Abstract
Phosphoglycerate kinase (PGK) is an enzyme that produces one ATP molecule in the glycolytic pathway. Clonorchis sinensis is largely dependent on glycolysis for energy production. We performed immunoelectron microscopy on adult C. sinensis by using mouse immune serum raised against recombinant C. sinensis PGK. A high density of gold particles was found in the microvilli of the intestinal epithelium and in lamellae of the sperm duct. PGK was common in the somatic cells of intra-uterine eggs and in excreted products. It was localized with moderate intensity in muscular fibers of the subtegumental muscle layer, and in the myoepithelia of the intestine and excretory bladder. We suggest that PGK plays an essential role in C. sinensis energy production for movement via muscle contraction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clonorchis sinensis is a member of the class Trematoda, which includes the schistosomes. Schistosomes are anaerobic parasitic helminths that utilize glucose as their main energy source. They consume glucose every hour at the equivalent of 26% of their dry body weight (Lee et al. 1995). C. sinensis absorbs glucose from its environment (Seo and Lee 1971) and utilizes it for energy production through the glycolytic pathway (Kang et al. 1969). Phosphoglycerate kinase (PGK) catalyzes the conversion of 1,3-diphosphoglycerate and ADP into 3-phosphoglycerate and ATP. PGK is highly conserved from eubacteria to eukaryotic animals, implying that this enzyme is vital in these organisms (Fabry et al. 1990).
PGK has been localized in organs containing muscular tissues in diverse animals. In trematodes, PGK is associated with microtubules found in the tegument, and has been suggested to play a role in producing and supplying energy for repairing damaged tegumental cells (Goudot-Crozel et al. 1989; Lee et al. 1995). Immunohistochemically, PGK has been localized to muscle tissues of the oral and ventral suckers, and of the subtegument in C. sinensis (Hong et al. 2000). This study was performed to elucidate its ultrastructural distribution and to obtain insight into its physiological function in adult C. sinensis.
Materials and methods
Metacercariae of C. sinensis were collected from topmouth gudgeons, Pseudorasbora parva, by artificial digestion, and then fed to New Zealand white rabbits. Adult C. sinensis were recovered from rabbit bile ducts after 7 months. The flukes were fixed in 2% paraformaldehyde/0.4% glutaraldehyde, pH 7.4 for 1 h at room temperature. Fixed flukes were dehydrated in an alcohol series, embedded in LR gold resin (London Resin, UK) and polymerized under UV light. Ultrathin sections were cut at 90 nm and mounted on a nickel grid. The ultrathin sections were immunogold-labeled by following a procedure described previously (Yu and Chai 1995). Mouse immune serum raised against C. sinensis recombinant PGK was used as the primary antibody at a dilution of 1:500–1:1,000. The secondary antibody used was goat anti-mouse IgG, 5 nm colloidal gold-conjugated (Sigma). Pre-immune serum was employed as a negative control and run in parallel to the main experiment. The stained sections were observed under a transmission electron microscope (Jeol 1200EXII, Tokyo, Japan).
Results
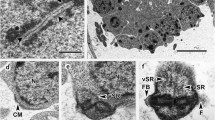
Cytoplasmic processes of the intestinal epithelium were most densely labeled with gold colloids; the cytoplasm and nucleus of intestinal epithelial cells were moderately labeled. Myoepithelial cells were moderately labeled (Fig. 1). In the wall of the excretory bladder, moderate gold-labeling was observed in the microvilli, in the cytoplasm of the epithelial cells and in the myoepithelial cells.
In the vas deferens, gold particles were found at a moderate density in the cytoplasm and nuclei of the epithelial cells. The lamellae of the epithelium and sperm were also labeled moderately (Fig. 2). Secretory granules in the epithelial cells were unlabeled.
Intra-uterine eggs containing miracidia showed the highest density of gold particle labeling in adult C. sinensis. Somatic cells with high electron density were found to be heavily labeled. In intra-uterine eggs, labeling was found in the secretory-excretory product (Fig. 3). Small bodies of low electron density were unlabeled.
The tegumental surface was highly convoluted. The tegumental syncytium contained many electron-dense bodies. Secretory bodies consisting of 10–20 secretory granules appeared in the syncytium and subtegumental muscle layers and tissues. Gold particles were found with moderate density in the tegumental syncytium and the subtegumental muscle fibers. Cytoplasm and the cytoplasmic processes of cytons were labeled with gold particles with a higher density than muscle fibers. The nuclei of cytons appeared gold-labeled with moderate density. Mesenchymal tissue under the tegument was gold-labeled at low density (data not shown).
Discussion
In adult C. sinensis, large amounts of exogenous glycogen were detected in the muscle fibers of subtegument, oral and ventral suckers, pharynx and proximal uterus (Seo and Lee 1971), suggesting that glycogen is metabolized through glycolysis and supplies energy for muscle contraction (Hong et al. 2000). In this study, PGK was localized in myoepithelial cells surrounding the intestine and sperm ducts (vas deferens). From this finding, we suggest that the glycolytic pathway provides energy for the muscular tissues for organ motility and worm locomotion.
In schistosomes, several glycolytic enzymes, such as PGK, glyceraldehyde 3-phosphate dehydrogenase and triose phosphate isomerase, have been localized in the tegument (Goudot-Crozel et al. 1989; Shoemaker et al. 1992; Lee et al. 1995). Actins have been found in the cytosol of the syncytium under regenerating tegument (Matsumoto et al. 1988), and were associated with enzymes of the glycolytic pathway (Arnold et al. 1971; Bronstein and Knull 1981; Poglazov 1983). Collectively, the repair of damaged tegument utilizes the glycolysis pathway to provide the required energy (Lee et al. 1995).
In C. sinensis, PGK was localized in cell nuclei. During DNA replication, DNA polymerase α replicates the leading strand in the nucleus. Of the DNA polymerase α, primer recognition proteins, namely, PGK and annexin 2 were found in the nucleus of hepatic and splenic cells (Kumble and Vishwanatha 1991). It is, therefore, possible in C. sinensis that PGK plays a role in DNA replication.
During spermatogenesis in mammals, PGK is expressed from spermatids at stage 12 to spermatozoa which begin to be motile (Vandeberg et al. 1981; Bluthmann et al. 1982). In C. sinensis, PGK was heavily localized in the testes and sperm in the seminal vesicle and the seminal receptacle (Hong et al. 2000), as well as in sperm in the sperm duct in the present study, suggesting that the glycolytic pathway supplies energy and/or the intermediates needed for sperm physiology.
Because of the high concentration of PGK in the intra-uterine eggs of C. sinensis, it is probable that miracidia in the eggs may accumulate PGK to produce the energy required for swimming after hatching.
References
Arnold H, Henning R, Pette D (1971) Quantitative comparison of the binding of various glycolytic enzymes of F-actin and the interaction of aldolase with G-actin. Eur J Biochem 22:121–126
Bluthmann H, Cicurel L, Kuntz GW, Haedenkamp G, Illmensee K (1982) Immunohistochemical localization of mouse testis-specific phosphoglycerate kinase (PGK-2) by monoclonal antibodies. EMBO J 1:479–484
Bronstein WW, Knull HR (1981) Interaction of muscle glycolytic enzymes with thin filament proteins. Can J Biochem 59:494–499
Fabry S, Heppner P, Dietmaier W, Hensel R (1990) Cloning and sequencing the gene encoding 3-phosphoglycerate kinase from mesophilic Methanobacterium bryanti and thermophilic Methanothermus fervidus. Gene 91:19–25
Goudot-Crozel V, Caillol D, Djabali M, Dessein AJ (1989) The major parasite surface antigen associated with human resistance to schistosomiasis is a 37-kDa glyceraldhyde 3P-dehydrogenase. J Exp Med 170:2065–2080
Hong SJ, Seong KY, Sohn WM, Song KY (2000) Molecular cloning and immunological characterization of phosphoglycerate kinase from Clonorchis sinensis. Mol Biochem Parasitol 108:207–216
Kang IK, Lee SH, Seo BS (1969) Study on the 14C-glucose metabolism by Clonorchis sinensis. Paper chromatographic analyses in combination with autoradiography. Korean J Parasitol 7:43–52
Kumble KD, Vishwanatha JK (1991) Immunoelectron microscopic analysis of the intracellular distribution of primer recognition proteins, annexin 2 and phosphoglycerate kinase, in normal and transformed cells. J Cell Sci 99:751–758
Lee KW, Shalaby KA, Medhat AM (1995) Cloning of the gene for phosphoglycerate kinase from Schistosoma mansoni and characterization of its gene product. Mol Biochem Parasitol 71:221–231
Matsumoto Y, Perry G, Levine RJC, Blanton R, Mahmound AAF, Aikawa M (1988) Paramyosin and actin in schistosomal tegument. Nature 333:76–78
Poglazov BF (1983) Actin and coordination of metabolic process. Biochem Int 6:757–765
Seo BS, Lee SH (1971) Autoradiographic studies on the distribution of 14C-labeled carbohydrates in Clonorchis sinensis. Chin J Microbiol 4:200–209
Shoemaker C, Gross A, Gebremichael A, Harn D (1992) cDNA cloning and functional expression of the Schistosoma mansoni protective antigen triose-phosphate isomerase. Proc Natl Acad Sci U S A 89:1842–1846
Vandeberg JL, Lee CY, Goldberg E (1981) Immunohistochemical localization of phosphoglycerate kinase in mouse testes. J Exp Zool 217:435–441
Yu JR, Chai JY (1995) Localization of actin and myosin in Cryptosporidium parvum using immunogold staining. Korean J Parasitol 33:155–164
Acknowledgement
This work was supported in part by the Research Institute for Biomedical and Pharmaceutical Sciences of Chung-Ang University (2002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hong, SJ., Shin, JK., Kang, SY. et al. Ultrastructural localization of phosphoglycerate kinase in adult Clonorchis sinensis . Parasitol Res 90, 369–371 (2003). https://doi.org/10.1007/s00436-003-0857-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-003-0857-y