the Mg2+-dependent ecto-ATPase activity of epimastigotes and trypomastigotes (Fig. 1). In the cytochemical analysis of the control trypomastigotes, the reaction product appeared as an electron-dense layer homogeneously distributed throughout the plasma and flagellar membranes (Fig. 2A). However, analysis by transmission electron microscope showed a substantial variability in the intensity of labeling among trypomastigotes of the same sample (data not shown). A faint or no reaction was observed after suramin treatment (Fig. 2B).
Biochemical detection of Mg2+-dependent ecto-ATPase activity in Trypanosoma cruzi. Epimastigotes (black columns) or trypomastigotes (white columns) at 1×108 cells/ml were incubated for 1 h in a reaction medium containing 10 mM Hepes (pH 7.2), 116 mM NaCl, 5.4 mM KCl, 5.5 mM d-glucose, 5 mM Tris-ATP (with [γ-32P]ATP at 104 Bq/nmol ATP) and 5 mM MgCl2 in the absence or presence of 500 µM suramin. Data are means±SE of three determinations with different cell suspensions. Pi Inorganic phosphate
Cytochemical detection of Mg2+-dependent ecto-ATPase activity in T. cruzi. A General aspect of trypomastigote forms, showing the presence of reaction product indicative of the Mg2+-dependent ecto-ATPase activity, in association with the flagellar and cell-body membranes. B No reaction product was seen when parasites were previously treated with 500 µM suramin for 1 h. FP Flagellar pocket, K kinetoplast. Bar 1 µm
Mg2+-dependent ecto-ATPase and parasite–host cell interaction
When the interaction of epimastigotes with macrophages was performed at 4 °C, the parasites only adhered to the macrophage surface (Table 1). Adhesion of epimastigotes and trypomastigotes to macrophages was inhibited about 63% and 45%, respectively, when parasites were treated with DIDS and about 54% and 66%, respectively, when epimastigotes and trypomastigotes were treated with suramin. DIDS and suramin did not change the mean number of adhered epimastigotes and trypomastigotes per macrophage. The internalization of parasites made during 1 h at 37 °C also changed when the parasites were pre-incubated in the presence of the drugs. There was an accentuated decrease, of about 50% and 38% for DIDS and about 35% and 48% for suramin, in the infected macrophages with epimastigote and trypomastigote forms, respectively. No significant changes were observed in the mean number of parasites per infected macrophage (Table 1). The addition of suramin during the trypomastigote–macrophage interaction decreased adhesion and internalization at the same rate as described for pre-treated trypomastigotes. The interaction between trypomastigote forms and resident macrophages in the presence of ATP was also analyzed. As shown in Table 2, ATP in concentrations above 100 μM significantly increased both the percentage of infected macrophages and the endocytic index. No significant differences were observed in the mean number of trypomastigotes per infected macrophage (Table 2).
Suramin treatment and parasite growth
Epimastigote growth was inhibited by suramin and a reduction of 70% was observed on day 5 of culture (Fig. 3). Suramin did not induce metacyclogenesis. The number of trypomastigote forms after 7 days of growth in LIT medium supplemented or not with 500 µM suramin did not differ (data not shown). The cellular integrity and viability of epimastigotes during growth was evaluated by analysis of cell motility and trypan blue dye exclusion. They were not affected by any of the conditions used in the assays.
Suramin effect on epimastigote growth, shown by the growth curve of epimastigote forms of T. cruzi cultivated in LIT medium (Camargo 1964) for 7 days at 28 °C in the absence (black circles) or presence (white circles) of 500 µM suramin. Values shown are means of determinations in triplicate from three different experiments. mL Milliliters
Modulation of Mg2+-dependent ecto-ATPase activity by suramin
After 24 h of cultivation, the ATPase activity was about 126 nmol Pi/h for 108 cells and, after 48 h and 72 h, the enzymatic activity had a significant reduction (about 48%, 80%, respectively; Fig. 4). Epimastigote forms cultivated in LIT medium containing 500 µM suramin showed an increase in Mg2+ATPase activity, when compared with epimastigotes grown in the absence of suramin during the same period of time (Fig. 4). The ATPase activity of parasites grown in the presence of the drug also decreased with the time of cultivation. This decrease, however, was less accentuated when compared with control cells (Fig. 4). Epimastigotes (108 cells) grown with suramin during 72 h hydrolyzed ATP at 100.3±10.4 nmol Pi/h (in contrast with control cells which hydrolyzed ATP at 25.9±1.9 nmol Pi/h), which represents only 25% of the ATPase activity obtained with suramin-treated parasites. Growth in the presence of suramin increased the Mg2+-dependent ecto-ATPase activity and did not select a resistant population of epimastigotes. A further treatment with suramin caused an inhibition of the Mg2+-dependent ecto-ATPase activity similar to that obtained for control cells (data not shown). Figures 4 and 5 show that epimastigotes grown with suramin during 72 h present a Mg2+-dependent ecto-ATPase activity more than three times higher than control cells, while no differences were observed in the other phosphomonoesterase activities (Fig. 5).
Effect of long-term treatment with suramin on Mg2+-dependent ecto-ATPase activity. Epimastigotes (at 1×108 cells/ml) were grown for 24, 48 and 72 h in LIT medium supplemented with 10% fetal calf serum (FCS) in the absence (black columns) or presence (white columns) of 500 µM suramin and then incubated for 1 h at 28 °C in the reaction medium described in Fig. 1
Effect of long-term treatment with suramin on the activity of distinct ecto-enzymes of T. cruzi. Epimastigotes (at 1×108 cells/ml) were grown for 72 h in LIT medium supplemented with 10% FCS in the absence (black columns) or presence (white columns) of 500 µM suramin and then incubated for 1 h at 28 °C in distinct incubation media for the detection of Mg2+-dependent ecto-ATPase, ecto-phosphatase (pNPPase), 5′-nucleotidase (5′AMPase) and 3′-nucleotidase (3′AMPase). The values represent means of at least three independent experiments performed in triplicate
Enhancement of Mg2+-dependent ecto-ATPase activity and parasite adhesion
Scanning electron microscopic analysis of the epimastigote–macrophage interaction process showed an accentuated increase in the number of adhered epimastigotes grown in the presence of suramin during 72 h adhered to macrophages. Frequently, more than 4 parasites were observed adhered to the surface of resident macrophages when the interaction was carried out for 1 h at 37 °C. (Fig. 6). There was also a significant increase in the percentage of macrophages with attached suramin-treated parasites, as compared with control cells (Table 3). The increase was about 62% when the interaction was made during 30 min at 4 °C and was 375% during 1 h at 37 °C. At 4 °C, there was a slightly increase in the mean number of adhered parasites per macrophage. However, this number was significantly greater when interaction was made at 37 °C (Fig. 6). The binding indices for suramin-treated parasites at 4 °C and 37 °C were respectively 66% and 560% greater than that obtained for control cells submitted to the same experimental conditions (Table 3).
Scanning electron images of the interaction between epimastigotes and mouse resident macrophages. Epimastigotes grown in LIT medium for 72 h in the absence (A) or presence (B) of 500 μM suramin were incubated for 1 h at 37 °C with macrophages and processed for scanning electron microscopy. Suramin induced a significant increase in the number of adhered epimastigotes per macrophage. Bar 10 μm
Discussion
During the past two decades, considerable progress has been achieved in the study of ectonucleotidases in general (Zimmermann 1996) and ecto-ATPases in particular (Plesner 1995; Dombrowski et al. 1998). This progress is related to the finding that, in contrast to the established view, nucleotides can be found in significant concentrations outside cells (Gordon 1986; Dombrowski et al. 1998). Nucleotides released to the extracellular medium may exert their effects on other cells in the vicinity of the secretion site and modulate biological processes, by binding to specific cell-surface receptors (Dombrowski et al. 1998). The role of Mg2+-dependent ecto ATPase activity on the surface of parasites is still the subject of speculation. In some parasites, such enzymatic activity is considered an important extracellular signal in the regulation of virulence (Barros et al. 2000; Berrêdo-Pinho et al. 2001; Peres-Sampaio et al. 2001; Jesus et al. 2002). In Toxoplasma gondii the secreted E-type ATPase (NTPase) may play a role in initiating parasite egress, since treatment of infected host cells with dithiols (e.g. dithiotheitol) activates the ATPase activity and stimulates a mass parasite exodus, associated with a depletion of host-cell ATP levels (Silverman et al. 1998; Carruthers 1999). In T. gondii, two isoforms of an E-type ATPase were isolated. The avirulent strains expressed only the NTPase 1 isoform, which is a true apyrase, whereas the virulent strains expressed NTPase 1 and the NTPase 3 isoform, which cleaves predominantly nucleotide triphosphates (Bermudes et al. 1994). Recently, it was shown that the invasive ameba Entamoeba histolytica has a much higher Mg2+-dependent ecto-ATP diphosphohydrolase activity than non-invasive E. histolytica and the free-living ameba E. moshkovskii (Barros et al. 2000). This ameba E-type ATPase is stimulated more than 2-fold by d-galactose (Barros et al. 2000), an important molecule involved with E. histolytica adhesion (Radvin et al. 1980). So, it was proposed this enzyme may be a pathogenesis marker for this cell (Barros et al. 2000). In Leishmania amazonensis, virulent promastigotes showed a Mg2+-dependent ecto-ATPase activity 2-fold higher than that observed in avirulent promastigotes (Berrêdo-Pinho et al. 2001). In the present study, we observed that, albeit present in all trypomastigote forms, the amount of electron-dense precipitate indicative of Mg2+-dependent ecto-ATPase activity varied among distinct individuals in the same population. The lack of homogeneity in the distribution of a particular surface molecule on individuals of the same isolate or clone is characteristic of Trypanosoma cruzi (De Souza 1989) and can reflect differences in infectivity, as shown by Pereira et al. (1996).
The ecto-ATPase activity can be inhibited by the impermeant agent DIDS (Knowles 1988; Plesner 1995; Barbacci et al. 1996; Meyer-Fernandes et al. 1997) and by suramin, a polyanionic compound with known anti-parasitic activities that has been shown to be a P2 receptor-antagonist (Chen and Lin 1997). Suramin has also been found to interfere with the mitogenic signaling of P2 receptors to MAP kinases (Neary et al. 1998), to interfere with the binding of growth factors to their receptors at the cell surface (Middaugh et al. 1992) and to inhibit nuclear enzymes and other intracellular enzymatic systems (Voogd et al. 1993; Eisenberger and Reyno 1994). The Mg2+-dependent ecto-ATPase of T. cruzi is inhibited by suramin. This inhibition is dose-dependent and is expressed by an accentuated reduction in the ATP hydrolysis rate and a significant reduction in the electron-dense reaction product observed on the cell surface of epimastigote and trypomastigote forms (Fig. 2). Suramin also inhibits the growth of epimastigote forms without affecting their cell viability or metacyclogenesis rate. Since epimastigote growth in vitro is serum-dependent (O′Daly et al. 1987) and is not enhanced in the presence of exogenous ATP or adenosine (data not shown), we suggest that suramin exerts its growth-inhibitory effects in T. cruzi by preventing the binding of growth factors on the surface of epimastigote forms, as described previously for tumor cell lines (Hosang 1985; Abdiu et al. 1999), inducing a receptor-shedding (Galvani et al. 1995) or interfering with the uptake of host low-density lipoprotein through receptor-mediated endocytosis, as described for African trypanosomes (Vansterkenburg et al. 1993).
Interestingly, the growth of epimastigotes in the presence of suramin induces an increase in Mg2+-dependent ecto-ATPase activity not followed by an increase in other ecto-enzymatic activities, such as 3′ and 5′ nucleotidase and ecto-phosphatase. This fact discards the possibility that enhancement of Mg2+-dependent ecto-ATPase caused by suramin could be caused by subversion of the exocytic traffic of molecules, as described in macrophages (Pesanti 1978). The accumulation of physiologically active molecules on the cell surface by suramin was previously described in glioma cell lines, resulting in an increase in cellular attachment (Hinek et al. 1999). Epimastigotes over-expressing ecto-ATPase activity are not suramin-resistant, since their enzymatic activity is inhibited by a subsequent suramin treatment.
The idea that ecto-ATPase activity could be related to virulence seems to be also applicable to T. cruzi. In this study, we show that trypomastigotes, evolutive forms infective to mammalian hosts, were up to 20 times more efficient than non-infective epimastigotes in hydrolyzing ATP (Fig. 1). Recently, the association of ecto-ATPase activity with adhesion molecules raised the possibility that extracellular nucleotides may play important roles in regulating cell adhesion (Stout et al. 1995; Kirley 1997; Meyer-Fernandes 2002). As is well known, the attachment capacity of parasites is a prerequisite for entry into host cells, in the case of intracellular parasites, or for triggering the cytotoxicity mechanism of extracellular parasites, as described in E. histolytica and Trichomonas vaginalis (Espinosa-Cantellano and Martínez-Palomo 2000).
Another important finding is that the addition of up to 200 μΜ ATP to the interaction medium increased by about 30% the number of macrophages infected by trypomastigote forms. Taking account of the fact that ATP can be released by different kinds of cells, such as neutrophils and endothelial cells (Fredholm 1997), together with the role of these cells in the pathogenesis of Chagas′ disease (Chen et al. 2001; Petkova et al. 2001), we suggest that Mg2+-dependent ecto-ATPase activity on T. cruzi could stimulate the adherence of parasites to host cells and protect them from neutrophil attack, by releasing adenosine to inhibit superoxide production.
References
Abdiu A, Larsson SE, Wasteson A, Walz TM (1999) Suramin blocks growth-stimulatory effects of platelet-derived growth factor on malignant fibrous histiocytomas in vitro. Cancer Lett 146:1890–1894
Abuin G, Colli W, De Souza W, Alves MJM (1989) A surface antigen of Trypanosoma cruzi involved in cell invasion (Tc-85) is heterogeneous in expression and molecular constitution. Mol Biochem Parasitol 35:229–237
Acosta-Serrano A, Almeida IC, Freitas-Junior LH, Yoshida N, Schenkman S (2001) The mucin-like glycoprotein super-family of Trypanosoma cruzi: structure and biological roles. Mol Biochem Parasitol 114:143–150
Aurivillius M, Hansen OC, Lazrek MBS, Bock E, Öbrink B (1990) The cell adhesion molecule Cell-CAM 105 is an ecto-ATPase and a member of the immunoglobulin superfamily. FEBS Lett 264:267–269
Barbacci E, Filippini A, De Cesaris P, Ziparo E (1996) Identification and characterization of an ecto-ATPase activity in rat Sertoli cells. Biochem Biophys Res Commun 222:273–279
Barros FS, De Menezes LF, Pinheiro AAS, Silva EF, Lopes AHCS, De Souza W, Meyer-Fernandes JR (2000) Ectonucleotide diphosphoydrolase activities in Entamoeba histolytica. Arch Biochem Biophys 375:304–314
Bermudes D, Peck KR, Afifi MA, Beckers CJ, Joiner KA (1994) Tandemly repeated genes encode nucleoside triphosphate hydrolase isoforms secreted into the parasitophorous vacuole of Toxoplasma gondii. J Biol Chem 269:29252–29260
Berrêdo-Pinho M, Peres-Sampaio CE, Chrispim PPM, Belmont-Firpo R, Lemos AP, Martiny A, Vannier-Santos MA, Meyer-Fernandes JR (2001) A Mg-dependent ecto-ATPase in Leishmania amazonensis and its possible role in adenosine acquisition and virulence. Arch Biochem Biophys 391:16–24
Burleigh BA, Andrews NW (1998) Signaling and host cell invasion by Trypanosoma cruzi. Curr Opin Microbiol 1:461–465
Camargo EP (1964) Growth and differentiation in Trypanosoma cruzi. I. Origin of metacyclic trypanosomes in liquid media. Rev Inst Med Trop Sao Paulo 6:93–100
Carruthers VB (1999) Armed and dangerous: Toxoplasma gondii uses an arsenal of secretory proteins to infect host cells. Parasitol Int 48:1–10
Chen BC, Lin WW (1997) Inhibition of ecto-ATPase by the P2 purinoceptor agonists, ATPgammaS, alpha,beta-methylene-ATP, and AMP-PNP, in endothelial cells. Biochim Biophys Res Commun 233:442–446
Chen L, Watanabe T, Watanabe H, Sendo F (2001) Neutrophil depletion exacerbates experimental Chagas′ disease in BALB/c, but protects C57BL/6 mice through modulating the Th1/Th2 dichotomy in different directions. Eur J Immunol 31:265–275
Colli W (1993) Trans-sialidase: a unique enzyme activity discovered in the protozoan Trypanosoma cruzi. FASEB J 7:1257–1264
De Souza W (1984) Cell biology of Trypanosoma cruzi. Int Rev Cytol 86:197–283
De Souza W (1989) Components of the cell surface of Trypanosoma cruzi. Prog Protozool 1:87–184
Dombrowski K, Ke Y, Brewer KA, Kapp JA (1998) Ecto-ATPase: an activation marker necessary for effector cell function. Immunol Rev 161:111–118
Dubyak GR, El-Moatassim C (1993) Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol 34:C577–C606
Dutra PML, Dias FA, Rodrigues CO, Romeiro A, Attias M, De Souza W, Lopes AHCS, Meyer-Fernandes JR (2001) Platelet-activating factor modulates a secreted phosphatase activity of the trypanosomatid parasite Herpetomonas muscarum muscarum. Curr Microbiol 43:288–292
Dzhandzhugazyan KN, Bock E (1993) Demonstration of (Ca2+-Mg2+)-ATPase activity of the neural cell adhesion molecule. FEBS Lett 336:279–283
Dzhandzhugazyan KN, Kirkin AF, Straten P thor, Zeuthen J (1998) Ecto-ATP diphosphohydrolase/CD39 is overexpressed in differentiated human melanomas. FEBS Lett 430:227–230
Eisenberger MA, Reyno LM (1994) Suramin. Cancer Treat Rev 20:259–273
Espinosa-Cantellano M, Martínez-Palomo A (2000) Pathogenesis of intestinal amebiasis: from molecules to disease. Clin Microbiol Rev 13:318–331
Filippini A, Taffs RE, Agui T, Sitkovsky MV (1990) Ecto-ATPase activity in cytolytic T-lymphocytes—protection from cytolytic effects of extracellular ATP. J Biol Chem 265:334–340
Fredholm BB (1997) Purines and neutrophil leukocytes. Gen Pharmacol 28:345–350
Furuya T, Zhong L, Meyer-Fernandes JR, Lu H-G, Moreno SNJ, Docampo R (1998) Ecto-protein tyrosine phosphatase activity in Trypanosoma cruzi infective stages. Mol Biochem Parasitol 92:339–348
Galvani AP, Cristiani C, Carpinelli P, Landonio A, Bertolero F (1995) Suramin modulates cellular levels of hepatocyte growth factor receptor by inducing shedding of a soluble form. Biochem Pharmacol 50:959–966
Glynn IM, Chappel JB (1964) A simple method for the preparation of 32P-labelled adenosine triphosphate of high specific activity. Biochem J 90:147–149
Gordon JL (1986) Extracellular ATP: effects, sources and fate. Biochem J 233:309–319
Hinek A, Jung S, Rutka JT (1999) Cell surface aggregation of elastin receptor molecules caused by suramin amplified signals leading to proliferation of human glioma cells. Acta Neuropathol (Berl) 97:399–407
Hosang M (1985) Suramin binds to platelet-derived growth factor and inhibits its biological activity. J Cell Biochem 29:265–273
Jesus JB, Lopes AHCS, Meyer-Fernandes JR (2002) Characterization of an ecto-ATPase of Tritrichomonas foetus. Vet Parasitol 103:29–42
Kirley TL (1997) Complementary DNA cloning and sequencing of the chicken muscle ecto-ATPase. Homology with the lymphoid cell activation antigen CD39. J Biol Chem 272:1076–1081
Knowles AF (1988) Differential expression of ectoMg2+-ATPase and ectoCa2+-ATPase activities in human hepatoma cells. Arch Biochem Biophys 263:264–271
Lemos AP, Peres-Sampaio CE, Guimarães-Motta H, Silva JL, Meyer-Fernandes JR (2000) Effects of naturally occurring polyols and urea on mitochondrial F0F1ATPase. Z Naturforsch Teil C 5:392–398
Lemos AP, Pinheiro AA, Berrêdo-Pinho M, Fonseca de Souza AL, Motta MC, De Souza W, Meyer-Fernandes JR (2002) Ectonucleotide diphosphohydrolase activity in Crithidia deanei. Parasitol Res 88:905–911
Lowry OH, Lopes J (1946) The determination of inorganic phosphate in the presence of labile phosphate esters. J Biol Chem 162:421–428
Meirelles MNL, Juliano L, Carmona E, Silva SG, Costa EM, Murta AC, Scharfstein J (1992) Inhibitors of the major cysteinyl proteinase (GP57/51) impair host cell invasion and arrest the intracellular development of Trypanosoma cruzi in vitro. Mol Biochem Parasitol 52:175–184
Meyer-Fernandes JR (2002) Ecto-ATPases in protozoa parasites: looking for a function. Parasitol Int 51:299–303
Meyer-Fernandes JR, Dutra PML, Rodrigues CO, Saad-Nehme J, Lopes AHCS (1997) Mg2+-dependent ecto ATPase activity in Leishmania tropica. Arch Biochem Biophys 341:40–46
Meyer-Fernandes JR, Silva-Neto MA da, Soares MD, Fernandes E, Vercesi AE, De Oliveira MM (1999) Ecto-phosphatase activities on the cell surface of the amastigote forms of Trypanosoma cruzi. Z Naturforsch Teil C 54:977–984
Meyer-Fernandes JR, Lanz-Mendoza H, Gondim KC, Willott E, Wells MA (2000) Ectonucleotide diphosphohydrolase activities in hemocytes of larval Manduca sexta. Arch Biochem Biophys 382:152–159
Middaugh CR, Mach H, Burke CJ, Volkin DB, Dabora JM, Tsai PK, Bruner MW, Ryan JA, Marfia KE (1992) Nature of the interaction of growth factors with suramin. Biochemistry 31:9016–9024
Ming M, Chuenkova M, Ortega-Barria E, Pereira MEA (1993) Mediation of Trypanosoma cruzi invasion by sialic acid on the host cell and trans-sialidase on the trypanosome. Mol Biochem Parasitol 59:243–252
Moreno SNJ, Silva J, Vercesi AE, Docampo R (1994). Cytosolic free calcium elevation in Trypanosoma cruzi is required for cell invasion. J Exp Med 180:1535–1540
Nakaar V, Beckers CJM, Polotsky V, Joiner KA (1998) Basis for substrate specificity of the Toxoplasma gondii nucleoside triphosphate hydrolase. Mol Biochem Parasitol 97:209–220
Nakanishi M, Takihara H, Minoru Y, Yagawa K (1991) Extracellular ATP itself elicits superoxide generation in guinea pig peritoneal macrophages. FEBS Lett 282:91–94
Neary JT, McCarthy M, Kang Y, Zuniga S (1998) Mitogenic signaling from P1 and P2 purinergic receptors to mitogen-activated protein kinase in human fetal astrocyte cultures. Neurosci Lett 242:159–162
O′Daly JA, Rodriguez MB, Garlin G (1987) Trypanosoma cruzi: growth requirements at different temperature in fetal bovine serum or peptide supplemented media. Exp Parasitol 64:78–87
Ortega-Barria E, Pereira MEA (1991) A novel T. cruzi heparin-binding protein promotes fibroblast adhesion and penetration of engineered bacteria and trypanosomes into mammalian cells. Cell 67:411–421
Ouaissi MA, Taibi A, Cornette J, Velge P, Marty B, Loyens M, Esteva M, Rizvi FS, Capron A (1990) Characterization of major surface and excretory–secretory immunogens of Trypanosoma cruzi trypomastigotes and identification of potential protective antigen. Parasitology 100:115–124
Pereira MEA, Zhang K, Gong Y, Herrera EM, Ming M (1996) Invasive phenotype of Trypanosoma cruzi restricted to a population expressing trans-sialidase. Infect Immun 64:3884–3892
Peres-Sampaio CE, Thorp-Palumbo S, Meyer-Fernandes JR (2001) An ecto-ATPase activity present in Leishmania tropica stimulated by dextran sulfate. Z Naturforch Teil C 56:820–825
Pesanti EL (1978) Suramin effects on macrophage phagolysosome formation and antimicrobial activity. Infect Immun 20:503–511
Petkova SB, Huang H, Factor SM, Pestell RG, Bouzahzah B, Jelicks LA, Weiss LM, Douglas SA, Wittner M, Tanowitz HB (2001) The role of endothelin in the pathogenesis of Chagas′ disease. Int J Parasitol 31:499–511
Plesner L (1995) Ecto-ATPases: identities and functions. Int Rev Cytol 158:141–214
Radvin JI, Croft BY, Guerrant RL (1980) Cytopathogenic mechanisms of Entamoeba histolytica. J Exp Med 152:377–390
Scharfstein J, Schmitz V, Morandi V, Capella MM, Lima AP, Morrot A, Juliano L, Muller-Esterl W (2000) Host cell invasion by Trypanosoma cruzi is potentiated by activation of bradykinin B(2) receptors. J Exp Med 192:1289–1300
Schenkman S, Eichinger D (1993) Trypanosoma cruzi trans-sialidase and cell invasion. Parasitol Today 99:218–222
Schenkman S, Diaz C, Nussenzweig V (1991a) Attachment of Trypanosoma cruzi trypomastigotes to receptors at restricted cell surface domains. Exp Parasitol 72:76–86
Schenkman S, Jiang MS, Harth GW, Nussenzweig V (1991b) A novel cell surface trans-sialidase of Trypanosoma cruzi generates a stage-specific epitope required for invasion of mammalian cells. Cell 65:1117–1125
Schenkman S, Robbins ES, Nussenzweig V (1991c) Attachment of Trypanosoma cruzi to mammalian cells requires parasite energy, and invasion can be independent of the target cell cytoskeleton. Infect Immun 59:645–654
Schenkman S. Eichinger D, Pereira MEA, Nussenzweig V (1994) Structural and functional properties of Trypanosoma trans-sialidase. Annu Rev Microbiol 48:499–523
Silva LHP, Nussenzweig V (1953) Sobre uma cepa de Trypanosoma cruzi altamente virulenta para o camundongo branco. Folia Clin Biol 20:191–207
Silverman JA, Qi H, Riehl A, Beckers C, Nakaar V, Joiner KA (1998) Induced activation of the Toxoplasma gondii nucleoside triphosphate hydrolase leads to depletion of host cell ATP levels and rapid exit of intracellular parasites from infected cells. J Biol Chem 273:12352–12359
Souto-Padrón T, Campetella OE, Cazzulo JJ, De Souza W (1990) Cysteine proteinase in Trypanosoma cruzi: immunocytochemical localization and involvement in parasite–host cell interaction. J Cell Sci 96:485–490
Stout JG, Strobel RS, Kirley TL (1995) Properties of and proteins associated with the extracellular ATPase of chicken gizzard smooth muscle. A monoclonal antibody study. J Biol Chem 270:11845–11850
Vansterkenburg EL, Coppens I, Wilting J, Bos OJ, Fischer MJ, Janssen LH, Opperdoes FR (1993) The uptake of the trypanocidal drug suramin in combination with low-density lipoproteins by Trypanosoma brucei and its possible mode of action. Acta Trop 54:237–250
Vieira MC, Carvalho TU de, De Souza W (1994) Effect of protein kinase inhibitors on the invasion process of macrophages by Trypanosoma cruzi. Biochem Biophys Res Commun 203:967–971
Voogd TE, Vansterkenburg EL, Wilting J, Janssen LH (1993) Recent research on the biological activity of suramin. Pharmacol Rev 45:177–203
Wachstein M, Meisel E (1957) Histochemistry of hepatic phosphatases at a physiologic pH with special reference to the demonstration of bile canaliculi. Am J Clin Pathol 27:13–23
Yagi K, Nishino I, Eguchi M, Kitagawa M, Miura Y, Mizoguchi T (1994) Involvement of ecto-ATPase as an ATP receptor in the stimulatory effect of extracellular ATP on NO release in bovine aorta endothelial cells. Biochem Biophys Res Commun 203:1237–1243
Yoshida N, Blanco AS, Araguth MF, Russo M, Gonzalez J (1990) The stage-specific 90-kilodalton surface antigen of metacyclic trypomastigotes of Trypanosoma cruzi. Mol Biochem Parasitol 39:39–46
Yoshida N, Favoreto S Jr, Ferreira AT, Manque PM (2000) Signal transduction induced in Trypanosoma cruzi metacyclic trypomastigotes during the invasion of mammalian cells. Braz J Med Biol Res 33:269–278
Zheng LM, Zychlinsky A, Liu CC, Ojcius DM, Young JD (1991) Extracellular ATP as a trigger for apoptosis or programmed cell death. J Cell Biol 112:279–288
Zhong L, Lu H-G, Moreno SNL, Docampo R (1998) Tyrosine phosphate hydrolysis of host proteins by Trypanosoma cruzi is linked to cell invasion. FEMS Microbiol Lett 161:15–20
Zimmermann H (1996) Extracellular purine metabolism. Drug Dev Res 39:337–352
Acknowledgements.
The authors wish to thank Antonio Bosco Carlos, Eliandro Lima and Fabiano Ferreira Esteves for their valuable technical assistance. This work was supported by the Brazilian agencies PRONEX (grant 0885), CNPq, CNPQ/UFRJ (PIBIC), FAPERJ and FUJB/UFRJ.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bisaggio, D.F.R., Peres-Sampaio, C.E., Meyer-Fernandes, J.R. et al. Ecto-ATPase activity on the surface of Trypanosoma cruzi and its possible role in the parasite–host cell interaction. Parasitol Res 91, 273–282 (2003). https://doi.org/10.1007/s00436-003-0965-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-003-0965-8