Abstract
The reef-building sedentary polychaete, Sabellaria alveolata, is well known for its specialized anterior end, the operculum, which is exposed to the environment during vital activities, such as feeding or as it seals its tube when the animal withdraws. This region represents the most important sensory structure in Sabellariidae. It comprises two lobes, tentacular filaments, protective chaetae (paleae), a median organ, and a median ridge, and bears various pigmented spots. Worms swiftly withdraw into tubes triggered by abrupt light change (shadow reflex). We suspected that the pigmented spots were photoreceptive and responsible for the shadow response. To test this hypothesis, the median organ and median ridge of S. alveolata were investigated by applying light microscopy, confocal laser scanning microscopy, scanning, and transmission electron microscopy. Besides unciliated supportive cells, these organs consist of numerous multiciliated and glandular cells on their ventral surfaces. The multiciliated cells are of two types: epidermal supportive and receptor cells. The median organ is innervated directly from the brain by two longitudinal basiepithelial nerves. The presence of multiciliated cells and mucocytes suggests that the organ is sensory, involved in both feeding and tube-building behavior. Furthermore, we found two pairs of eyespots on the lateral posterior part of the median ridge, situated close to the neurite bundles anterior to the brain. These eyes are simple in structure and resemble larval-type eyes. They are composed of only two cells each, one rhabdomeric photoreceptor cell and one pigmented supportive cell. The pigmented eyes on the median ridge are clearly involved in photoreception and very likely involved in shadow reflex.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sabellariidae Johnston, 1865, is a group of marine sedentary annelids highly specialized for benthic life (Rouse and Pleijel 2001; Capa and Hutchings 2014; Faroni-Perez et al. 2016). All species are strictly tubicolous; they build tubes of cemented sand grains and other mineral particles, often attached to hard substrates of varying sizes, such as echinoid spines and rocks (Kirtley 1994). The majority of sabellariid species described inhabit intertidal or shallow depths, with some species restricted to the continental shelf or the deep sea (Capa et al. 2012; Faroni-Perez et al. 2016). Some species are gregarious and often occur in high numbers, whereas others live solitary or near conspecifics (Faroni-Perez et al. 2016). The larvae of Sabellaria alveolata (Linnaeus, 1767) exhibit gregarious behavior during settlement, resulting in tubes attached one to another, forming patchy aggregates or large reefs. It has been shown that the anterior sensory organs of the larvae are involved in this behavior (Pawlik 1986, 1988, 1990; Faroni-Perez et al. 2016).
The body of adult sabellariids shows a heteronomous segmentation, consisting of the operculum, thorax, parathorax, abdomen, and cauda (Rouse and Pleijel 2001; Capa and Hutchings 2014; Capa et al. 2015). The operculum is the most characteristic feature of sabellariids and represents their highly specialized anterior end or head region, which seals the tube, protecting the animals against unfavorable conditions, such as predation attempts (Capa et al. 2012; Capa and Hutchings 2014). Although some external morphological similarities are found with the operculum in Pectinariidae (Rouse and Pleijel 2001), the operculum in Sabellariidae is regarded as their most important apomorphy (Capa and Hutchings 2014). It comprises two lobes projecting anteriorly, with the opercular chaetae distally, oral filaments laterally, and a pair of palps ventrally. The operculum bears one to three rows of strong protective chaetae, the paleae, followed by rows of opercular papillae (Rouse and Pleijel 2001; Capa et al. 2015). In some genera, the two lobes may fuse during metamorphosis. At the junction of the opercular lobes, a crest is located running from the upper lip toward the opercular crown, called the median ridge, which may hold the median organ (median cirrus of Rouse and Pleijel 2001) anteriorly in certain species (Faroni-Perez et al. 2016). Thus, the whole structure is formed by the prostomium, peristomium, and most likely, two anterior segments (Wilson 1929; Dales 1952; Orrhage 1978).
The operculum is thought to be the most important protective and sensory structure in Sabellariidae (Kirtley 1994; Lechapt and Kirtley 1996; Rouse and Pleijel 2001; Capa et al. 2015; Faroni-Perez et al. 2016). The median organ (the dorsal hump in larvae) is considered to play a major sensory role in gregarious species during settlement and metamorphosis and is likely to be involved in reproductive strategies during benthic stages (Wilson 1968; Faroni-Perez et al. 2016). However, detailed ultrastructural studies are missing, except one study describing the diversity and evolution of this organ within the family (Faroni-Perez et al. 2016).
Sensory organs play an important role in the evolution and adaptive radiation of animals, since they allow the perception and response to environmental cues (Faroni-Perez et al. 2016). Several structures located anteriorly on the body and found in different ontogenetic stages of sabellariids have been regarded as sensory organs, for example, ocelli, palps, nuchal organs, the median ridge, and the median organ (Wilson 1929, 1977; Dales 1952; Eckelbarger 1976, 1978; Eckelbarger and Chia 1976; Orrhage 1978; Kirtley 1994; Lechapt and Kirtley 1996; Rouse and Pleijel 2001; Faroni-Perez et al. 2016).
Regarding the median organ, within sabellariids there is a great diversity considering size, shape, and the presence of other sensory structures, such as, ciliated receptor cells and putative eyespots (Orrhage 1978; Capa et al. 2015; Faroni-Perez et al. 2016). The benthic stages of the most solitary and deep-water species possess conspicuous median organs, whereas in gregarious members, the median organs are small or even absent (Faroni-Perez et al. 2016). The morphology of the median ridge and median organ are species-specific and show distinct ciliation and pigmentation patterns. Parts of the pigment spots have been thought to represent eye spots (Orrhage 1978; Capa et al. 2015; Faroni-Perez et al. 2016). As other tube-building polychaetes, such as, Sabellidae and Serpulidae, in the sabellariid S. alveolata an escape response was observed by retracting rapidly into the tubes when shaded (see video in supplementary material). Since this was shown without any mechanical stimuli, the responses of the worms are most likely solely triggered by the eyes present on either the median organ or the median ridge.
Members of the related Sabellidae (see Struck et al. 2015; Weigert and Bleidorn 2016, for phylogenetic relationships) often possess highly specialized eyes on their tentacular crowns functioning as alarm systems (Nilsson 1994; Bok and Nilsson 2016; Bok et al. 2016, 2017). Rows of pigment spots on either side of the median ridge were presumed to represent eyes (Orrhage 1978 and see Fig. 1f in; Faroni-Perez et al. 2016). The question then arises whether the pigment spots on the median organ and median ridge of S. alveolata represent photoreceptive structures comparable to those present in Sabellidae.

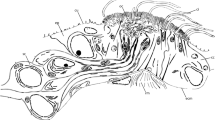
Redrawn from Orrhage (1978)
Schematic drawings of the anterior end of S. alveolata. a Ventral view showing opercular paleae (pa) and opercular papillae (op). Tentacular filaments (tf) on external side of the opercular lobes and palps (pp). Ventrally the median ridge (mr) extends anteriorly; 2 pairs of eyespots (es) on both sides of the posterior end of median ridge. Inner part of operculum ventrally with numerous cilia (ci). Mouth (m) followed by 2 thoracic segments (sg1, sg2), 3 parathoracic segments (pts) and numerous abdominal segments (as). b Lateral view with notopodia (not) on parathoracic segments (pts), as well as paddle-shaped notopodia and neuropodia (neu) with conical lobes. Each segment dorsally with a pair of branchiae (br) except for the first thorax segment (sg1). c Dorsal view showing operculum paleae (pa) organized in three rows and operculum papillae encircling the operculum. as abdominal segment, br branchia, ci cilia, es eyespot, m mouth, mr median ridge, neu neuropodium, not notopodium, op operculum papillae, pa paleae, pp palp, pts parathorax segment, sg1, 2, thorax segment 1, 2, tf tentacular filament.
To date, Sabellariidae were seldom studied by transmission electron microscopy (TEM) and, among their sensory structures, only the palps in late larvae of Phragmatopoma californica (Fewkes, 1889) have been studied (Amieva and Reed 1987; Amieva et al. 1987). Since the ultrastructure and morphology of the median ridge and median organ was unknown, and structural evidence is lacking to verify whether the pigment spots present on the median ridge, in fact, represent eyes likely involved in shadow reflex, an ultrastructural investigation was designed for S. alveolata. In this species, adult individuals possess a small median organ and a prominent median ridge. The present study combines light microscopy, scanning, and transmission electron microscopy in order to describe the structure of these organs.
The median organ and median ridge are mainly built up of ciliated and unciliated supportive cells, pigment cells, various gland cells, and a few ciliated receptor cells. In contrast to other species [i.e., Idanthyrsus australiensis (Haswell, 1883) Bok, unpubl. obs.], our results show that most pigmented structures do not represent eyes; we only found eyes at the base of the median ridge anterior to the brain. The structure of these simple eyes in Sabellariidae is described here for the first time.
Materials and methods
Adult specimens of S. alveolata were collected at Saint-Efflam (Brittany, France) during summer, 2017. Additional specimens were collected from reefs at Mont St. Michel Bay (Normandy, France) in the same year. Small parts of the reefs were broken off and intact tubes with living animals inside were raised in the laboratory under running seawater until processing. Animals were removed from their tubes by carefully crashing the walls. Only intact individuals were used for experiments at the Marine Biological Station of Roscoff (Brittany, France). Life observations were made immediately after removing the animals from their tubes.
For electron microscopy, small adult individuals were chosen. They were relaxed for about 15 min in 8% magnesium chloride (MgCl2 × 6 H2O) isotonic with seawater and fixed immediately in a solution of picric acid, paraformaldehyde, and glutaraldehyde (phosphate buffered, 0.075 M) and adjusted to the appropriate osmolality with sucrose (SPAFG, Ermak and Eakin 1976) for 2 h at 4 °C. Specimens from Mont St. Michel were fixed in 2.5% glutaraldehyde in 0.05 M phosphate buffer with 0.3 M NaCl. After five rinses in the appropriate buffer for 10 min each, specimens were stored in the same buffer containing 0.05% NaN3 at 4 °C until further processing, or they were used for whole mount light microscopy.
Specimens were post-fixed in 1% OsO4 (phosphate buffered) for 1 h at 4 °C. After being washed for 5 min in either 0.075 M buffer adjusted with sucrose or a 0.05 M buffer adjusted with NaCl samples that were dehydrated using an EtOH series (30% for 5 min at 4 °C, 50% for 5 min at 4 °C, 70% for 10 min at 4 °C, 80% for 10 min at 4 °C, 95% for 10 min at 4 °C, 95% for 10 min RT, 2 × 100%, 10 min RT). Specimens for SEM were then critical-point dried using CO2(l), mounted with adhesive tabs on aluminum stubs, coated with platinum/iridium and examined with a Zeiss Auriga (Oberkochen, Germany) scanning electron microscope.
For TEM, the smallest specimens available were chosen. Specimens for TEM and light microscopy were dissected into smaller parts. Only the anterior ends were used for further processing. These were transferred into a solution of ethanol and the intermedium propylene oxide (100% EtOH: propylene oxide, 1:1, 2 × 30 min), followed by pure propylene oxide (4 × 15 min). This solution was replaced by mixtures of the intermedium and the embedding medium, starting with propylene oxide: Araldite/Epon (PolyBed 812) 3:1 for 6 h, followed by 2:1 (12 h) and finally 1:1 (12 h). The intermedium was then allowed to evaporate overnight. Before final embedding took place, the specimens were transferred into drops of fresh Araldite/Epon for 5 min at 60 °C. After two repetitions, specimens were brought into the embedding molds. Polymerization was carried out at 60 °C for 72 h. Specimens were either cut in series of semi-thin (1 µm) or ultrathin sections (70 nm), using a UC 6 Leica ultra-microtome (Wetzlar, Germany).
Semi-thin sections were stained with toluidine blue (0.5% toluidine blue in a 1% aqueous solution of borax for 15 s at 60 °C), rinsed with H2O and mounted with an Entellan mounting medium. Pictures were taken with a Zeiss Axioskop light microscope equipped with a CCD camera (Invisitron Systems) and Vision (Spot) software. A series of ultrathin sections were placed on single slot grids coated with pioloform support films, then contrasted at 20 °C with 2% uranyl acetate (30 min) and 0.5% lead citrate (20 min) in a Nanofilm Surface Analysis Ultrastainer (Göttingen, Germany). Finally, the sections were examined with the Zeiss EM 902A and Libra 120 transmission electron microscopes (Oberkochen, Germany). Images were recorded using CCD cameras (Image SP®, 4 k, Moorenweis, Germany).
For confocal Laser Scanning Microscopy (cLSM), small animals were fixed in 4% paraformaldehyde in phosphate-buffered saline (PPS: 140 mM NaCl, 6.5 mM KCl, 2.5 mM Na2HPO4, 1.5 mM KH2PO4, 12% sucrose, pH 7.4, 4 °C, 2.5 h). After the fixation specimens were rinsed in PBS and stored in the same buffer, containing a few crystals of NaN3 to avoid the growth of bacteria and fungi. Prior to immunolabeling, specimens were incubated with PBT (9 ml PBS + 1 ml 1% Triton X-100) containing 6% BSA (bovine serum albumin) for 1 h, and finally, incubated with the primary antibody for 2–4 days at 4 °C.
The primary antibody was mouse anti-acetylated α-tubulin (monoclonal, clone 6-11-B-1; Sigma-Aldrich, Heidelberg, Germany, dilution 1:1000 in PBT). Following several washes (3 × in PBT, 20 min each), the secondary antibody was applied for 2–3 days at 4 °C (goat anti-mouse, Cy2 conjugated, Dianova, Hamburg, Germany, dilution 1:200). After being rinsed three times for 10 min in PBS, specimens were mounted in Fluoromount (Southern Biotech, Birmingham, USA). Specificity of immunoreactivity was controlled by incubating specimens in the same manner, but omitting the primary antibodies. Observations were made with a Zeiss Pascal 5 confocal laser scanning microscope (Zeiss, Jena, Germany). Z-stacks are displayed as maximum projections if not stated otherwise. All images were further processed using Adobe Photoshop® and Illustrator® to adjust brightness, contrast, and size for assembling and labeling the plates.
Results
Position and general structure of the median organ
The body of S. alveolata was divided into five distinct regions: opercular, thoracic, parathoracic, abdominal, and caudal, as is typical of Sabellariidae (Figs. 1a–c, 2a, b). The thorax comprised two, the parathorax three, and the abdomen 23–29 chaetigers in the specimens from St. Efflam (Fig. 2a, b). The caudal region appeared to be unsegmented. The paired branchiae are located dorsally, present from the second thoracic chaetiger to the posterior onto the abdomen, diminishing in size continuously. Anteriorly in the body, at the opercular region, the median organ and median ridge are located.
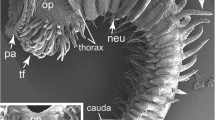
S. alveolata from Saint-Efflam, France. Light microscopy (a, c, e) and scanning electron microscopy images (b, d, f) from fixed specimens. a Whole specimen in ventral view with operculum paleae (pa) on anterior end, followed by two thoracic segments, three parathoracic segments (pts), numerous abdominal segments (as) and cauda (c). Anterior end bearing numerous tentacular filaments (tf) on the ventral side of the operculum. b Whole specimen in ventral view indicating three parathoracic segments (arrows) with notopodia bearing simple, capillary chaetae. Abdominal segments with paddle-shaped notopodia (not) and neuropodia (neu) with capillary chaetae. c Opercular region in ventral view indicating the position of the median ridge (mr) framed by pigmented band previously suggested to represent eyespots (arrows). Note the pigmented stripes (pig) on ventral side of opercular lobes adjacent to the median ridge. d Opercular region in ventral view showing tentacular filaments organized in bundles and rows on each side of the operculum. The median ridge (mr) protrudes from the body wall and terminates in a short median organ (mo). e Pigmentation pattern of operculum and median ridge (mr); note the strong pigmented band along the edges of the median ridge (arrows). f Median ridge (mr) and median organ (mo) in ventral view showing strong and dense ciliation (ci). Additional rows of cilia (ci) form bands on the operculum. as abdominal segment, c cauda, ci cilium, m mouth, mo median organ, mr median ridge, neu neuropodium, not notopodium, op operculum, pa operculum paleae, pig pigment, pp palp, pts parathorax segment, tf tentacular filament
The opercular region consisted of two lobes, partially fused dorsally (Figs. 1a–c, 2c, d). Each lobe bears 8–10 parallel rows of contractile tentacular filaments along its ventrolateral external margin. A row comprises 8–10 ciliated tentacular filaments with an opposite slight flap and groove in between, which conveys particles to a longitudinal stream ending in the mouth (Fig. 2d, f, suppl. Figure 1a–i). Anterior-distal rows of single tentacular filaments run along the ventrolateral internal flap margin of the longitudinal stream (suppl. Figure 1a–i). The external and internal sides of the stream joined the mouth by lateral and superior lips, respectively.
The median ridge is located anteriorly to the superior lip between the palps and extends distally, terminating at the dorsal fusion zone of the opercular lobes (Figs. 1a, 2c–g). Densely arranged pigment spots border the median ridge laterally (suppl. Figure 1a–i). Anteriorly the median ridge continues as the median organ, both of which are pigmented and not distinctly set off. The median organ is small with a rounded tip and is as densely ciliated as the median ridge (Fig. 2d–f). The ventral side of the opercular lobes is marked with stripes of pigment, varying among specimens in zebra-like patterns, and numerous rows of cilia (Fig. 2c, e, suppl. Figure 1a–i). The pigmentation pattern in the conserved specimens did not differ from that observed in living animals. The cerebral pigmented eyes are almost invisible or indistinguishable from this pigmentation when viewed externally under a microscope (Fig. 2c).
General histological organization of the median ridge and median organ
In the median ridge and median organ, four main layers were distinguishable and formed by the cuticle, the epidermis, the extracellular matrix (ECM), and the musculature. The epidermis is the outermost cellular layer composed of cuboidal supportive cells with glandular and ciliated cells in between. Externally, it bears the cuticle (Figs. 3b, e, 4a, b, 6a–e). Posteriorly, two pairs of eyespots are present on both sides of the median ridge (Figs. 3d–g, 4b, 5a, suppl. Figure 2a–d). They are situated basally in the epidermis, close to two prominent neurite bundles, extending longitudinally through the median ridge (Figs. 4b, 5a). The eyes are covered by epidermal cell processes. A prominent ECM separates the epidermis, basiepithelial nerve bundles, and eyespots from the underlying longitudinal and circular musculature (Figs. 4a, b, 5a). Between the neurite bundle and eye spots, the ECM forms a prominent fold, which extends ventrally and separates the eye area from the nerve proper (Fig. 5a). In the region of the eyes, a few extracellular spaces were observed (Fig. 5a; asterisks). These eyes are the only photoreceptive structures found in the median organ and median ridge. Likewise, staining with antibodies against anti-acetylated α-tubulin did not reveal any signs of unpigmented ciliated photoreceptor cells.

C redrawn from Orrhage (1978)
S. alveolata from Mont St. Michel Bay (France). Transverse semi-thin sections of the anterior end. Toluidine blue staining. Relative positions of sections are indicated in c. a Anteriormost end of the median ridge (mr). The three rows of operculum paleae (outer, middle and inner row: opa, mpa, ipa) are built up by two chaetigerous sacs. Palps (pp) in front of the median ridge and surrounded by numerous tentacular filaments (tf) cut at different levels. b Enlargement of adjacent section from anterior end of the median ridge; note the cilia (ci) and pigment granules (pig) as well as glandular cells (gc) in the epidermis of the median ridge. Ventral surface of median ridge with a few multiciliated cells (mc). c Scheme showing position of transverse sections. d Section through the anterior end where the two operculum lobes fuse. Epidermis of the median ridge with prominent pigment spots on each side (encircled). Palps with a blind ending blood vessel (bv). e Detail of median ridge; somewhat more posterior position as d. Each side of the median ridge with one small eyespot (es). Epidermis with numerous pigment granules (pig) and multiciliated cells (mc). f, g Left and right eyespot (es) composed of pigment cup (pic) and photoreceptor cell (prc); arrowhead points to border between cell body and microvilli; sections somewhat posterior to e. bv blood vessel, ci cilia, es eye spot, gc gland cell, hc hyaline cartilage, ipa inner paleae, mc multiciliated cell, mpa middle paleae, mr median ridge, nv nerve, opa outer paleae, pic pigment cup, pig pigment, pp palp, prc photoreceptor cell, tf tentacular filaments.
Low magnification TEM images of the median ridge of S. alveolata from Mont St. Michel Bay (France), transverse sections. a Anterior part of median ridge (inset shows relative position). Epidermis build up of multiciliated cells (mc) with electron-dense cytoplasm, unciliated electron-lucent supportive cells and a few glandular cells (gc). Numerous pigment granules (pig) within epidermal cells. Note ECM (arrows) separating the epidermis from the underlying dorsal circular and longitudinal muscle fibers (muc, mul). b Posterior part of median ridge (see inset for position). Epidermal supportive cells with numerous pigment granules (pig); eyespot (encircled) embedded basally and laterally in the median ridge. Note the two intraepithelial nerves (nv, boxed) innervating the median ridge, in close proximity to the eyespot. Epidermis separated by an ECM from the longitudinal and circular musculature (black and white arrows). Musculature forms two additional prominent longitudinal bundles at the lateral ridges. Stippled box indicates position of Fig. 5a. ci cilia, cu cuticle, muc circular musculature, mul longitudinal musculature, mv microvilli, n nucleus, nv nerve, pig pigment granule
S. alveolata from Mont St. Michel Bay (France). a Enlargement of right opercular nerve and eye from Fig. 4b. TEM. Nerve composed of three neurite bundles (ne) separated by glial cells (glc) and accompanied by a few neuronal somata (so). Nerve partly separated from epidermis proper by prominent fold of ECM (ecm, arrows). Eye situated close to this fold, composed of pigment cell (pic) and rhabdomeric photoreceptor cell (prc) covered by epidermal cells (ep) and cuticle (cu). Note glial cell processes close to the eyes (arrowheads) and intercellular spaces within epidermis (asterisks). b Anti-acetylated α-tubulin-like immuno-reactivity of the anterior end. CLSM. Two main ventral nerves (mvn) emanate from the brain (b) and run into the dorsal ridge. Brain with circumoesophageal connectives (cc) and several additional opercular nerves (arrows). c Schematic drawing illustrating innervation of the median ridge by two prominent nerves (mvn). Palp nerves and additional opercular nerves are summarized and simplified (arrowheads). b brain, cc circumoesophageal connectives, cu cuticle, ecm ECM, ep epidermis, glc glial cell, muc circular musculature, mul longitudinal musculature, mv microvilli, mvn main ventral nerve, n1 nucleus of pic, n2 nucleus of prc, ne neurite bundle, pic pigment cell, prc photoreceptor cell, so neuronal soma
The paired prominent neurite bundle passing the eyes comprises the innervation of the median ridge and median organ (Fig. 5a–c). It directly emanates from the neuropil of the brain and only innervates these organs, whereas other regions of the operculum and the palps are innervated separately (Fig. 5b, c). Each nerve supplying the median ridge comprises about 300 fibers of different diameter (0.6–2.5 µm) arranged in three to four neurite bundles, separated by glial cells (Fig. 5a). The innermost bundle is the largest and consists of about 170 neurites, and the adjacent neurite bundles are less voluminous. The neurite bundles are accompanied by a few neuronal somata, which are located between the epidermal supportive cells and the fold, formed by the ECM separating the nerve from the eyes (Fig. 5a). Shortly after entering the median ridge, the neurite bundles split into numerous smaller fibers, supplying the entire region (Fig. 5b).
The musculature of the median ridge consists of circular and longitudinal muscle fibers (Fig. 4a, b). Anteriorly, circular muscle fibers form a smooth layer, and the epithelium is thicker ventrally rather than laterally. Ventrally, the epithelial cells are about 2.8 times higher than in the periphery (Fig. 4a). Posteriorly, in the median ridge, the layer of circular muscle fibers is thinner and embedded into a double layer of longitudinal muscles fibers forming an outer and an inner part (Fig. 4b). The outer or ventral layer of longitudinal muscle fibers bears the epithelium of cuboidal cells of equal height. Laterally, this outer layer of longitudinal muscle is absent and the epithelial lining forms a pair of grooves extending into the ridge which house the lateral nerves and the eyes (Fig. 4b). Here the epidermis is two–three times higher. Thus, the neurite bundles are surrounded by muscle tissue, except on the lateral side (Figs. 4b, 5a).
Epidermis
The epidermis of the median ridge, the median organ, and ventrally on the opercular lobes, consists of only a few different cell types in S. alveolata. These comprise supportive cells, ciliated cells, gland cells, and sensory cells (Figs. 4a, b, 6a–e). The sensory cells are ciliated as well, with cilia penetrating the cuticle (Fig. 6a, c). However, these are comparatively rare and will be described in more detail in a forthcoming paper. Sensory cells with cilia not penetrating the cuticle were not encountered. A part of the supportive cells contain electron-dense vesicles, which are regarded as pigment granules and thus, represent the pigment cells (Figs. 4a, 6b, d). The epidermis is covered by an inconspicuous cuticle (1.5–2.5 µm thick), which is penetrated by numerous microvilli, motile cilia, sensory cilia, and the openings of the glandular cells (Fig. 6a–e). The cuticle hardly contains any material and is composed of scarce fine fibrils. Grid-like arranged layers of parallel collagen fibers are lacking. The microvilli traversing the cuticle are branched, and their slightly swollen tips exceed the electron-denser surface of the cuticle for about 100 nm.
S. alveolata from Mont St. Michel Bay (France). Epidermis of median ridge and ventral part of operculum. TEM. Inset: position of transverse sections. a Epidermis next to median ridge. Cuticle (cu) penetrated by microvilli (mv), cilia (ci) and openings of glandular cells (gc). Cuticle devoid of collagen fibers. Boxed area enlarged in c. b Epidermis of median ridge. Electron-dense multiciliated cells (mc) surrounded by electron-lucent cells without cilia. Both cell types linked to each other by zonulae adhaerentes (arrows) and contain numerous pigment granules (pig). Boxed area enlarged in d. c Detail of the gland cell (gc) opening from a, adjacent to putative sensory cell. Cells are adjoined to each other by zonula adhaerentes (za, arrows). Microvilli (mv) and cilia (ci) penetrate the cuticle, cilia with basal plate (bp) and accessory centriole. d Detail of multiciliated cell from b; cilia (ci) and microvilli (mv) penetrate the cuticle (cu). Note branching of some microvilli (mv) on their distal end. e Detail of an electron-lucent cell with bundles of intermediate filaments (if) entering branched microvilli (mv). bb basal body, bp basal plate, ci cilium, cu cuticle, ecm ECM, gc gland cell, if intermediate filament, mc multiciliated cell, mi mitochondrion, mu muscle fiber, mv microvillus, n nucleus, pig pigment granule, sj septate junction, za zonula adhaerens
Gland cells are frequent on the median organ and ventrally on the opercular lobes in the vicinity of the median ridge (Figs. 3b, e, 6a, c). Their secretion is electron-lucent and somewhat heterogeneous (Fig. 6a, c). Their apices are about 2 µm wide and form open pores in the cuticle surrounded by a circle of microvilli. These pore apertures are typically located close to the multiciliated supportive or to receptor cells (Figs. 3e, 6c). Apically adhering junctions interconnect the gland cells with adjacent epidermal cells (Fig. 6c). As is typical of epithelia, zonulae adherentes were observed in all distal parts of the epithelial cells. Very likely, they are basally followed by septate junctions as well, but this could not be identified with certainty in all the sections examined.
In the multiciliated epidermal cells, the large nuclei have a basal position, whereas numerous mitochondria and pigment granules of homogeneous content are located more apically (Fig. 6b). The cytoplasm of the multiciliated cells is more electron-dense compared to the electron-light cytoplasm of the adjacent non-ciliated supportive cells. The cilia of the multiciliated cells, measuring 250 nm in diameter, have axonemes with a typical 9 × 2 + 2 arrangement of microtubules. The basal plate of the cilia is located above the level of the apical cell membrane, thus representing a type 2 basal body (Fig. 6c, d). Numerous branching microvilli emerge between the cilia.
The non-ciliated supportive cells possess large basal nuclei, apically located mitochondria, and some cells contain pigment granules morphologically similar to those present in the multiciliated cells (Fig. 6b). These cells bear numerous microvilli as well, penetrating the cuticle and forming an apical layer of microvillar tips (Fig. 6a, b, d). Certain supportive cells from the median ridge possess a well-developed basal–apical system of intermediate filaments, which enter the microvilli for about 1–2 µm and then terminate in small hemidesmosomes (Fig. 6e).
Eyes
Position and general structure
Pigment spots occur laterally along the median ridge, randomly in single or double lines, with a higher posterior concentration near the superior lip than anteriorly near the median organ (Figs. 2e, 3e, suppl. Figure 1a–i). Only the posterior-most spots are eyes and are distinctly marked off from the surrounding tissues, whereas the other pigment spots show more diffuse borders (Fig. 3e–g, suppl. Figure 2a–d). The two pairs of pigmented eyes found are situated at the base of the epithelium laterally in the median ridge and anterior to the neuropil of the brain. Each eye consists of only two cells, a pigmented cell and a photoreceptor cell (Figs. 3f, g, 5a, 7a). The former exhibits a cup-like arrangement of shading pigment granules. The eyes are inverse in design and the pigment cup opens ventrally. This arrangement allows detecting changes in light intensity from ventro-frontally if the operculum is exposed in feeding position (Fig. 5a). Both cells, pigmented supportive and photoreceptor cell, are interconnected by a zonula adhaerens, followed by a septate junction (Fig. 7a–c). In peripheral histological sections, this junctional zone is also visible as a dense line (Fig. 3g; arrowhead).
S. alveolata from Mont St. Michel Bay (France). Eyes, TEM, transverse sections. a Eye situated on the right side of the median ridge. Eye composed of two cells: a pigmented supportive cell (pic) and a rhabdomeric photoreceptor cell (prc) form a cavity into which rhabdomeric microvilli (mv) project. Membranes of the two cells highly folded (arrows) and apically interconnected by zonulae adhaerentes (za). Note glial cell processes (glc) and intercellular spaces close to the junction of both cell types (asterisks), pigment cell with a few microvilli emerging close to prc (double arrow). Cell body of photoreceptor cell with numerous mitochondria (mi) and spherical nucleus (n2); elongated nucleus (n1) of pigment cell located eccentrically. b, c Junctional complexes composed of septate junctions (sj) and zonulae adhaerentes (za) between pigment supportive cell and photoreceptor cell. d Rhabdomeric microvilli (mv) close to pigment supportive cell with shading pigment granules (pig). Note electron-dense membrane of pigment cell (arrows). mi mitochondrion, mv rhabdomeric microvilli, n1 nucleus of pigment cell, n2 nucleus of photoreceptor cell, pic pigmented supportive cell, pig pigment granule, prc photoreceptor cell, sj septate junction, za zonula adhaerens
Photoreceptor cell
The photoreceptor cell is rhabdomeric. It comprises a large cell body, measuring 8 µm in diameter. The apical part of the cell projects toward the pigment cup and gives rise to numerous microvilli. The basal side faces toward the cuticle and is covered by a layer of epidermal supportive cells (Figs. 5a, 7a). The epidermal overlay forms a coat 12–14 µm thick above the photoreceptor cell. Both cell types form processes which are highly interdigitated. The optical cavity, with its sensory microvilli, is thus situated about 20 µm below the epithelial surface. It is surrounded by the pigment cup and is filled with densely arranged microvilli (Figs. 5a, 7a). The microvilli, measuring 120–150 nm in diameter, extend from the apical membrane of the photoreceptor cell in a brush-like pattern into the 6.5 µm-deep pigment cup (Fig. 7c, d). Branching of microvilli was not observed on the available sections.
The photoreceptor cell contains numerous mitochondria, which are more abundant apically. The spherical nucleus, with few and evenly dispersed heterochromatin, is situated basally close to the epidermal cells (Figs. 5a, 7a). It is accompanied by a small Golgi complex. Cilia or vestiges were not observed, but a centriole was found 2.7 µm below the apical membrane. In the apical region, numerous tubular cisternae of smooth endoplasmic reticulum (ER) and numerous multivesicular bodies were observed. In the optic cavity, similar material accumulates opposite to the photoreceptor cell, close to the apex of the pigment cell (Fig. 7a). The cell body of the photoreceptor cell is partly surrounded by glial cell processes (Figs. 5a, 7a).
Pigment supportive cell
The pigment cup consists of a single pigment cell. It contains numerous pigment granules, with varying sizes and electron-density, forming about eight dense layers of granules (Fig. 7a, d). Mitochondria are sparse, and the nucleus is flattened with high heterochromatin content and is more electron-dense than in the photoreceptor cell (Fig. 7a). The nucleus is situated outside the pigment-containing part in a pocket beside the photoreceptor cell. The apical membrane facing the optical cavity is associated with a thin layer of electron-dense filaments, giving the membrane a seemingly thicker appearance (Fig. 7d). Most of the apical membrane is smooth and a few microvilli projecting into the optical cavity were only encountered in a small area close to the junction with the receptor cell (Fig. 7a; double arrows). Cilia were not observed in the pigment cell.
Discussion
General aspects
The present results on the ultrastructure of the anterior sense organs in S. alveolata revealed two pairs of pigmented spots laterally on the median ridge, as photoreceptor elements that, in all probability, play an important role in the shadow response. The pigmented spots recognized as eyes are located close to the brain, and are likely the main sensory organs, involved in the observed escape reflexes. The eyes found are of the simplest, comprising a simple set of only one photoreceptor cell and one pigment cell (Purschke et al. 2006; Richter et al. 2010).
A median ridge, presumed to represent a major part of the sensory system in Sabellariidae, is present in all sabellariid species. However, a conspicuous prolongation, called the median organ or median cirrus, is only present in certain sabellariid species (Capa et al. 2012, 2015; Capa and Hutchings 2014; Faroni-Perez et al. 2016). During ontogeny, the median organ develops from an area called the dorsal hump in the competent larvae, whereas the median ridge has been described only for metamorphosed worms (Amieva and Reed 1987; Faroni-Perez et al. 2016). Within Sabellariidae, these anterior structures show a broad diversity of morphological traits, considering size, shape, ciliation pattern, and pigment spots (Faroni-Perez et al. 2016).
While solitary and deep-water sabellariids usually have a conspicuous median organ, gregarious species lack or have a small organ (Faroni-Perez et al. 2016). Among the gregarious sabellariids, a median organ is present in certain littoral species of Sabellaria and Idanthyrsus. However, some morphological variability of the median organ was observed within a given species (e.g., in I. australiensis; see Faroni-Perez et al. 2016). Similarly, S. alveolata individuals showed an evident morphological variability in size of the median organ, amount and density of lateral pigment spots on the median ridge, as well as in the ventral pigmented stripes on the opercular lobes (LFP, pers. obs., see suppl. Figure 1a–i).
The median ridge and median organ of S. alveolata bear a dense ciliation, similarly to a congeneric from Brazil (Faroni Perez et al. 2016). However, a comparatively sparse ciliation can be found in other species (e.g., Lygdamis nasutus Capa, Faroni-Perez, Hutchings, 2015; see Capa et al. 2015). In general, similar organs with such a well-developed external ciliation often play a role in chemo-or mechanoreception, but also in feeding and in tube-building species in a particle selection (Eckelbarger 1977, 1978; Smith and Chia 1985; Amieva and Reed 1987; Amieva et al. 1987; Dubois et al. 2005; Riisgrad and Nielsen 2006). Moreover, the dense pattern of cilia ventrally on the opercular lobes may also be involved in generating microcurrents ventilating local water.
The larval dorsal hump, which forms the adult median organ, was found to play a major sensory role in gregarious species during settlement and substrate selection (Wilson 1968; Faroni-Perez et al. 2016). This was further supported by prominent nerves, which were shown to emanate directly from the brain (Capa et al. 2015; Faroni-Perez et al. 2016) and led to the hypotheses that the median organ functions primarily as a sensory organ, and at least part of the pigment spots represent eyes. Together with the escape reflex behavior observed, which occurred without mechanical or chemical stimuli through currents (see video in supplementary material), this hypothesis appeared to be the best model for deducing a sensory function of the median ridge and median organ and motivated the present research.
Epidermis and cuticle
In addition to the description of the eyes in S. alveolata, the present study unveiled structural peculiarities of the epidermis. In contrast to other polychaetes of comparable size, the cuticle in S. alveolata is structurally similar to a general larval cuticle in lacking a prominent grid of collagen fibers (Hausen 2005; Purschke et al. 2014). Our unpublished observations in other parts of the anterior end (e.g., tentacular filaments, palps, and opercular papillae) indicated that throughout the anterior end, the cuticle is similar to those herein described for the median organ, median ridge, and opercular lobes. Although not closely related, in members of the so-called basal radiation and in small species, such as interstitial polychaetes, the absence of collagen fibers in the cuticle was also reported (Kristensen and Nørrevang 1982; Hausen 2001, 2005; Purschke et al. 2014). Even on the branchiae of species with a collagenous cuticle, the cuticle is considerably thinner and contains less or no collagen fibers (Purschke et al. 2017). A similar thin cuticle bare of collagen has been described on the palps in the late larvae of the sabellariid P. californica (Amieva and Reed 1987; Amieva et al. 1987). All these results likely indicated that a thinner cuticle, lacking prominent collagen fibers, may favor organisms for the exchange of molecules with the adjacent environment or its protective role was, in part, taken over by their elaborated tubes. In the case of S. alveolata these tubes are not left after settlement and cannot be rebuilt. Further studies are called for clarifying the adaptive significance of this feature. The general epidermal organization was as typical of polychaetes except for the richness in pigment cells (Hausen 2005).
Sensory structures
The median organ and median ridge are equipped with sensory cells, and a pair of prominent nerves connects them with the brain. Thus, a sensory function can generally be assumed. However, the comparatively low number of sensory cells found on the median organ and median ridge, together with the equally small-sized nerve supplying this structure, sensing the environment is probably not its main function. Given a 1:1 representation of axons and receptor cells present in the afferent nerves and the necessity for the presence of a few efferent neurites as well, the total number of receptor cells did not appear to be very high (Purschke 2016). For example, the number of neurites at the base of the median organ with approximately 300 fibers was a little less than present in a single dorsal cirrus of a given parapodium in Eurythoe complanata (Pallas, 1766) and about 1/6 less than in the parapodial cirri of Harmothoe Kinberg, 1856 (see Horridge 1963; Lawry 1967; Purschke et al. 2017). Nonetheless, in comparison to sedentary sabellariids, the fireworm and scale worm are annelids with different life strategies, such as non-gregarious behavior, reproductive biology, and most importantly, foraging and mobility. This requires a greater investment in the sensory system. However, within sabellariids, variability in sensory organs is to be expected and likely related to continuous adaptation toward different life history traits and bathymetry (Faroni-Perez et al. 2016).
Generally, when sabellariids are in the feeding position, the ventral side of the opercular region is the most externally exposed part of the body, not only for vision, but also for light perception, triggering seasonality on gametogenesis, spawning, and recruitment (Dubois et al. 2007; Culloty et al. 2010; Faroni-Perez 2014; Aviz et al. 2018). The median ridge clearly is photoreceptive, although the number of eyes present in S. alveolata was considerably lower than expected. The pigmented eyes are the only pigmented photoreceptive elements found on the opercular region. Currently, the best explanation is that these structures must be responsible for the escape reflex when the anterior end is shaded. However, considering the extraordinary size of the species for TEM investigations, and the variability of the pigmentation pattern on the opercular lobes among the specimens, it could not be completely excluded that more photoreceptive structures are present. Indeed, while juveniles were used for TEM, during the ontogeny, the number of pigment spots seemed to increase, and certain specimens showed prominent eye-like pigment spots on the operculum (see supplementary Fig. 1a–i). So, it cannot be excluded that the number of eyes present increases during growth.
Unpigmented photoreceptor elements may have been overlooked as such structures are usually minute and hard to find (Purschke et al. 2006, 2017; Purschke 2016). Although present more often than formerly expected, only in case of ciliary photoreceptor cells are these found with conventional antibody staining against tubulin (e.g., Purschke and Müller 1996; Wilkens and Purschke 2009; Lehmacher et al. 2016). However, the study did not provide evidence for the presence of such structures. On the other hand, rhabdomeric photoreceptor cells and other ectopic photoreceptor cells were mostly discovered by chance if no model organisms, such as Platynereis dumerilii (Audouin & Milne Edwards, 1833), are chosen in which the opsin genes are known (Ayers et al. 2018).
Broadly comparable results of unpigmented photoreceptors, very likely involved in shadow reflex, were found in the cephalocordate Branchiostoma Costa, 1834 (Pergner and Kozmik 2017; Lacalli 2018). Along the anterio-dorsal axis of the dorsal nerve cord in Branchiostoma, a multiple photoreceptor system was described built up of an array of rhabdomeric photoreceptor cells without shading pigment. In addition, these may also play a role in circadian rhythms. In the polychaete P. dumerilii at least two different sensory pathways were found in the optical system responsible for the shadow reflex (Ayers et al. 2018), one of which employees a Go-type opsin and is present in the cirri, and the other represents a ciliary-type opsin exhibited in sensory structures present in the head. Unfortunately, the morphology—rhabdomeric, ciliary or otherwise—of the Go-opsin expressing cells is unknown in P. dumerilii. Recently, photoreceptor elements were described in the parapodial cirri of the amphinomid E. complanata. These exhibit rhabdomeric morphology and do not comprise shading pigment, but their opsin is still unknown (Purschke et al. 2017).
In annelids several types of eyes can be distinguished with respect to their structure, presence in a certain body region, or their occurrence during ontogeny (Purschke et al. 2006; Purschke 2016). Due to their close proximity to the brain, the eyes of S. alveolata are believed to represent cerebral eyes as, for example, present in Sabellidae (Ermak and Eakin 1976; Purschke et al. 2006; Arendt et al. 2009; Bok and Nilsson 2016). Among these eyes, larval and adult eyes may be distinguished by their so-called molecular fingerprint and, under certain circumstances, by their different structure or their developmental history. Whereas the larval eyes are of the inverse type and usually comprise only two cells, one pigmented supportive cell and one rhabdomeric photoreceptor cell, typical adult eyes are multicellular and converse. Until recently adult eyes of this type were known to be restricted to Amphinomida, Sipuncula and Errantia, whereas members of Sedentaria (to which Sabellariidae belong) usually possess pigmented cerebral eyes of the larval type and sometimes in high numbers (Purschke et al. 2014). However, recently it could be shown that adult eyes must have been present in the last common ancestor of Sedentaria, and the pattern observed in many sedentary species most likely represents a derived situation (Wilkens and Purschke 2009; Vodopyanov and Purschke 2017). Thus, some of the small eyes described in Sedentaria very likely represent miniaturized adult eyes. The eyes of S. alveolata structurally correspond to larval eyes, however, merely by a structural analysis it is yet too early to assign them as larval or adult eyes. Only if such small eyes still exhibit characteristics of adult eyes or their developmental history is known, such a decision can be made (Wilkens and Purschke 2009; Purschke 2011; Purschke and Nowak 2015; Martínez et al. 2017).
It is well known that early in ontogeny, sabellariid larvae develop a first left-sided eyespot, then a pair develops on the right side and later, the fourth eyespot develops on the left side posteriorly to the first and nearer to the mid-dorsal line (Wilson 1929, 1977; Dales 1952; McCarthy et al. 2002). If these eyes are retained through adulthood, then the positional dorsal–ventral shifts onto the opercular structures that occur during metamorphosis may explain the present findings. Alternatively, the larval helical rotation swimming behavior in sabellariids may indicate a non-visual scanning with the absence of synaptic circuitry and larval eyespots are sensory–motor units, whereas body rotation or other scanning movements were not observed in adults prior to the shadow reflex scape behavior, indicating spatial vision of light (Randel and Jékely 2015).
Nevertheless, it has been shown that small and simple eyes, as observed for S. alveolata, are perfect sensory organs for discriminating the direction of light (Jékely et al. 2008; Randel and Jékely 2015). This may be the primary reason for the prevalence of larval-type eyes in many sedentary taxa, since phototaxis and escape reflexes might be the most important role of photoreception in these often tube-dwelling annelids.
However, the fan worms Sabellidae and Serpulidae are examples where special and highly elaborated eyes function as arm systems evolved de novo and completely independent from cerebral eyes (Nicol 1950; Nilsson 1994; Bok and Nilsson 2016; Bok et al. 2016, 2017). In these taxa, the latter are comparable in structure to those of S. alveolata (Ermak and Eakin 1976). Obviously, such alarm eyes evolved several times independently in these groups but not all sabellid and serpulid species possess such a system (Bok et al. 2016). In sabellariids, the opercular characters, including the median ridge and median organ, show considerable diversity among genera and species. Therefore, it is likely that depending on the taxa, other eye patterns could have evolved and certain pigmented structures observed in other species, in fact, represent eyes (Faroni-Perez et al. 2016; Bok et al. unpubl. obs.). It yet remains to be determined whether similar evolutionary traits as described for Sabellidae can be detected in certain Sabellaridae, or even ciliary, instead of rhabdomeric, photoreceptor cells are involved in escape reflex. The best candidates appear to be taxa, with well-developed median organs, such as Lygdamis and Idanthyrsus (Capa et al. 2015; Faroni-Perez et al. 2016). For example, in L. nasutus, the cirrus-like median organ bears a row of rounded pigment spots and terminates in a plate-like structure, externally resembling sabellid compound eyes (Capa et al. 2015; Bok et al. 2016).
Conclusions
The data revealed that the median ridge and median organ in S. alveolata represents a sensory structure. Due to the presence of pigmented eyes at its base close to the brain, it is most likely involved in the escape reflex when the animals are shaded. These eyes are comparatively simple and correspond to cerebral eyes typically found in sedentary polychaetes. Very likely, the median organ has additional functions, as indicated by its dense ciliation of motile cilia. This study is a starting point for more revealing investigations. For example, the larval eyes in sabellariids may undergo ontogenetic transformation and can be retained or replaced by adult eyes. Moreover, still undetected ectopic photoreceptive structures may be present and involved in light perception as a visual system for shadow reflexes or regulating circadian rhythms.
References
Amieva MR, Reed CG (1987) Functional morphology of the larval tentacles of Phragmatopoma californica (Polychaeta: Sabellaridae): composite larval and adult organs of multifunctional significance. Mar Biol 95:243–258
Amieva MR, Reed CG, Pawlik JR (1987) Ultrastructure and behavior of the larva of Phragmatopoma californica (Polychaeta: Sabellariidae): identification of sensory organs potentially involved in substrate selection. Mar Biol 95:59–266
Arendt D, Hausen H, Purschke G (2009) The ‘division of labour’ model of eye evolution. Philos Trans R Soc B 364:2809–2817
Aviz D, Pinto AJA, Ferreira MAP, Rocha A, Rosa Filho JS (2018) Reproductive biology of Sabellaria wilsoni (Sabellariidae: Polychaeta), an important ecosystem engineer on the Amazon coast. J Mar Biol Assoc UK 98:743–754
Ayers T, Tsukamoto H, Gühmann M, Rajan VBV, Tessmar-Raible K (2018) A Go-type opsin mediates the shadow reflex in the annelid Platynereis dumerilii. BMC Biol 16:41
Bok MJ, Nilsson D-E (2016) Fan worm eyes. Curr Biol 26:R907–R908
Bok MJ, Capa M, Nilsson D-E (2016) Here, there and everywhere: the radiolar eyes of fan worms (Annelida, Sabellidae). Integr Comp Biol 56:784–795
Bok MJ, Porter ML, Nilsson D-E (2017) Phototransduction in fan worm radiolar eyes. Curr Biol 27:R681–R701
Capa M, Hutchings P (2014) Sabellariidae Johnston, 1865. In: Westheide W, Purschke G, Böggemann M (eds) Handbook of zoology online. A natural history of the phyla of the animal kingdom; Annelida: Polychaetes. De Gruyter, Berlin. http://www.degruyter.com/view/db/zoology/bp_029147-6_63. Accessed 15 Oct 2014
Capa M, Hutchings P, Peart R (2012) Systematic revision of Sabellariidae (Polychaeta) and their relationship with other polychaetes using morphological and DNA sequence data. Zool J Linn Soc 164:245–284
Capa M, Faroni-Perez L, Hutchings P (2015) Sabellariidae from Lizard Island, Great Barrier Reef, including a new species of Lygdamis and notes on external morphology of the median organ. Zootaxa 4019:184–206
Culloty SC, Favier E, Ní Riada M, Ramsay NF, O’Riordan RM (2010) Reproduction of the biogenic reef-forming honeycomb worm Sabellaria alveolata in Ireland. J Mar Biol Assoc UK 90:503–507
Dales RP (1952) The development and structure of the anterior region of the body in the Sabellariidae, with special reference to Phragmatopoma californica. Q J Microsc Sci 93:435–452
Dubois S, Barillé L, Cognie B, Beninger PG (2005) Particle capture and processing mechanisms in Sabellaria alveolata (Polychaeta: Sabellariidae). Mar Ecol Prog Ser 301:159–171
Dubois S, Comtet T, Retière C, Thiébaut E (2007) Distribution and retention of Sabellaria alveolata larvae (Polychaeta: Sabellariidae) in the bay of Mont-Saint-Michel, France. Mar Ecol Prog Ser 346:243–254
Eckelbarger KJ (1976) Larval development and population aspects of the reef-building polychaete Phragmatopoma lapidosa from the east coast of Florida. Bull Mar Sci 26:117–132
Eckelbarger KJ (1977) Larval development of Sabellaria floridensis from Florida and Phragmatopoma californica from southern California (Polychaeta: Sabellariidae), with a key to the sabellariid larvae of Florida and a review of development in the family. Bull Mar Sci 27:241–255
Eckelbarger KJ (1978) Metamorphosis and settlement in the Sabellariidae. In: Chia F-S, Rice ME (eds) Settlement and metamorphosis of marine invertebrate larvae. Elsevier, New York, pp 145–164
Eckelbarger KJ, Chia FS (1976) Scanning electron microscopic observations of the larval development of the reef-building polychaete Phragmatopoma lapidosa. Can J Zool 54:2082–2088
Ermak TH, Eakin RM (1976) Fine structure of the cerebral and pygidial ocelli in Chone ecaudata (Polychaeta: Sabellidae). J Ultrastruct Res 54:243–260
Faroni-Perez L (2014) Seasonal variation in recruitment of Phragmatopoma caudata (Polychaeta: Sabellariidae) in the southeast coast of Brazil: validation of methodology for categorizing age classes. Iheringia 104:5–13
Faroni-Perez L, Helm C, Burghardt I, Hutchings P, Capa M (2016) Anterior sensory organs in Sabellariidae (Annelida). Invertebr Biol 135(4):423–447
Hausen H (2001) Untersuchungen zur Phylogenie “spiomorpher” Polychaeten (Annelida). Logos, Berlin, pp 1–142
Hausen H (2005) Comparative structure of the epidermis in polychaetes (Annelida). Hydrobiologia 535/536:25–35
Helm C, Bok MJ, Hutchings P, Kupriyanova E, Capa M (2018) Developmental studies provide new insights into the evolution of sense organs in Sabellariidae (Annelida). BMC Biology 18:149. https://doi.org/10.1186/s12862-018-1263-5
Horridge GA (1963) Proprioceptors, bristle receptors, efferent sensory impulses, neurofibrils and number of axons in the parapodial nerve of the polychaete Harmothoe. Proc R Soc Lond Ser B 157:199–222
Jékely G, Colombelli J, Hausen H, Guy K, Stelzer E, Nédélec F, Arendt D (2008) Mechanism of phototaxis in marine zooplankton. Nature 456:395–399
Kirtley DW (1994) A review and taxonomic revision of the family Sabellariidae Johnston, 1865 (Annelida; Polychaeta) (series 1). Sabecon Press Science, Los Angeles, pp 1–223
Kristensen RM, Nørrevang A (1982) Description of Psammodrilus aedificator sp.n. (Polychaeta), with notes on the Arctic interstitial fauna of Disko Island, W. Greenland. Zool Scr 11:265–279
Lacalli T (2018) Amphioxus, motion detection, and the evolutionary origin of the vertebrate retinotectal map. EvoDevo 9:6. https://doi.org/10.1186/s13227-018-0093-2
Lawry JV Jr (1967) Structure and function of the parapodial cirri of the polynoid polychaete, Harmothoe. Zeitschrift für Zellforschung und Mikroskopische Anatomie 82:345–361
Lechapt JP, Kirtley DW (1996) Bathysabellaria spinifera (Polychaeta: Sabellariidae), a new species from deep water off New Caledonia, Southwest Pacific Ocean. Proc Biol Soc Wash 109:560–574
Lehmacher C, Ramey-Balci PA, Wolff LI, Fiege D, Purschke G (2016) Ultrastructural differences in presumed photoreceptive organs and molecular data as a means for species discrimination in Polygordius (Annelida, Protodriliformia, Polygordiidae). Org Divers Evol 16:559–576
Martínez A, Purschke G, Worsaae K (2017) Protodrilidae Hatschek, 1888. In: Westheide W, Purschke G, Böggemann M (eds) Handbook of zoology online. A natural history of the phyla of the animal kingdom; Annelida: Polychaetes. http://www.degruyter.com/view/Zoology/pb029147-6_8. Accessed 19 Sept 2017
McCarthy DA, Forward RB Jr, Young CM (2002) Ontogeny of phototaxis and geotaxis during larval development of the sabellariid polychaete Phragmatopoma lapidosa. Mar Ecol Prog Ser 241:215–220
Nicol JAC (1950) Responses of Branchiomma vesiculosum (Montagu) to photic stimulation. J Mar Biol Assoc UK 29:303–320
Nilsson DE (1994) Eyes as optical alarm systems in fan worms and ark clams. Philos Trans R Soc Lond B 346:195–212
Orrhage L (1978) On the structure and evolution of the anterior end of the Sabellariidae (Polychaeta, Sedentaria). With some remarks on the general organisation of the polychaete brain. Zool Jb Anat 100:343–374
Pawlik JR (1986) Chemical induction of larval settlement and metamorphosis in the reef building tube worm Phragmatopoma californica (Polychaeta: Sabellariidae). Mar Biol 91:59–68
Pawlik JR (1988) Larval settlement and metamorphosis of two gregarious sabellariid polychaetes: Sabellaria alveolata compared with Phragmatopoma californica. J Mar Biol Assoc UK 68:101–124
Pawlik JR (1990) Natural and artificial induction of metamorphosis of Phragmatopoma lapidosa californica (Polychaeta: Sabellariidae), with a critical look at the effects of bioactive compounds on marine invertebrate larvae. Bull Mar Sci 46:512–536
Pergner J, Kozmik Z (2017) Photoreceptors of amphioxus—insights into evolution of vertebrate opsins, vision and circadian rhythmicity. Int J Dev Biol 61:665–681. https://doi.org/10.1387/ijdb.170230zk
Purschke G (2011) Sipunculid-like ocellar tubes in a polychaete, Fauveliopsis cf. adriatica (Annelida, Fauveliopsidae): implications for eye evolution. Invertebr Biol 130:115–128
Purschke G (2016) Annelida: basal groups and pleistoannelida. In: Schmidt-Rhaesa A, Harzsch S, Purschke G (eds) Structure and evolution of invertebrate nervous systems. Oxford University Press, Oxford, pp 254–312
Purschke G, Müller MC (1996) Structure of photoreceptor-like sense organs in Protodriloides species (Polychaeta, Protodrilida). Cah Biol Mar 37:205–219
Purschke G, Nowak K (2015) Ultrastructure of pigmented eyes in Dorvilleidae (Annelida, Errantia, Eunicida) and their importance for understanding the evolution of eyes in polychaetes. Acta Zool 96:67–81
Purschke G, Arendt D, Hausen H, Müller MCM (2006) Photoreceptor cells and eyes in Annelida. Arthropod Struct Dev 35:211–230
Purschke G, Bleidorn C, Struck T (2014) Systematics, evolution and phylogeny of Annelida—a morphological perspective. Mem Mus Vic 71:247–269
Purschke G, Hugenschütt M, Ohlmeyer L, Meyer H, Weihrauch D (2017) Structural analysis of the branchiae and dorsal cirri in Eurythoe complanata (Annelida, Amphinomida). Zoomorphology 136:1–18
Randel N, Jékely G (2015) Phototaxis and the origin of visual eyes. Philos Trans R Soc 371:1–12
Richter S, Loesel R, Purschke G, Schmidt-Rhaesa A, Scholtz G, Stach T, Vogt L, Wanninger A, Brenneis G, Döring C, Faller S, Fritsch M, Grobe P, Heuer CM, Kaul S, Møller OS, Müller CHG, Rieger V, Rothe BH, Stegner MEJ, Harzsch S (2010) Invertebrate neurophylogeny: suggested terms and definitions for a neuroanatomical glossary. Front Zool 7:29
Riisgrad HU, Nielsen C (2006) Feeding mechanism of the polychaete Sabellaria alveolata revisited: comment on Dubois et al. (2005). Mar Ecol Prog Ser 328:295–305
Rouse GW, Pleijel F (2001) Polychaetes. Oxford University Press, New York, 345 pp
Smith PR, Chia F-S (1985) Larval development and metamorphosis of Sabellaria cementarium Moore, 1906 (Polychaeta: Sabellariidae). Can J Zool 63:1037–1049
Struck TH, Golombek A, Weigert A, Franke FA, Westheide W, Purschke G, Bleidorn C, Halanych KM (2015) The evolution of annelids reveals two adaptive routes to the interstitial realm. Curr Biol 25:1993–1999
Vodopyanov S, Purschke G (2017) Fine structure of the cerebral eyes in Flabelligera affinis (Annelida, Sedentaria, Cirratuliformia): new data prove the existence of typical converse annelid multicellular eyes in a sedentary polychaete. Zoomorphology 136:307–325
Weigert A, Bleidorn C (2016) Current status of annelid phylogeny. Org Divers Evol 16:345–362
Wilkens V, Purschke G (2009) Pigmented eyes, photoreceptor-like sense organs and central nervous system in the polychaete Scoloplos armiger (Orbiniidae, Annelida) and their phylogenetic importance. J Morphol 270:1–15
Wilson DP (1929) The larvae of the British sabellarians. J Mar Biol Assoc UK 15:221–269
Wilson DP (1968) The settlement behaviour of the larvae of Sabellaria alveolata (L.). J Mar Biol Assoc UK 48:387–435
Wilson DP (1977) The distribution, development and settlement of the sabellarian polychaete Lygdamis muratus (Allen) near plymouth. J Mar Biol Assoc UK 57:761–792
Acknowledgements
We are grateful to the head of our department, Professor Dr. A. Paululat, Osnabrück, for various kinds of support. Professor Dr. T. Bartolomaeus and Dr. E. Tilic, Bonn, Germany, kindly helped fixing specimens from Mont St. Michel. Thanks are also due to K. Etzold and W. Mangerich, Osnabrück, for various kinds of technical assistance, particularly for introducing CM to electron microscopy techniques. Funding was provided by National Council for Scientific and Technological Development, Brazil (CNPq – SWE 201233/2015-0) and Muséum National d’Histoire Naturelle.
Note added in proof
While this paper was in type setting another study on a sabbellariid median organ was published (Helm et al. 2018): The authors regard the median organ as a structure comparable to nuchal organs and describe the eyes of Idanthyrsus australensis differing in structure, number and position from those described here for S. alveolata.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
We neither used endangered species nor were the investigated animals collected in protected areas. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (MP4 61832 KB)
Rights and permissions
About this article
Cite this article
Meyer, C., Faroni-Perez, L. & Purschke, G. Anterior sense organs in Sabellaria alveolata (Annelida, Sedentaria, Spionida) with special reference to ultrastructure of photoreceptor elements presumably involved in shadow reflex. Zoomorphology 138, 39–54 (2019). https://doi.org/10.1007/s00435-018-0422-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-018-0422-y