Abstract.
Intraerythrocytic bodies identified as haemogregarine gamonts were found in 29% of 97 brown tree snakes (Boiga irregularis) examined during a haematological survey of reptiles in Australasia during 1994–1998. The morphological characteristics of the parasites were consistent with those of Haemogregarina boigae Mackerras, 1961, although the gamonts were slightly larger and lacked red caps but contained distinctive polar grey capsules. Gamonts did not distend host cells but laterally displaced their nuclei. They were contained within parasitophorous vacuoles and possessed typical apicomplexan organelles, including a conoid, polar rings, rhoptries and micronemes. Schizonts producing up to 30 merozoites were detected in endothelial cells of the lungs of 11 snakes. The absence of erythrocytic schizogony suggests the parasites belong to the genus Hepatozoon. Electron microscopy also revealed the presence of curious encapsulated organisms in degenerating erythrocytes. These stages did not possess apical complex organelles and were surrounded by thick walls containing circumferential junctions and interposed strips reminiscent of oocyst sutures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Haemogregarines (Apicomplexa: Haemogregarinidae) are common haemoprotozoan parasites of reptiles but are generally non-pathogenic to their hosts. The main genera infecting reptiles are Haemogregarina, Hepatozoon, Karyolysus and Hemolivia (cf. Mackerras 1961; Petit et al. 1990; Barnard and Upton 1994). Conventionally, haemogregarine species have been differentiated on the basis of host occurrence, gamont morphology and site of schizogonous development. However, the life cycles of most species are not known and host specificity has been assumed rather than demonstrated. The reliability of gamont morphology for species differentiation is also questionable, due to their pleomorphy. The present study describes the morphology and ultrastructure of haemogregarines detected in brown tree snakes (Boiga irregularis) during the course of a large-scale survey conducted on haemoparasites of reptiles in the southwestern Pacific. Brown tree snakes are endemic to Australasia but were accidentally introduced to several Pacific islands where they have become pest species.
Materials and methods
Brown tree snakes were collected opportunistically from coastal regions in central and northern Queensland and the Northern Territory in Australia, from the Solomon Islands and from the island of Guam in the southwestern Pacific Ocean during 1994–1998. All collections were made under wildlife and animal ethics permits issued by regional authorities. Animals were caught manually during the evenings when they came out to feed. Blood was collected from the caudal vein, two blood smears prepared and the remaining blood fixed in 3% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.2). Snakes were released at the site of capture, except for 11 animals which were transported back to the laboratory and maintained in terraria. These snakes were kept for varying periods of time (from 1 week to 2 years) and then killed by barbiturate overdose for pathological examination. Blood smears were fixed in methanol, stained with Giemsa (1:15 in Sorensen's buffer, pH 7.2) for 10 min and examined under oil immersion. Aliquots of glutaraldehyde-fixed blood were gently centrifuged, the cell pellets washed in buffer and then embedded in 2% agarose to facilitate handling. Gel blocks were post-fixed in 1% osmium tetroxide, prestained in 0.5% uranyl acetate in 50% ethanol, dehydrated and embedded in Epon resin. Ultrathin (50 nm) sections were cut, stained with uranyl acetate and lead citrate and examined using a TEOL101 electron microscope. Tissue samples collected at post-mortem (lung, liver, spleen, kidney, heart, skeletal muscle) were fixed in buffered 10% formalin and 3% glutaraldehyde and processed for histology and electron microscopy using standard preparative techniques.
Results
A total of 97 brown tree snakes were collected during the survey: 64 from northern Australia, 21 from Guam and 12 from the Solomon Islands. Haemogregarines were detected in blood smears from 28 snakes, all originating from central Queensland in Australia. Parasitaemia ranged over 1–90% and repeat sampling of captive snakes indicated infections could persist under laboratory conditions for up to 2 years. No clinical signs of disease were apparent in any of the snakes. No haemosporidia, piroplasms or trypanosomes were detected in blood smears. Low numbers of coccidian oocysts and strongyle eggs were occasionally detected in faecal samples from 12 snakes. Ticks recovered from seven snakes were identified as Amblyomma and Aponomma spp.
Intra-erythrocytic gamonts
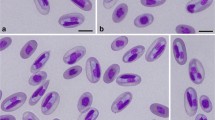
The haemogregarine developmental stages detected in the blood smears were all intraerythrocytic gamonts and no schizonts were detected in red blood cells. The gamonts did not distend host cells but caused marked lateral displacement of their nuclei. Infected erythrocytes usually stained paler than uninfected erythrocytes, but there was no evidence of haemoglobin denaturation or granulation. Mature gamonts were crescent-shaped with lightly basophilic granular cytoplasm, dense centrally located nuclei and prominent polar caps staining light blue-grey (Fig. 1). They were contained within parasitophorous vacuoles and most infected cells contained single gamonts, although infections by two gamonts were occasionally observed. The gamonts ranged over 16.0–17.3 μm in length (mean ± standard deviation = 16.3±0.78; n=30) and 4.0–4.8 μm in width (4.4±0.32; n=30). The gamonts were monomorphic, being remarkably uniform in size, shape, appearance, location and effect on the host cell. Electron microscopy revealed the presence of characteristic apicomplexan organelles in the anterior half of the gamonts (Fig. 2), including a conoid, 2–8 rhoptries and up to 50 micronemes (Fig. 3). The conoid was located at the apex of the gamonts and appeared as a truncated cone of fibrils within the anterior polar ring. The rhoptries were elongate, electron-dense, saccular organelles ranging over 100–200 nm in cross-sectional diameter, while the micronemes were smaller, electron-dense, elliptical organelles ranging over 80–120 nm in diameter. Gamonts were bounded by a trilaminar pellicle supported by longitudinal subpellicular microtubules which were interrupted at the polar rings. The parasite nucleus was elongate and located in the posterior half of the gamont. The gamonts were located within membrane-bound parasitophorous vacuoles separating the parasites from the host cell cytoplasm and haemoglobin. Shrinkage artifacts frequently exaggerated the vacuolar space between gamonts and host-cell cytoplasm; and the vacuole membrane was occasionally observed to adhere to the gamont pellicle (Fig. 3). The cytoplasm of infected erythrocytes was homogeneous and the haemoglobin content appeared unchanged, compared with that of adjacent uninfected cells.
Light micrograph of erythrocytes from the brown tree snake, Boiga irregularis, showing crescent-shaped gamonts of Hepatozoon boigae laterally displacing host-cell nuclei. Bar 5 μm
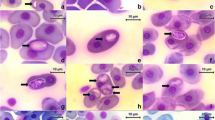
Electron micrograph of B. irregularis erythrocyte infected with Hp. boigae gamont, showing parasite with conspicuous nucleus and apical complex organelles. Bar 1 μm
Electron micrograph of anterior region of Hp. boigae gamont located within parasitophorous vacuole in B. irregularis erythrocyte. Note presence of prominent apical complex organelles (conoid, micronemes, rhoptries) enclosed within parasite pellicle. Bar 0.5 μm
Tissue schizonts
Schizonts at different stages of maturity were observed in capillaries in the respiratory tracts of the 11 snakes from which post-mortem samples were collected (Fig. 4). The schizonts were evident as multinucleate ovoid bodies ranging over 10–40 μm in diameter. They markedly distended the vascular endothelial cells and laterally displaced the host-cell nuclei. Transmission electron microscopy of the turgid cells confirmed their identity as capillary endothelial cells (Fig. 5). Cross-sections of mature schizonts revealed the presence of up to 30 merozoites, ranging in width over 1.2–2.4 μm and located within large membrane-bound parasitophorous vacuoles. The majority of merozoites were peripherally located around a central mass of electron-lucent vacuoles (Fig. 6).
Light micrograph of lung tissue from the brown tree snake, Boiga irregularis, showing two multinucleate schizonts of Hp. boigae located within endothelial cells. Bar 20 μm
Electron micrograph showing cross-section through Hp. boigae schizont located within parasitophorous vacuole in endothelial cell in snake lung. Bar 2 μm
Electron micrograph through mature schizont of H. boigae showing up to 20 merozoites and numerous electron-lucent vacuoles. Bar 2 μm
Thick-walled intra-erythrocytic forms
During the course of the transmission electron microscopic investigations, curious thick-walled intra-erythrocytic stages were detected in seven brown tree snakes (exclusively in four snakes, but in conjunction with typical haemogregarine gamonts in another three). Infected erythrocytes showed extensive degenerative changes in haemoglobin content, with a marked loss in electron density and considerable granulation (Fig. 7). The erythrocyte membrane appeared serrated due to the presence of numerous small protrusions projected outwards from the cell (Fig. 8). The erythrocyte nuclei were frequently triangular in appearance, their chromatin content pale and diffuse (except around the margin) and they were laterally displaced by the parasitophorous vacuole. Shrinkage artifacts within the vacuole were common. The contained organisms were stellate in appearance and ranged over 2–4 μm in diameter. They possessed a large, centrally located nucleus, ribosome-rich cytoplasm and several large, electron-dense granules. They lacked any typical apical complex organelles and no mitochondria were observed. The most distinctive feature of these stages was the thick wall completely surrounding them. The wall was electron-dense and ranged in thickness over 100–160 nm (Fig. 9). It contained two prominent circumferential sutures or junctions which divided the wall into halves (Fig. 9). The junctions contained lip-like thickenings of the wall (up to 320 nm thick), with two interposed electron-dense strips forming a 250-nm channel through the wall (Fig. 10). Repeated examination of blood smears from these snakes by light microscopy failed to reveal any stages which were unequivocally referable to the thick-walled stages detected by electron microscopy.
Electron micrograph of thick-walled gamont located within parasitophorous vacuole in B. irregularis erythrocyte with irregular membrane, diffuse haemoglobin and turgid nucleus. Bar 1 μm
Electron micrograph through erythrocyte infected with thick-walled gamont showing membrane protrusions and diffuse haemoglobin content. Bar 200 nm
Electron micrograph of thick-walled gamont showing central nucleus, cytoplasm with electron-dense vacuoles and granules and prominent thick wall with conspicuous junctions at opposite ends. Bar 400 nm
. Electron micrograph of suture-like junction in thick wall, showing lip-like terminal swellings and electron-dense lines in close apposition. Bar 100 nm
Discussion
Haemogregarine parasites were a common finding in brown tree snakes, being detected at a prevalence of 29% in the 97 snakes examined. However, marked differences were observed in the geographic distribution of infected snakes. All infections were found in snakes from northern Australia (prevalence of 44%) and none in those from Guam or the Solomon Islands. The absence of infections in island populations suggests either that the snakes which colonized the islands after their accidental introduction in the 1950s were not infected, or that the transmission of infections could not be sustained due to a lack of suitable vectors. Haemogregarines may be transmitted by a range of haematophagous invertebrates, including leeches, acarines (ticks, mites) and insects (mosquitoes, flies, lice); and vertebrates acquire infections through vector bites or the ingestion of infected vectors. Regrettably, the vectors and transmission cycles of most haemogregarine species are unknown. Future studies should be conducted on snake populations in their natural home range of northern Australia. Haemogregarines have previously been detected in 26 species of Australian snakes, including seven colubrid species, eight boid species and 11 elapid species (O'Donoghue and Adlard 2000). Boiga irregularis is a colubrid snake and haemogregarines have been detected on several occasions in snakes collected in Queensland and the Northern Territory (cf. Mackerras 1961).
Infections varied markedly in their intensity (parasitaemia ranging over 1–90%), but no clinical signs of disease were noted, even in the most heavily infected snakes. Gamonts occupied approximately half the volume of infected erythrocytes but usually did not distend the host cell. The erythrocyte nucleus was laterally displaced and the cytoplasm stained paler than uninfected cells. Nonetheless, it is not known whether these changes manifest any haematological abnormalities, as normal values for most blood parameters have yet to be established for this snake species. Gamonts are essentially dormant stages awaiting ingestion by vectors, so their cytopathologic effects may be limited. They are non-dividing stages and do not release daughter cells via host cell lysis. However, the longevity of infected cells is not known. The persistence of infections in the captive snakes for up to 2 years in the absence of vector challenge suggests that infected cells are not lysed or prematurely cleared from the circulation. Alternatively, infections might persist through constant replenishment by asexual multiplicative stages of parasite in host tissues. In this study, numerous schizonts were detected in the lungs of the 11 snakes kept from 1 week to 2 years prior to post-mortem. No gross or histopathological changes were detected, suggesting that the tissue stages were transient or did not elicit host inflammatory responses. Mature schizonts distended the host endothelial cells into the lumen of the blood vessels and merozoites were presumably liberated by host cell lysis. However, no ischaemic or haemorrhagic changes were observed in host tissues on gross or histopathological examination. There is therefore no direct evidence that haemogregarine infections caused significant pathology in brown tree snakes during their schizogonous proliferation in tissues or their gamont formation in erythrocytes.
The identification of the haemogregarine genus and species infecting the snakes was problematic. Different parasite species have conventionally been described on the basis of host occurrence and gamont morphology. However, little is known about the actual host-specificity of the parasite species and the gamonts exhibit considerable pleomorphism. To date, a total of 22 Haemogregarina spp have been described from Australian snakes, including six from colubrids, eight from boids and eight from elapids (O'Donoghue and Adlard 2000). Two Haemogregarina spp have previously been described from brown tree snakes, Hg. boigae and Hg. mirabilis. At the light microscopy level, the gamonts detected in this study were consistent with previous descriptions of those of Hg. boigae Mackerras, 1961, except that they were slightly longer and did not have prominent red caps, but contained conspicuous and distinctive polar grey capsules. The gamonts were the same size as those of Hg. mirabilis, but the latter were paler staining and lacked polar caps. Species described from other colubrid snakes (cf. Mackerras 1961) include Hg. calligaster from the northern tree snake, Dendrelaphis calligastra (forms smaller gamonts without polar caps), Hg. dendrophidis from the common tree snake, D. punctulata (forms pale gamonts without polar caps), Hg. aspidomorphi from the slate-grey snake, Stegonotus plumbeus (forms thin gamonts with anterior nuclei and pale polar caps), and Hg. stegonoti, also from S. plumbeus (forms large gamonts with thick membranes and recurved tails). While the gamonts found in this study were most similar to those of Hg. boigae, the sites of development of the schizonts suggest that the parasites may not belong to the genus Haemogregarina.
The current classification of haemogregarine taxa is based on a combination of characters, including parasite developmental cycle, host occurrence, vector and route of transmission (cf. Telford 1984). Most workers recognize three families: Haemogregarinidae (containing the genera Haemogregarina, Cyrilia and Desseria), Karyolysidae (containing the genera Karyolysus and Hemolivia) and Hepatozoidae (containing the genus Hepatozoon). Siddall (1995) suggested that chelonian haemogregarines be regarded as Haemogregarina sensu stricto, whereas fish haemogregarines be classified as Cyrilia or Desseria, or left as Haemogregarina sensu lato. Smith (1996) subsequently suggested that all members of the genus Haemogregarina in snakes, crocodilians, lizards, amphibians, birds and mammals be transferred to the genus Hepatozoon. The key characters used to differentiate genera are sporogonic development, vector transmission and sites of development. Haemogregarinids produce oocysts with naked sporozoites in the gut of leech vectors, vertebrates are infected by vector bite and schizogony occurs in host erythrocytes. Hepatozoids produce oocysts containing numerous sporocysts in the haemocoel of various vectors (insects, acarines, leeches), vertebrates are infected by ingesting vectors and schizogony occurs in vascular endothelial cells in host tissues. Karyolysids produce sporokinetes and motile spores in the gut and ova of mite vectors, vertebrates become infected by ingesting mites and schizogony occurs in host vascular endothelial cells. Although the vectors and complete developmental cycles of the parasites detected in the brown tree snakes remain to be determined, the presence of extra-erythrocytic rather than intra-erythrocytic schizonts suggests that they belong to the genus Hepatozoon. Accordingly, it is suggested that Hg. boigae from brown tree snakes be reassigned to Hp. boigae (Mackerras, 1961) nov. comb., pending further investigations on vector identity and developmental cycle.
The ultrastructural detection of the curious thick-walled, electron-dense stages in degenerating host erythrocytes presented an enigma. No corresponding thick-walled stages were found by light microscopy despite repeated examination, suggesting that they were rare and only detected fortuitously. In contrast, they were detected in seven snakes and were particularly numerous in ultra-thin sections of erythrocytes from four snakes. The reason for the discordance between the light and electron microscopic findings is not known but is not considered to be attributable to preparative artifacts, as the organisms were unique and regular in form. The infected host cells also showed profound degenerative changes, with marked haemoglobin depletion and numerous knob-like protrusions of the cell membrane. Similar protrusions have been described on reptilian and amphibian erythrocytes infected with Karyolysus spp (Beyer 1977), Haemogregarina spp (Desser and Weller 1973; Paterson et al. 1988) and Hepatozoon spp (Nadler and Miller 1985) and they even resembled those found on human erythrocytes infected with Plasmodium falciparum (Aikawa et al. 1983). Similar cytoplasmic changes have also been detected in erythrocytes infected with Karyolysus spp (Beyer 1977) and Hepatozoon spp (Nadler and Miller 1985). However, all previous studies described gamonts with typical apicomplexan features rather than the thick-walled stages found in this study, which were completely devoid of apical complex organelles (notably a conoid, rhoptries, micronemes). While the possession of these organelles may vary depending on the stage of parasite development, the occurrence of a wall 100–160 nm thick with bipolar junctions was unique.
An extensive literature search failed to reveal an organism with a comparable wall. Hervas et al. (1997) described some unusual electron-dense stages of Hp. canis in dog erythrocytes, but the walls were 60 nm thick and no junctions were observed. The junctions did show some structural similarities to excystation suture lines described in the inner sporocyst walls of Toxoplasma gondii, Sarcocystis and Isospora spp (Scholtyseck 1973; Speer et al. 1998). The suture lines possessed prominent lip-like thickenings with interposed strips, but they were usually only 140 nm thick (compared to 320 nm observed in this study). Comparisons made with the walls of other spore-forming protists revealed few similarities. Haplosporidia infect aquatic invertebrates and form spores with coats 100–200 nm thick, with an apical operculum and sometimes a filamentous tail (cf. Perkins 1990). Myxozoans infect invertebrates, fish, amphibians and reptiles and form multicellular spores with walls 200–500 nm thick, containing prominent cell junctions between apposing valves (cf. Lom 1990). Microsporans infect a wide range of invertebrate and vertebrate hosts and form unicellular spores with walls 150–200 nm thick, composed of distinct endo- and exo-spore layers (cf. Canning 1990). A range of viruses, rickettsia, bacteria and fungi have been found to form smaller intra-erythrocytic inclusions in reptiles, but the structures did not have comparable walls and their internal features were distinctive (Telford 1984; Desser and Yekutiel 1986; Desser and Barta 1988, 1989; Barnard and Upton 1994).
As we are unable to reconcile their ultrastructure with any previously published reports, the identity of the thick-walled intra-erythrocytic stages in the brown tree snakes remains speculative. It is not known whether they were encapsulated gamonts awaiting uptake by a suitable vector, aberrant developmental stages forming a wall more usually associated with sporogonic development, hitherto undescribed novel stages of Hp. boigae, or developmental stages of another unrelated organism. Molecular characterization studies may reveal their phylogenetic relationships, but genotypic research on haemogregarines is still in its infancy due to difficulties encountered in purifying parasite DNA and developing useful PCR primers (Perkins and Keller 2001).
References
Aikawa M, Rabbege JR, Udeinya I, Miller EH (1983) Electron microscopy of knobs in Plasmodium falciparum infected erythrocytes. J Parasitol 69:435–437
Barnard SM, Upton SJ (1994) A veterinary guide to the parasites of reptiles, vol 1: Protozoa. Kreiger, Malabar, Fla.
Beyer T (1977) Electron microscope study of Karyolysus sp. (Sporozoa: Adeleida: Haemogregarinidae) and of changes induced in the infected host cell. Protistologia 13:57–66
Canning EU (1990) Phylum Microspora. In: Margulis L, Corliss JO, Melkonian M, Chapman DJ (eds) Handbook of Protoctista. Jones and Bartlett, Boston, pp 53–72
Desser SS, Barta JR (1988) Ultrastructural observations on Thrombocytozoons ranarum Tchacarof 1963, an intrathrombocytic yeast of frogs. Can J Microbiol 34:1096–1098
Desser SS, Barta JR (1989) The morphological features of Aegyptianella bacterifera: an intraerythrocytic rickettsia of frogs from Corsica. J Wildl Dis 25:313–318
Desser SS, Weller I (1973) Structure, cytochemistry and locomotion of Haemogregarina sp. from Rana berlandieri. J Protozool 20:65–73
Desser SS, Yekutiel D (1986) Blood parasites of amphibians and reptiles in Israel. Isr J Zool 34:77–90
Hervas J, Carrasco L, Sierra MA, Mendez A, Gomez-Villamandos JC (1997) Ultrastructural findings in natural canine hepatozoonosis. J Vet Med B 44:119–125
Lom J (1990) Phylum Myxozoa. In: Margulis L, Corliss JO, Melkonian M, Chapman DJ (eds) Handbook of Protoctista. Jones and Bartlett, Boston, pp 36–52
Mackerras MJ (1961) The haematozoa of Australian reptiles. Aust J Zool 9:61–122
Nadler SA, Miller JH (1985) Fine structure of Hepatozoon mocassini (Apicomplexa: Eucoccidiorida) gamonts and modifications of infected erythrocyte plasmalemma. J Protozool 32:275–279
O'Donoghue PJ, Adlard RD (2000) Catalogue of protozoan parasites recorded in Australia. Mem Queensl Mus 45:1–163
Paterson WB, Desser SS, Barta JR (1988) Ultrastructural features of the apical complex, pellicle and membranes investing the gamonts of Haemogregarina magna (Apicomplexa: Adeleina). J Protozool 35:73–80
Perkins FO (1990) Phylum Haplosporidia. In: Margulis L, Corliss JO, Melkonian M, Chapman DJ (eds) Handbook of Protoctista. Jones and Bartlett, Boston, pp 19–29
Perkins SL, Keller AK (2001) Phylogeny of nuclear small subunit rRNA genes of hemogregarines amplified with specific primers. J Parasitol 87:870–876
Petit G, Landau I, Baccam D, Lainson R (1990) Description et cycle biologique d'Hemolivia stellata n.g., n. sp., hemogregarine de crapauds bresiliens. Ann Parasitol Hum Comp 65:3–15
Scholtyseck E (1973) Ultrastructure. In: Hammond DM, Long PL (eds) The coccidia: Eimeria, Isospora, Toxoplasma and related genera. Butterworths, London
Siddall ME (1995) Phylogeny of adeleid blood parasites with a partial systematic revision of the haemogregarine complex. J Eukaryot Microbiol 42:116–125
Smith TG (1996) The genus Hepatozoon (Apicomplexa: Adeleina). J Parasitol 82:565–585
Speer, CA, Clark S, Dubey JP (1998) Ultrastructure of the occysts, sporocysts, and sporozoites of Toxoplasma gondii. J Parasitol 84:504–512
Telford SR (1984) Reptilian haemoparasites. In: Hoff GL, Frye FL, Jacobson ER (eds) Diseases of amphibians and reptiles. Plenum, New York
Acknowledgements.
The authors would like to thank Lien Bahn for technical assistance with electron microscopy. This study was supported by an Australian Postgraduate Award scholarship awarded to K.J. and research grants from the Australian Research Council and the United States Department of Agriculture in Hawaii.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jakes, K.A., O'Donoghue, P.J. & Whittier, J. Ultrastructure of Hepatozoon boigae (Mackerras, 1961) nov. comb. from brown tree snakes, Boiga irregularis, from northern Australia. Parasitol Res 90, 225–231 (2003). https://doi.org/10.1007/s00436-003-0849-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-003-0849-y