Abstract
Morphological adaptations for feeding are a conspicuous feature of avian evolution. Accipitrids exhibit a wide range of prey preferences. Skulls of 97 species which were assigned to seven dietary groups in the present study, were compared from the dorsal, lateral, and ventral views using geometric morphometrics. Landmarks were placed on the overall shape of cranium, bill, orbit, nostril, and attachment area for different jaw muscles. The results suggested considerable variations on the shape of bill and cranium, as well as the size of jaw closing muscles, by which can distinguish most of the groups. Scavengers were found to have a more slender and shallower skull, smaller orbits and longer maxilla whereas piscivores have a larger palatine. Mammalivores are characterized by reduced attachment area for the M. adductor mandibular externus superficialis, a relatively large palatine, long maxilla, and caudally positioned quadrate. Insectivores tend to have larger and more anteriorly oriented orbits, a relatively large attachment area for the M. adductor mandibular externus superficialis, and relatively broad and thin bills. Avivores are distinctive in their broad and protrudent caudal cranium. These morphological characteristics have some functional implications, and shed light on further biomechanical research. Moreover, phylogeny and size significantly contribute to skull shape variation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Avian feeding strategies are highly diverse and the morphological adaptations for feeding are a conspicuous feature of avian evolution (Gill 1994). The feeding apparatus is comprised of skeletal, muscular, and neural systems, which work together to ensure proper function. The avian skull, which is mainly composed of the bill and cranium, exhibits a remarkable degree of morphological variation (Zusi 1993). There has been considerable research on the relationship between bill morphology and diet, particularly in finches (Bowman 1961; Bhattacharyya 1994; Grant 1999; Grenier and Greenberg 2005; Nebel et al. 2005; Grant and Grant 2006; van de Pol et al. 2009), on the relationship between the avian orbit, eye structure and feeding behavior (O’Rourke et al. 2010), and on the relationship between the structure of the cranium, which provides the main attachment area for the jaw muscles, and feeding behavior (Herrel et al. 2005a, b, 2010; Sustaita 2008; van der Meij and Bout 2004, 2008; Soons et al. 2010; Sustaita and Hertel 2010; Rayfield 2011).
The Accipitridae are a highly diverse group of raptors that vary greatly in body form and size. They are characterized by hooked bills, sharp talons, and enlarged eyes with acute vision (del Hoyo et al. 1994). They typically seize prey with their hindlimbs and kill it with their bill and foot. Accipitrids have diverse dietary preferences that include birds, insects, fish, mammals, reptiles, and carrion (del Hoyo et al. 1994). This diversity in morphology and food preferences makes them an ideal group in which to study the relationship between morphology and ecology. The skull, jaw muscles, and consequently the bite force capacity are important for an ecomorphological study.
Traditional linear measurement analysis is fundamental for the quantitative comparison of morphological variation. It has been suggested that the skull morphology of diurnal raptors is closely related to their dietary preferences (Hertel 1995), and that Old and New World vultures can be categorized into three distinct guilds of feeding behavior (Hertel 1994). However, traditional morphometrics is limited in capturing substantial information of geometry. By contrast, landmark-based morphometric methodis advantageous for separating shape information from size variation and in providing a visual representation of shape variation (Zelditch et al. 2004). Geometric morphometric techniques have been used widely in research on both extant and extinct species (Acosta and Tambussi 2006; Foster et al. 2008; Brusaferro and Insom 2009; Kulemeyer et al. 2009; Marugán-Lobón and Buscalioni 2004, 2006, 2009; Degrange and Picasso 2010; Brusatte et al. 2012, Si et al. 2015; Bright et al. 2016; Tokita et al. 2016).
A 3-D geometric morphometric research on raptors shown that shape of beak and cranium were largely controlled by non-dietary factors including integration and allometry; meanwhile, diet also has impact in shaping the skull morphology, especially for scavengers, insectivores and avivores which were distinct from many other dietary groups (Bright et al. 2016). Unfortunately, most of the investigation on skull shape diversification, nether linear morphometric nor geometric morphometric method, pay no attention to jaw muscles. However, it is important to consider bone and muscle characteristics together, because bite force is influenced by the geometry of the skull and jaw closing muscles, and the relative size of the jaw closing muscles (van der Meij and Bout 2008). In a 2-D geometric morphometric work to explore the relationship among skull shape, ecology, and phylogeny in scavenging raptors, origin and size of jaw muscle were considered when selecting landmarks, but provided no information on raptors in other trophic guilds (Si et al. 2015). In this paper, we investigate 97 accipitrid species to identify patterns of variation in skull shape, to assess the extent to which this variation could reflect adaptation to a particular diet or foraging mode. Landmarks were placed on the overall shape of cranium, bill, orbit, nostril, and attachment area for different jaw muscles. This study will be useful for the dietary and ecological interpretation of fossil species.
Materials and methods
Morphological variation of skulls of 97 species in the family Accipitridae was analyzed using two-dimensional geometric morphometrics. According to their preferred prey (Temeles 1985; del Hoyo et al. 1994; Hertel 1994, 1995; Gamauf et al. 1998; Roulin and Wink 2004), these species were assigned to one of seven dietary categories : scavenger, piscivore, insectivore (species which prey on invertebrates), mammalivore, avivore, herpetivore, or generalist (Table S1). These categories are not absolute and we are not suggesting that all species are obligate feeders within their respective dietary group. However, we think this classification is generally valid with regard to examining the correlation between skull morphology and diet, and these dietary categories have been adopted and used in previous researches (Hertel 1995; Bright et al. 2016).
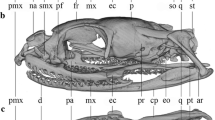
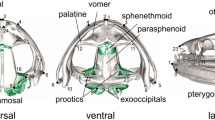
Landmark-based geometric morphometrics are based on the configurations of landmarks in a coordinate system. Landmarks were digitized on high resolution digital images which were obtained using a Canon Eos 60D digital camera. To capture as much shape information as possible, each specimen was photographed with a scale bar in lateral, dorsal and ventral views, with the palate (dorsal and ventral views) or the mid-sagittal plane (lateral view) of the skull parallel to the photographic plane. Skulls that were incomplete or where landmarks could not be reliably digitized were excluded from the analysis. A total of 286, 276, and 292, lateral, ventral and dorsal views were analyzed, respectively. Landmarks were digitized for only one side of the skull to avoid possible variation because of lateral asymmetry. A total of 20 lateral landmarks, 12 ventral landmarks, and 10 dorsal landmarks were defined and marked on the images using the program tpsDig2 (Fig. 1; Table 1). Many of these landmarks were chosen to reflect functional features, and to indicate the lengths of lever arms for muscular actions, and sizes of muscles. Muscle size was roughly reflected by the size of its origin. The positions of landmarks 16–18 in the dorsal view recorded the approximate size of attachment area of M. adductor mandibular externus superficialis; landmarks 2–6 in the ventral view indicated the size of the pterygoid bone and the attachment of M. pterygoideus.
Shape information was obtained after generalized least squares Procrustes superimposition, namely the generalized procrustes analysis (GPA), which removes from landmark coordinates variation due to scaling, position and orientation (Rohlf and Slice 1990; Zelditch et al. 2004). To display the main patterns of variation across the entire skull among accipitrids, we carried out separate principal component analyses (PCA) for data of the three different views. PCA transforms the variables (Procrustes coordinates) into a set of new variables; the principal components (PCs), which are uncorrelated with each other and successively account for the maximum possible amount of variation. The thin-plate spline (TPS) technique was used to visualize shape changes. Permutation tests of difference in means based on Procrustes distances were performed to compare the differences among groups favoring different diets in the lateral, dorsal, and ventral views. These analyses and visualizations were carried out in Morpho J (Klingenberg 2011).
1000 trees for the possible phylogenetic affinities of 88 studied species were retrieved at http://birdtree.org/ (Jetz et al. 2012). These trees take the Hackett et al. (2008) topology as a backbone. A strict consensus tree was then built in Mesquite 2.75 (Maddison and Maddison 2011). This consensus tree was used in the following phylogenetic analyses. To test if phylogeny significantly correlated with skull shape, phylogenetic signal in the morphometric data set was assessed by four different methods. First, a Permutation test was conducted in MorphoJ. Second, Pagel’s lambda (Pagel 1999) test was carried out using the R package GEIGER (Harmon et al. 2008). The influence of the phylogeny increases with lambda from 0 (no phylogenetic signal) and 1 (strong phylogenetic signal). To determine whether lambda is significantly different from zero, we used a likelihood ratio test in R (Harmon et al. 2008). Third, Blomberg’s K test (Blomberg et al. 2003) was performed in R using the PHYTOOLS package (function phylosig). Values of K close to 1 indicate trait similarity is proportional to divergence and a Brownian motion model of evolution fits the data; K > 1 indicates that close relatives are more similar than expected, and K < 1 indicates more divergence between taxa than expected under a Brownian model. Fourth, we performed the computation of Kmult using the function physignal in the R package geomorph (Adams 2014). MESQUITE was used to map PC 1 and 2 which contained the shape information onto the reference phylogeny. To control for phylogeny, phylogenetic ANOVA including a post-hoc test (using Holm–Bonferroni method) on means was conducted in R using the package PHYTOOLS (Revell 2012) to see if skull shape (represented by PCs) significantly different between any two dietary groups. Because materials used in this study are different in size, allometry is an important potential factor to influence the skull shape. We estimated evolutionary allometry using multivariate regression of the independent contrasts of Procrustes coordinates as the shape variables, on independent contrasts of log-transformed centroid size as the size measure.
Results
The first two PCs produced by PCA of the lateral data summarized the majority (62.12%) of the total shape variation. PC1 mainly explained variation in the length of the bill and the size of the orbit (Fig. 2). PC1 scores of different dietary groups decreased in the following order: avivores, insectivores, herpetivores, generalists, mammalivores, piscivores, scavengers. Scavengers had the longest maxilla, shortest cranium, smallest orbit. Besides, while the cranium varied, the landmarks which described the attachment area of M. adductor mandibular externus superficialis did not show much variation. This indicated that the attachment area of M. adductor mandibular externus superficialis of scavengers was large relative to their small cranium. PC2 largely described shape changes in the relative size of maxilla and cranium, attachment area of M. adductor mandibular externus superficialis, size of antorbital fenestra, the position and size of nostrils and the position of quadrate. The cranium and the attachment area of M. adductor mandibular externus superficialis became smaller with the increasing PC2 score while the size of antorbital fenestra became larger. Scavengers occupied a unique area in the shape space with no overlap with other dietary categories. The piscivores could be clearly separated to a lesser extent with a small cranium, smaller attachment area of M. adductor mandibular externus superficialis, a heavier and more curved bill, and a more caudal positioned quadrate. The area occupied by herpetivores overlapped much with mammalivores. The insectivores generally have a higher cranium, larger M. adductor mandibular externus superficialis attachment area, and thinner and less curved bill than the mammalivores. The dietary generalists cluster in the middle of the other groups. Avivores had the largest PC1, suggested that their skulls were obviously different from scavengers.
The first two PCs produced by PCA of the ventral data also explained most of the shape variation (69.43%). Shape changes associated with PC1 were the length of the proc. maxillaris of the palatine to the tip of the maxilla, the position of the pars lateralis of the palatine and quadrate, and the width of the cranium (Fig. 3). Scavengers had the largest PC1 with the longest distance of proc. maxillaris of palatine to the tip of the maxilla, a narrower skull, a more caudally positioned pars lateralis of palatine and the quadrate. PC2 primarily related to the length of proc. maxillaris of palatine and size of pars lateralis of palatine; along the increase of PC2, pars lateralis of palatine decreased from piscivores and mammalivores to insectivores and avivores. Scavengers were also the first group that could be separated, followed by piscivores. PC2 of piscivores was small with a relatively longer proc. maxillaris of palatine and larger pars lateralis of palatine. Herpetivores and generalists are in more or less the same position relative to the other groups as they were by PCA of the lateral surface data, but more overlap between insectivores and mammalivores is apparent. From a ventral perspective, the insectivores have a reduced palatine, which is the opposite of the mammalivores. The avivores had the smallest PC1 and a relatively large PC2 with an anteriorly positioned quadrate, a small palatine, and enlargement of caudal cranium.
PC1 of the dorsal surface mainly explained variation in the length of the premaxilla, the size and orientation of the orbit, and the width of the cranium (Fig. 4). Scavengers were again the most distinct group with the largest PC1 and the longest premaxilla, smallest orbit and narrowest cranium. Piscivores had the next largest PC1. PC2 principally showed variation in width of the premaxilla and the position of the anterior orbit. Insectivores and mammalivores took the extremes of the PC2; insectivores tend to have less curved and broader bills, whereas mammalivores were the opposite. There was considerable overlap between herpetivores and mammalivores and generalists are clustered in the central zone between the other dietary groups.
Permutation tests (10,000 permutation rounds) for Procrustes distances among different dietary groups were conducted to compare the shape differences among groups. The results showed that shape variations among different groups were significant (Table 2). Examination of pairwise Procrustes distances between dietary categories showed patterns consistent with the ones observed in the scatter plots of the scores along the first two principal components.
Skull shape variation was significantly correlated with phylogeny (Tables 3, 4). To illustrate current patterns of phenotypic variation among taxa, and reconstruct evolutionary pathways that have led to these patterns, the phylogenetic tree was projected onto the shape space of the lateral, ventral and dorsal views (Figs. 5, 6, 7), respectively. Phylomorphospace revealed considerable criss-crossing of branches, and disparity in lineage density. In general, the lineage density of basal clades (e.g. Elaninae, Perninae, Aegypiinae and Gypaetinae) was notably lower than that of derived clades. In more ancestral clades, variation of the lateral view followed the transformation of PC1, that was, changes in the length of the bill, the size of the orbit, and the height of the cranium; whereas the more derived lineages diversified substantially along PC2 in morphospace that associated with the range difference of attachment area of M. adductor mandibular externus superficialis (Fig. 5). In dorsal view, the basal clades occupied the lower part of the morphospace and can be distinguished from derived lineages by PC2, which mainly described the length of the upper bill. Moreover, within basal lineages, there appears to be diversification in the direction of the first principal component (the horizontal direction in Fig. 7). More compact occupation in the phylomorphospaces of some lineages, especially Buteoninae, suggested that there was a clear phylogenetic signal. Visual inspection also found some homoplasy, apart from Aegypiinae and Gypaetinae, Milvinae which is more closely related to buteonine taxa than to other kites (Perninae and Elaninae) in the phylogeny (Lerner and Mindell 2005; Griffiths et al. 2007), occupied similar space with the basal pernine kites (Figs. 5, 7).
Phylogenetic ANOVA with post hoc test showed that skull shape was only significantly different between scavengers and other dietary groups in dorsal view (p < 0.05); no significant difference between any two non-scavenging groups was recovered. In lateral view, significant difference was only detected between scavengers and insectivores (p = 0.04); but in ventral view, skull shape of scavengers was only much different from that of avivores (p = 0.02).
Size predicted 15.4, 11.15 and 23.02% of shape variation in the dorsal, lateral and ventral views, respectively, thus indicating that there was clear allometry (Figs. S1–S3). In addition, all permutation tests showed that allometry was highly significant (p < 0.0001). With increasing skull size, the shape changes associated with allometry presented as a relatively larger bill, and a shorter cranium in dorsal view; higher cranium and larger attachment area of M. adductor mandibular externus superficialis in lateral view; larger palatine bone, and narrower cranium in ventral view.
Discussion
Significant differences among seven groups of raptors indicate strong correlation between skull morphology and dietary preference. Both bill and cranium showed considerable variations on their shape. The upper bill varied mainly in the length and depth, while changes of cranium were greatly reflected in its size relative to the total skull, the attachment area for the origin of jaw muscles, the size and orientation of the orbits. Consistent with Hertel’s work (1994), scavengers were quite different from others in having slender and lower skull, smaller orbit and longer bill. The main function of the bills of scavengers is to rip flesh from a carcass in contrast to killing active preys (Hertel 1995; Sustaita 2008; Sustaita and Hertel 2010). A relatively long upper bill which is also curved enhances the ability to rip meet apart, and might be advantageous to intrude deeply into the carrions of much larger body size than themselves (Hertel 1994, 1995; Kulemeyer et al. 2009). Variation on orbit size was thought to be associated with the mobility and the size of prey. Scavengers tend to have small orbit, which might be the result of feeding on dead and immobile prey. Orbits of other groups of raptors were large, especially in insectivores and reflected their large eyes with acute vision and broad visual field, which enable them to examine surrounding environment without moving the neck and avoid being exposed to prey species (Jones et al. 2007). Besides, the relative large area occupied by vultures in the shape space of the first two principal components indicated that there was also much variation among scavengers.
Piscivores were distinguishable in three views to a lesser degree than scavengers. They were found to have a relatively large palatine. Palatine is the main bone for the origin of M. pterygoideus which can close the upper and lower jaws simultaneous (Beecher 1951; Burton 1974; Donatelli 2012). A large palatine might be an indication of a well-developed M. pterygoideus in fish-eating raptors. From architectural perspective, M. pterygoideus is larger in mass and physiological cross-sectional area (PCSA), longer in fiber length in raptors, compared with M. adductor mandibular externus superficialis (Wang et al. 2017). As muscle excursion and velocity are directly proportional to muscle fiber length (Lieber and Ward 2011), relatively long fibers in M. pterygoideus are capable to achieve greater velocities. Thus, we presume that the well-developed M. pterygoideus of piscivores is indicative of a fast bite suitable for pulling and tearing flesh from prey. On the other hand, a well-developed M. pterygoideus also helps to keep the skull steady by balancing the forces produced by resistant food items and the large shear force produced by the retraction of the adductor complex (Bout and Zweers 2001; Gussekloo and Bout 2005).
The shape variations of the skull of mammalivores include the reduced attachment area of M. adductor mandibular externus superficialis, larger palatine, larger and more robust bill, and more caudally positioned quadrate, compare with other non-scavenging groups. The more caudally positioned quadrate might be helpful in increasing the bite force as in finches (van der Meij and Bout 2008); It also increases the outlever and give the lever system of the jaw-closing, as a whole, an advantage in velocity transfer. A relatively high inlever-outlever ratio means that M. adductor mandibular externus superficialis exerts stronger force, but slower movement, when the jaw closes. This was certified by many researches (Beecher 1951; Bowman 1961; Bock 1964; Zweers 1974; Van der Meij and Bout 2004; Sustaita 2008; Donatelli 2012). Accordingly, a larger and heavier bill might be a kind of reinforcement associating with the excessive stresses because of strong bite force (Bowman 1961; Soons et al. 2010).
The orbits of insectivores are larger and more anterior in position, indicating a large binocular field of vision (Heesy 2004). The principal function of binocular field is in guiding the bill or feet towards food objects (Martin 2007). Insectivores always catch preys by quick stooping attacks above prey items and then pinning them against the ground. This kind of hunting strategy might require the image to be as stable as possible (Kulemeyer et al. 2009; O’Rourke et al. 2010). The main function of M. adductor mandibular externus superficialis is to raise the lower jaw, and the muscle provided the greatest contribution to the bite force (Van der Meij and Bout 2008). A large M. adductor mandibular externus superficialis is advantageous in producing strong bite force and a large attachment area for this muscle suggests that increased bite force is a key performance feature of the skulls of insectivores which typically catch and kill prey entirely with their bills (Csermely et al. 1998; Fowler et al. 2009). Another advantage of the large area of origin of the adducting muscle is likely to spread the force of the muscle over a wider area of bone, thereby reducing stress on individual bones, as found in other birds (Bowman 1961; Bock 1964). A shorter maxilla in insectivores might be the result of the relatively small size of their insect prey and the requirement for more flexibility. Avivores were found similar to insectivores in the size of jaw muscles’ origin, but their caudal part of the cranium was broader and more protrudent. This feature implies a well-development of cerebellum which provided important role during movement, especially for raptors preying on flying birds.
The skulls of herpetivores were similar to those of mammalivores, which could reflect the thicker and tougher skins of their respective prey compared to those than of avivores (Hertel 1995). Generalists occupied the middle of the morphospace with respect to all other dietary groups in all the three views. This is consistent with the relatively high degree of overlap in skull shape and diet between generalists and the other dietary groups.
Consistent with previous research of raptors (Bright et al. 2016), we also found that variation of skull shape was closely related to skull size in Accipitridae. The significant allometry suggested that size was undoubtedly an effective mechanism through which accipitrids may diversify their prey preference and feeding behaviors. Some trends in allometry that we detected in Accipitridae, such as lengthening of the upper bill, and enlargement of jaw closing muscle attachment sites, in larger skull, are shared by other avian lineages, such as Hawaiian honeycreepers (Tokita et al. 2016).
The present study revealed a clear phylogenetic signal in the landmark data on the skull shape of Accipitridae. The considerable criss-crossing of clades indicated low disparity of forms relative to the number of species (Sidlauskas 2008), indicating that raptors are thoroughly exploring a tightly constrained morphological space, through either extensive convergence or, alternatively, limited shape change from a basal morphological state (Bright et al. 2016). Higher lineage density of derived clades indicated an initial division of morphospace among different subfamilies followed by morphological oscillation around the centers of the morphospace distributions for those different subfamilies (Sidlauskas 2008).
The present study combined the skeletal and muscular features to compare skull shape across feeding types. Our results confirm some conclusions of previous studies of the morphology of the feeding apparatus in raptors (Hertel 1994; Bright et al. 2016), reveal considerable variation on cranium shape, a judgment different from that of Bright et al’s (2016), and most importantly uncover substantial changes of jaw closing muscles. All functional interpretations related to skeleton and muscle shape in the study were indirect, and based on general principle. To demonstrate the relationship between form and function, specific biomechanical works are needed in the future, such as how the bite force and speed are affected by the jaw closing muscles and bill shape.
References
Acosta HC, Tambussi C (2006) Skull morphometry of Pygoscelis (Sphenisciformes): inter and intraspecific variations. Polar Biol 29:728–734
Adams DC (2014) A generalized K statistic for estimating phylogenetic signal from shape and other high-dimensional multivariate data. Syst Biol 63:685–697
Baumel JJ, Witmer LM (1993) Osteologia. In: Baumel JJ, King AS, Breazile JE, Evans HE, Van den Berge JC (eds) Handbook of avian anatomy: nomina anatomica avium, vol 23. Nuttall Ornithological Club, Cambridge, Massachusetts, pp 45–132
Beecher WJ (1951) Adaptations for food-getting in the American blackbirds. Auk 68:411–440
Bhattacharyya BN (1994) Diversity of feeding adaptations in certain columbid birds: A functional morphological approach. J Biosci 19:415–427
Blomberg SP, Garland T Jr, Ives AR (2003) Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57:717–745
Bock WJ (1964) Kinetics of the avian skull. J Morphol 114:1–42
Bout RG, Zweers GA (2001) The role of cranial kinesis in birds. Comp Biochem Physiol 131A:197–205
Bowman RI (1961) Morphological differentiation and adaptation in the Galapagos finches. Univ Calif Publ Zool 58:1–302
Bright JA, Marugán-Lobón J, Cobb SN, Rayfield EJ (2016) The shapes of bird beaks are highly controlled by nondietary factors. PNAS 113:5352–5357
Brusaferro A, Insom E (2009) Morphometric analysis of the kingfisher cranium (AVES). Ital J Zool 76:53–63
Brusatte SL, Sakamoto M, Montanari S, Harcourt Smith WEH (2012) The evolution of cranial form and function in theropod dinosaurs: insights from geometric morphometrics. J Evol Biol 25:365–377
Burton PJK (1974) Jaw and tongue features in Psittaciformes and other orders with special reference to the anatomy of the Tooth-billed pigeon (Didunculus strigirostris). J Zool 174:255–276
Csermely D, Bertè L, Camoni R (1998) Prey killing by Eurasian Kestrels: the role of the foot and the significance of bill and talons. J Avian Biol 29:10–16
Degrange FJ, Picasso MBJ (2010) Geometric morphometrics of the skull of Tinamidae (Aves, Palaeognathae). Zoology 113:334–338
del Hoyo J, Elliott A, Sargatal J (1994) Handbook of the birds of the world vol 2. New World vultures to Guineafowl. Lynx Edicions, Barcelona
Donatelli RJ (2012) Jaw musculature of the Picini (Aves: Piciformes: Picidae). Int J Zool 2012:1–12
Foster DJ, Podos J, Hendry AP (2008) A geometric morphometric appraisal of beak shape in Darwin’s finches. J Evol Biol 21:263–275
Fowler DW, Freedman EA, Scannella JB (2009) Predatory functional morphology in raptors: interdigital variation in talon size is related to prey restraint and immobilisation technique. PLoS One 4(11):e7999
Gamauf A, Preleuthner M, Winkler H (1998) Philippine birds of prey interrelations among habitat, morphology and behavior. Auk 115(3):713–726
Gill FB (1994) Ornithology, 2nd edn. W. H. Freeman, New York
Grant PR (1999) Ecology and evolution of Darwin’s finches. Princeton University Press, Princeton
Grant PR, Grant BR (2006) Evolution of character displacement in Darwin’s finches. Science 313:224–226
Grenier JL, Greenberg R (2005) A biogeographic pattern in sparrow bill morphology: parallel adaptation to tidal marshes. Evolution 59:1588–1595
Griffiths CS, Barrowclough GF, Groth JG, Mertz LA (2007) Phylogeny, diversity, and classification of the Accipitridae based on DNA sequences of the RAG-1 exon. J Avian Biol 38:587–602
Gussekloo SWS, Bout RG (2005) Cranial kinesis in palaeognathous birds. J Exp Biol 208:3409–3419
Hackett SJ et al (2008) A phylogenomic study of birds reveals their evolutionary history. Science 320:1763–1768
Harmon LJ, Weir JT, Brock CD, Glor RE, Challenger W (2008) GEIGER: investigating evolutionary radiations. Bioinformatics 24:129–131
Heesy CP (2004) On the relationship between orbit orientation and binocular visual field overlap in mammals. Anat Record 281:1104–1110
Herrel A, Podos J, Huber SK, Hendry AP (2005a) Evolution of bite force in Darwin’s finches: a key role for head width. J Evol Biol 18:669–675
Herrel A, Podos J, Huber SK, Hendry AP (2005b) Bite performance and morphology in a population of Darwin’s finches: implications for the evolution of beak shape. Funct Ecol 19:43–48
Herrel A, Soons J, Aerts P, Dirckx J et al (2010) Adaptation and function of the bills of Darwin’s finches: divergence by feeding type and sex. Emu 110:39–47
Hertel F (1994) Diversity in body size and feeding morphology within past and present vulture assemblages. Ecology 75:1074–1084
Hertel F (1995) Ecomorphological indicators of feeding behavior in recent and fossil raptors. Auk 112:890–903
Jetz W, Thomas GH, Joy JB, Hartmann K, Mooers AO (2012) The global diversity of birds in space and time. Nature 491:444–448
Jones MP, Pierce KE, Ward D (2007) Avian vision: a review of form and function with special consideration to birds of prey. J Exot Pet Med 16:69–87
Klingenberg CP (2011) MorphoJ: an integrated software package for geometric morphometrics. Mol Ecol Resour 11:353–357
Kulemeyer C, Asbahr K, Gunz P, Frahnert F et al (2009) Functional morphology and integration of corvid skulls—a 3D geometric morphometric approach. Front Zool 6:2
Lerner H, Mindell D (2005) Phylogeny of eagles, old world vultures, and other Accipitridae based on nuclear and mitochondrial DNA. Mol Phylogenet Evol 37:327–346
Lieber R, Ward S (2011) Skeletal muscle design to meet functional demands. Philos Trans R Soc B 366:1466–1476
Maddison W, Maddison D (2011) Mesquite: a modular system for evolutionary analysis, version 2.75. See http://mesquiteproject.org
Martin GR (2007) Visual fields and their functions in birds. J Ornithol 148(Suppl2):S547–S562
Marugán-Lobón J, Buscalioni ÁD (2004) Geometric morphometrics in macroevolution: morphological diversity of the skull in modern avian forms in contrast to some theropod dinosaurs. In: Ashraf M, Elewa T (eds) Morphometrics: applications in biology and paleontology. Springer, Berlin, pp 157–173
Marugán-Lobón J, Buscalioni AD (2006) Avian skull morphological evolution: exploring exo- and endocranial covariation with two-block partial least squares. Zoology 109:217–230
Marugán-Lobón J, Buscalioni AD (2009) New insight on the anatomy and architecture of the avian neurocranium. Anat Record 292:364–370
Nebel S, Jackson DL, Elner RW (2005) Functional association of bill morphology and foraging behaviour in calidrid sandpipers. Anim Biol 55:235–243
O’Rourke CT, Hall MI, Pitlik T, Fernández-Juricic E (2010) Hawk eyes I: diurnal raptors differ in visual fields and degree of eye movement. PLoS One 5(9):e12802
Pagel M (1999) Inferring the historical patterns of biological evolution. Nature 401:877–884
Rayfield EJ (2011) Strain in the ostrich mandible during simulated pecking and validation of specimen-specific finite element models. J Anat 218:47–58
Revell LJ (2012) phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol Evol 3:217–223
Rohlf FJ, Slice D (1990) Extension of the Procrustes method for the optimal superimposition of landmarks. Syst Zool 39:40–59
Roulin A, Wink M (2004) Predator-prey relationships and the evolution of colour polymorphism: a comparative analysis in diurnal raptors. Biol J Lin Soc 81:565–578
Si G, Dong Y, Ma Y, Zhang Z (2015) Shape similarities and differences on the skull of scavenging raptors. Zool Sci 32:171–177
Sidlauskas B (2008) Continuous and arrested morphological diversification in sister clades of characiform fishes: a phylomorphospace approach. Evolution 62:3135–3156
Soons J, Herrel A, Genbrugge A, Aerts P et al (2010) Mechanical stress, fracture risk, and beak evolution in Darwin’s ground finches (Geospiza). Philos Trans R Soc B 365:1093–1098
Sustaita D (2008) Musculoskeletal underpinnings to differences in killing behavior between North American accipiters (Falconiformes: Accipitridae) and falcons (Falconidae). J Morphol 269:283–301
Sustaita D, Hertel F (2010) In vivo bite and grip forces, morphology and prey-killing behavior of North American accipiters (Accipitridae) and falcons (Falconidae). J Exp Biol 213:2617–2628
Temeles EJ (1985) Sexual size dimorphism of bird-eating hawks: the effect of prey vulnerability. Am Nat 125(4):485–499
Tokita M, Yano W, James HF, Abzhanov A (2016) Cranial shape evolution in adaptive radiations of birds: comparative morphometrics of Darwin’s finches and Hawaiian honeycreepers. Philos Trans R Soc B 372:20150481
van de Pol M, Ens BJ, Oosterbeek K, Brouwer L et al (2009) Oystercatchers’bill shapes as a proxy for diet specialization: more differentiation than meets the eye. Ardea 97:335–347
Van der Meij MAA, Bout RG (2004) Scaling of jaw muscle size and maximal bite force in finches. J Exp Biol 207:2745–2753
Van der Meij MAA, Bout RG (2008) The relationship between shape of the skull and bite force in finches. J Exp Biol 211:1668–1680
Wang H, Yan J, Zhang Z (2017) Sexual dimorphism in jaw muscles of the Japanese sparrowhawk (Accipiter gularis). Anat Histol Embryol 46:558–562
Zelditch ML, Swiderski D, Sheets D, Fink WL (2004) Geometric morphometrics for biologists: a primer. Elsevier Academic Press, London
Zusi RL (1993) Patterns of diversity in the avian skull. In: Hanken J, Hall BK (eds) The skull. University of Chicago Press, Chicago, pp 391–437
Zweers GA (1974) Structure, movement and myography of the feeding apparatus of the Mallard (Anas platyrhynchosL.). Netherlands J Zool 24:323–467
Acknowledgements
We are grateful to H. F. James and C. M. Milensky (American Natural History Museum) for kindly providing us access to the collections under their care; R. Moorhouse for language improvement and helpful suggestions; C. Klingenberg for the course on geometric morphometrics; and three anonymous reviewers for constructive comments that improved the manuscript. This work was supported by the National Natural Science Foundation of China (31272259), and Support Project of High-level Teachers in Beijing Municipal Universities in the Period of 13th Five–year Plan (IDHT20180518).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
435_2018_406_MOESM4_ESM.docx
Supplementary Table S1 List of accipitrid species and museum specimens used in geometric morphometric analysis of skull variation; abbr = abbreviation of scientific name. a = avivore, g = generalist, I = insectivore, m = mammalivore, p = piscivore, h = herpetivore, s = scavenger. Numerals indicate the number of digital images of each skull surface (lateral, ventral, dorsal) used in analyses (DOCX 27 KB)
Rights and permissions
About this article
Cite this article
Sun, Y., Si, G., Wang, X. et al. Geometric morphometric analysis of skull shape in the Accipitridae. Zoomorphology 137, 445–456 (2018). https://doi.org/10.1007/s00435-018-0406-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-018-0406-y