Abstract
Pelophylax esculentus is the fertile hybrid of P. ridibundus and P. lessonae. During gametogenesis, one of the parental genomes is removed from the germ line cells, whereas the other one is clonally transmitted to the gametes. In hybrids, development of gonads is delayed in comparison with parental species. This may result from complex processes of genome elimination in female tadpoles at Gosner stages 28–46, potentially responsible for increased degeneration of germ cells in developing gonads from the very beginning of sexual differentiation to ovaries with diplotene oocytes, respectively. In this work, we revealed that germ cells died by apoptosis, as detected by expression of active caspase-3 using immunohistochemical method. The main group of degenerating germ cells was primary oogonia, however, in P. lessonae and P. ridibundus also secondary oogonia and diplotene oocytes were found. The number of degenerating germ cells was significantly higher in ovaries of P. esculentus. In hybrids, positive correlations were demonstrated between Gosner stage and gonadal volume, Gosner stage and the number of degenerating germ cells, gonadal volume and number of degenerating germ cells. These observations suggest that increased rate of apoptosis in germ cells, probably as the result of improper genome elimination, may be responsible for delayed maturation of ovaries in P. esculentus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Pelophylax esculentus complex contains the following taxa: P. ridibundus (genotype RR), Pallas 1771, P. lessonae (LL), Camerano 1882, and P. esculentus (RL), Linneus 1758. Pelophylax esculentus is the fertile hybrid of P. ridibundus and P. lessonae (Berger 1983) that propagate from generation to generation by hybridogenesis (Graf and Polls Pelaz 1989). Hybridogenesis is regarded as a form of genome parasitism, i.e., for hybrid offspring to be able to survive in a habitat, the hybrids must co-occur with one of the parental species (Graf and Polls Pelaz 1989; Joly 2001). Because of different habitat preferences of P. ridibundus and P. lessonae, naturally occurring populations are composed of the hybridogenetic taxon and one of the parental species (Rybacki and Berger 1994, 2001; Semlitsch et al. 1996; Socha and Ogielska 2010). There are mixed populations of P. lessonae and P. esculentus, forming a system labeled as L-E, P. ridibundus and P. esculentus, forming a system labeled as R-E (Rybacki and Berger 1994; Semlitsch et al. 1996). These and rarer populations consisting of all three taxa labeled as R-E-L (Lada et al. 1995; Hoffmann et al. 2015) and all-hybrid populations (E-E) exist across central Europe (Günther and Plötner 1990; Jakob et al. 2010; Rybacki and Berger 2001). The constant existence of hybrids is made possible through the process of elimination of one of the genomes (R or L) from early germ cells of the hybrid RL, reduplication of the remaining genome to restore diploid number of chromosomes before meiosis, and production of clonal haploid gametes (for review see Graf and Polls Pelaz 1989; Ogielska 2009). Studies carried out during development of female gonads have shown gradual loss of one of the chromosome sets (Tunner and Heppich-Tunner 1991). Earlier studies of one of us (Ogielska 1994) showed the presence of micronuclei (originally named nucleus-like bodies, NLB) in oogonia before meiosis that were supposed to be fragments of the interphase chromosomes rejected from the cell nucleus. Further studies proved that elimination of a genome occurs during the extended phase of the mitotic divisions of primary oogonia (Ogielska 2009). Oogenesis of P. ridibundus and P. lessonae begins at early stages of larval life before the completion of metamorphosis (Ogielska and Wagner 1990, 1993; Ogielska and Kotusz 2004). Development of ovaries of P. esculentus is extended by at least 1 year in relation to the parental species due to prolonged phase of oogonial proliferation when genome elimination takes place (Wagner and Ogielska 1990, 1993).
The aim of the study was qualitative and quantitative analysis of degenerations of germ line cells, and comprehensive statistical examination of the effect of germ cell degeneration on early development of tadpole ovaries in P. lessonae, P. ridibundus and P. esculentus. We consider that germ cells with improper genome elimination are likely to degenerate via apoptosis pathway. Degeneration and death of cells in an apoptotic manner depend on a major enzyme caspase-3, which is a cystic protease and occurs in the majority of cells as an inactive proenzyme (Elmore 2007). Expression of active caspase-3 is a valuable marker of apoptosis (Chmielewska et al. 2015) and thereby can serve in assessment of cell degeneration in juvenile ovaries. Activation of caspase-3 is followed by activation of endonucleases, which cause degradation of the chromosomal DNA, as well as activation of other proteases that cause reorganization of the cytoplasm and cytomorphological changes in form of, e.g., chromatin condensation, fragmentation of the nucleus, and pyknosis, leading eventually to the formation of apoptotic bodies.
Materials and methods
Parental individuals of P. lessonae (LL) were obtained from Urwitałt near Mikołajki (Mazury) (N53°48′20.62″ E 21°38′29.22″) and P. ridibundus (RR) were from the Barycz River Valley (N 51°31′53.64″ E17°20′8.67′’). The genotypes were confirmed by serum albumin introne-1 PCR according to Hauswaldt et al. (2012). Tadpoles of various phenotypes (LL, RR, RL) sourced from controlled in vitro crosses according to Berger et al. (1994). Ovaries were dissected from 21 individuals: four P. lessonae, five P. ridibundus and twelve P. esculentus. All ovaries were fixed in Bouin’s solution, routinely processed in paraffin and sectioned into 7-µm-thick sections. Staging of somatic development of tadpoles followed Gosner (1960), and staging of ovary development followed Ogielska and Kotusz (2004). Ovarian stages I–III represent undifferentiated gonad, at stage IV the secondary ovarian cavity and nest of secondary oogonia appeared, at stage V leptotene–pachytene, and beginning with stage VI, diplotene oocytes appeared. At stages VII–IX, number of diplotene oocytes increase considerably, and stage X represents fully grown ovary full of diplotene oocytes.
Immunohistochemical staining of apoptotic cells in tissue sections was performed using primary rabbit antibodies recognizing active caspase-3 (diluted 1:600, Abcam ab13847, recommended for X. leavis). Dako REAL™ EnVision™/HRP system and diaminobenzidine (DAB) (Dako, Denmark) were used for the visualization of antibodies, according to manufacturer’s instructions. Sections were additionally stained with hematoxylin acc. to Delafield. Specimens were examined and photographed under a Zeiss Axioskop 20 light microscope with an AxioCam ERc5s video camera, and AxioVision 4.8 software (Zeiss) was used for image analysis. Cells were assessed as apoptotic when active caspase-3 staining (DAB, brown color) was strong in both cytoplasm and cell nuclei. Counting of apoptotic cells was performed by selecting three nonconsecutive sections of gonads in order to eliminate the possibility of counting the same cell more than once on adjacent sections. Areas of consecutive sections of whole gonads were calculated in AxioVision 4.8 software (Zeiss). Volume of each section was calculated by multiplying the area of ovary section (µm2) by the thickness of section (7 µm). Volume of the whole ovary represents the sum of volumes of all sections. The total number of degenerating apoptotic germ cells in whole ovary (L) was estimated according to the following formula:
Ld—the sum of degenerating cells with strong active caspase-3 signal, counted within three nonconsecutive sections, Vs—volume of the three nonconsecutive sections, evaluated for apoptotic cells (area multiplied by thickness),Vg—volume of the whole gonad (the sum of volumes of all sections).
Histological identification of germ cells based on germ cell size, morphology and surrounding cells context. Primary oogonia were single cells with diameter 15–20 µm, surrounded by somatic cells; secondary oogonia and leptotene–pachytene meiocytes were cells with diameter 15–20 µm, forming isogenic groups (“nests”) connected by cytoplasmic bridges, and diplotene oocytes were cells with diameter bigger than 20 µm, round nucleus containing several nucleoli, and surrounded by follicular cells.
Statistical analysis (see Online Resource 1) was performed using STATISTICA 12.5 software (StatSoft, Poland). Groups of data were compared using the Mann–Whitney U test. Correlations between variables were analyzed using Spearman’s correlation test. Results of the analyses are presented as mean ± SD. Statistical significance was defined when P < 0.05.
For publication purposes, brightness and contrast adjustments were performed in CorelDRAW 13.
Results
General morphology of gonads of P. lessonae, P. ridibundus and P. esculentus
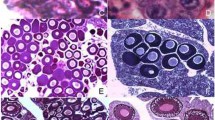
Ovaries were examined at stages IV–VI. Stage IV ovaries in P. lessonae (LL) and P. ridibundus (RR) were at the beginning of sexual differentiation, with primary ovarian cavity filled with somatic cells and thin ovarian cortex composed of primary oogonia (Fig. 1a, b). At gonadal stage V, the secondary ovarian cavity was well developed, and the ovarian cortex contained primary oogonia and nests of secondary oogonia and early meiocytes (Figs. 1c, 2a), whereas at stage VI, numerous diplotene oocytes appeared (Fig. 1d, e).
Comparison of the histological ovarian structure of P. lessonae (a, c, d), P. ridibundus (b, e) and P. esculentus (f). Degenerating germ cells are visualized by immunohistochemical staining of active caspase-3 expression (dark brown). Tissues counterstained with hematoxylin. (a, b) Ovary at gonadal stage IV, (c) ovary at gonadal stage V, (d–f) ovary at gonadal stage VI. (a–c) Inserts show the magnification of marked area. Black arrowhead diplotene oocytes, white arrowhead apoptotic primary oogonia, black arrow apoptotic diplotene oocyte, asterisk secondary ovarian cavity, cs circular staining of active caspase-3 located in cytoplasm underlying the nuclear membrane in primary oogonia, fb fat body, po primary oogonia. Scalebar 100 µm
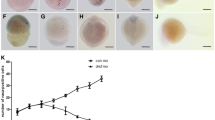
Degenerating germ cells in ovaries of P. lessonae (a, b) and P. esculentus (c, d). Immunohistochemically stained tissue sections show strong signal of active caspase-3 (brown) in apoptotic cells. Tissues counterstained with hematoxylin. a So nest of secondary oogonia containing apoptotic cells; b black arrow apoptotic diplotene oocyte; (c, d) degenerating primary oogonia showing active caspase-3 staining (brown), black arrow cell without pycnotic nucleus, white arrowhead pycnotic nuclei visible in the cells; black arrowhead cell with pyknotic nucleus without active caspase-3 staining; white arrow degenerating early diplotene oocyte with condensed chromatin in nucleus and inflated nucleolus, without active caspase-3 staining. Asterisk secondary ovarian cavity; cs circular staining of active caspase-3 located in cytoplasm underlying the nuclear membrane in primary oogonia; m meiocytes. Scalebar 100 µm
In P. esculentus (RL) ovaries, the secondary ovarian cavity at stage V was as distinct as in RR and LL, but the cortex was evidently thinner due to the lower number of secondary oogonia and meiotic oocytes forming cysts (not shown). In one individual, there was a single germ cells layer in the cortex, while secondary oogonia and meiocyte nests were absent. At stage VI, we found only few diplotene oocytes (Fig. 1f) and the ovarian cortex composed of primary oogonia. Mitotic activity of primary oogonia (not shown) was higher in hybrids than in parental species RR and LL of the same age, although secondary oogonia and meiocyte nests were much less abundant.
Comparison of the structure of stage VI ovaries of all three taxa demonstrated that diplotene oocytes were numerous in the gonads of parental species RR and LL (Fig. 1d, e) but were rare in the hybrids RL (Fig. 1f). This resulted in size of the lumen of the secondary ovarian cavity, which were partly or completely filled with protruding cortex containing diplotene oocytes in ovaries of parental species, whereas in hybrids the lumen was almost empty and thereby remained distinct (Fig. 1d–f).
Degenerating germ cells in P. lessonae and P. ridibundus
Active caspase-3 expression was very strong within the cell nucleus and cytoplasm of degenerating cells (Fig. 1b–d), although apoptotic cells were rare or absent in two individuals (Table 1). Ovaries at stages IV–V contained degenerating primary oogonia (Fig. 1b, c—white arrowhead), which constituted 30–100% of apoptotic cells, and in one individual secondary oogonia in nests (Fig. 2a) representing c. a. 70% of cells with caspase-3 signal. Diplotene oocytes were the dominant class of degenerating cells in the ovaries at stage VI (Figs. 1d, 2b—black arrows), roughly about 70% of cells displaying caspase-3 signal, while primary oogonia were the other 30% of cells. In a portion of primary oogonia, we also noticed “circular staining” of active caspase-3 localized in the cytoplasm underlying the nuclear envelope, but not in the nuclei (Fig. 1a–c—inserts, Fig. 2b–cs). Ovaries at stage IV used to have a lot of oogonia demonstrating the presence of “circular staining,” while the apoptotic oogonia with strong active caspase-3 signal were rare. In stage V–VI gonads, the “circular staining” of oogonia was present to the less extent.
Degenerating germ cells in P. esculentus
Germ cell degeneration was noted in all examined ovaries. Active caspase-3 expression was found only in primary oogonia at ovarian stages IV–V (Fig. 2c). Along with apoptotic changes, primary oogonia often displayed pyknotic nuclei (Fig. 2c—white arrowheads), but in some cells it was not distinct (Fig. 2c—black arrow). However, we also noticed germ line cells, both single (Fig. 2c) and groups of cells in cysts (not shown), showing other signs of degeneration, e.g., pyknotic nuclei (Fig. 2c—black arrowhead) or cell shrinking (not shown), but devoid of active caspase-3 staining. At stage VI ovaries, the only class of degenerating germ cells showing active caspase-3 staining was primary oogonia (Figs. 1f, 2d—white arrowheads). Some portion of primary oogonia showed “circular staining” of active caspase-3 in the cytoplasm around nuclei (Fig. 2d—cs), similarly as was shown in the ovaries of the parental species. In one case, we observed an early diplotene oocyte with shrunken chromatin and inflated nucleolus; however, this cell did not show active caspase-3 staining (Fig. 2d—white arrow). There were no degenerating secondary oogonia, meiocytes or diplotene oocytes displaying the positive expression of active caspase-3.
Size of ovaries and number of degenerating germ cells
Volumes of ovaries and the total number of degenerating germ cells are shown in Table 1. In P. lessonae, gonads showed an increase in volume with more advanced Gosner stage. The least developed gonad at stage IV had volume of 0.0099 mm3, whereas the most advanced stage VI ovaries were almost eight times bigger, i.e., 0.0758 and 0.0795 mm3 (Gosner stages 33 and 36 and 39, respectively). Ovaries of P. ridibundus at gonadal stages IV and VI showed a difference in terms of volume. Younger gonad (IV gonadal stage), with volume of 0.0026 mm3, was several times smaller than the older one (VI gonadal stage, 0.0203 mm3) (Gosner stages 32 and 46, respectively). The number of degenerating germ cells in the ovaries of P. lessonae and P. ridibundus ranged from 0 to 65 (0–4 cells per gonad section). Two individuals, among six analyzed, did not show degeneration symptoms, whereas in four individuals, we observed from 17 to 65 degenerating germ cells.
In P. esculentus, ovarian volumes ranged from 0.0046 to 0.0723 mm3 (see Table 1). As expected, the smallest volume was found in an ovary at stage IV and the largest—in an ovary at gonadal stage VI, and the sizes of ovaries reflected the youngest and the oldest tadpoles (Gosner stages 34 and 46, respectively). However, stages V and VI were found in tadpoles at various Gosner stages (38–45 and 40–46, respectively). Volumes of ovaries at stage V ranged from 0.0147 to 0.0502 mm3 and those at stage VI ranged from 0.0110 to 0.0723 mm3. Number of degenerating germ cells varied considerably between individuals. Ovaries at different developmental stages contained degenerating germ cells in the range of 37–1075 cells (0–36 cells per section). The smallest number of degenerating germ cells (37 cells) was assessed in gonads at stage V (Gosner stage 38). The youngest gonad at stage IV contained almost two times more degenerating germ cells (75 cells). The most numerous degenerating germ cells were found at stage V (1075 cells) (Gosner stage 43).
Statistical analysis
Due to the low number of individuals representing parental species, they were treated as one group (P. lessonae/ridibundus) in further analyses. In this group, the mean of degenerating apoptotic germ cells was 23 (SD = 24.150), and the median was 21.5 (25% percentile = 0; 75% percentile = 30, Fig. 3). The ovaries of hybrid P. esculentus were characterized by decidedly more numerous degenerating germ cells, with the mean 250 (SD = 289.820), and the median 172 (25% percentile = 106; 75% percentile = 244, Fig. 3). The number of degenerating apoptotic germ cells was significantly higher in P. esculentus than in P. lessonae/ridibundus (Fig. 3, Mann–Whitney U test, P = 0.0015), and degenerating cells were 10 times more frequent in the hybrid than in the species. There was no correlation (Spearman’s rank correlation, R = 0.3127) between the number of degenerating germ cells and gonad volumes, taking into account all specimens of the three taxa. Within the group of the parental species P. lessonae/ridibundus, only a weak trend was found between the gonadal stage and Gosner stage, but with no statistical significance (see Table 2 in Online Resource 1). In the hybrid P. esculentus (see Table 3 in Online Resource 1), a very strong positive correlation was found between the Gosner stage and volume of gonads (R = 0.615), as well as between the number of degenerating germ cells and gonadal volume (R = 0.727, see Fig. 4 in Online Resource 1). In addition, along with the age of tadpoles (older Gosner stages), the number of degenerating germ cells in the ovaries significantly increased (R = 0.729).
Discussion
Histological analysis of the ovaries of P. lessonae and P. ridibundus showed typical structure of female gonads in frogs (Ogielska and Kotusz 2004). Morphologically, they were composed of the ovarian cortex containing various classes of germ cells, and the secondary ovarian cavity. It is noteworthy that we observed relationship between the size of the secondary ovarian cavity and the presence of diplotene oocytes. More numerous diplotene oocytes caused apparent smaller ovarian cavity in P. lessonae and P. ridibundus at gonadal stage VI. This was due to the fact that secondary ovarian cavity was filled with diplotene oocytes protruding from the cortex. At the same time, we noticed the increase in gonad volume together with somatic development of the tadpole (Gosner stage). In P. esculentus, such significant positive correlation was also found, but in this case the volume increased as a result of enlarging secondary ovarian cavity and not the cortex (no protruding diplotene oocytes). Similar relationship was demonstrated in the study of Ogielska and Wagner (1993), where ovaries at older gonadal stages than VI were examined.
Hybridogenetic P. esculentus also demonstrated compliance with stages of ovarian development described for the species (Ogielska and Kotusz 2004). However, observations showed differences in the structure of the ovaries at the same gonadal stage when compared with P. lessonae and P. ridibundus. In particular, this related to the thickness of the ovary cortex, which in P. esculentus was definitely thinner, as was reported also by Ogielska and Wagner (1993). This was manifested by the far smaller number of diplotene oocytes observed in the ovaries at gonadal stage VI. Characteristic feature of hybrid ovaries was the presence of numerous mitotic oogonia (not shown in this study) and very low number or complete lack of nests of secondary oogonia and meiocytes. The observations confirmed that the ovaries of hybrids were delayed in their development compared to the parental species (Ogielska and Wagner 1990, 1993). It should also be noted that at gonadal stages V–VI the secondary ovarian cavity was not filled with cells, moreover at stage VI ovaries only single diplotene oocytes were present. The increased total volume of hybrid gonads did not correspond to an increase in the cell number neither the thickening of the cortex, contrary to P. lessonae and P. ridibundus as judged from morphology.
Using immunohistochemical staining of active caspase-3, a major enzyme involved in apoptotic processes (Elmore 2007), we show that germ line cells in Pelophylax ovaries are degenerating by programmed cell death. Apoptotic germ cells that were detected in gonads of the parental species were classified as primary oogonia, nests of secondary oogonia and early meiocytes, as well as early previtellogenic diplotene oocytes. Degenerating primary oogonia were described here for the first time, whereas the occasional degeneration of secondary oogonia and early meiocytes in nests was mentioned previously by Ogielska and Wagner (1993). Intrafollicular atresia of diplotene oocytes was already described in P. lessonae, P. ridibundus and Rana temporaria (Ogielska et al. 2010, 2013); however, again it was very rare in previtellogenic oocytes. In contrast to the morphological description given previously, this study has proved that germ line cells are directed to the programmed cell death during larval development. Degeneration of primary oogonia in tadpoles was previously mentioned by our team (Ogielska et al. 2010, 2013), and now we support the detailed information about the frequency of apoptotic cells in ovaries of three Pelophylax taxa. Moreover, we reveal that oogonial cells in P. esculentus gonads often degenerate due to the programmed cell death, instead of differentiating into early meiotic oocytes.
In P. esculentus, degenerating germ cells in most cases showed strong expression of active caspase-3. Primary oogonia were the dominant class of degenerating germ cells in ovaries at stages IV–VI and only they showed positive and strong expression of active caspase-3. On the other hand, we also found some nonapoptotic primary and secondary oogonia, as well as single diplotene oocytes, apparently degenerating in a different cell death process, since they did not express active caspase-3. P. esculentus had significantly more degenerating apoptotic germ cells (represented only by primary oogonia) than P. lessonae or P. ridibundus. In our opinion, it may reflect a physiological process of removing abnormal primary oogonia that is highly increased in the hybrid ovaries. These observations support earlier findings that elimination of chromosomes is restricted to the phase of primary oogonia proliferation (Tunner and Heppich-Tunner 1991; Ogielska 1994). Genome elimination may be a very imprecise process, causing chromosomal abnormalities or chromatin degeneration, which in turn may cause increased frequency of apoptotis in germ line cells. In our interpretation, degenerating primary oogonia were those with incorrectly eliminated chromosomes, as was formerly suggested by Ogielska (2009). The increased expression of active caspase-3, observed in hybrids, can activate restriction enzymes inside the cell nucleus (Elmore 2007), probably as a response to improper genome exclusion. In the ovaries of P. lessonae or P. ridibundus, much fewer degenerating cells were observed than in P. esculentus at the same Gosner stage. In the parental species, this may indicate that the older the individual (and more advanced gonads) the fewer degenerating primary oogonia are present in the ovaries. The explanation might be that the phase of oogonial proliferation in amphibian ovaries is restricted only to young individuals (Ogielska et al. 2013). On the other hand, we have shown the strong positive correlations between the number of degenerating germ cells and somatic developmental Gosner stage, as well as gonad volume in hybrid ovaries. We can assume that morphological maturation of P. esculentus gonads, apparently slower than in the parental species, is accompanied by the increased rate of apoptosis in primary oogonia. Our results are in accordance with the timing of genome elimination process, which takes place in hybrid females before and shortly after metamorphosis (Tunner and Heppich-Tunner 1991).
Interestingly, we have also found the different pattern of active caspase-3 staining in oogonial cells of all taxa, confined to the cytoplasm surrounding the cell nucleus (“circular staining”). Since these cells did not display any degeneration features we cannot confirm that they are committed to apoptosis. It is rather not likely, because in parental species numerous oogonia showed this type of signal; however, there was no massive cell degeneration at stage IV–VI ovaries. Activation of caspase-3 without entering apoptotic pathway was previously described in mouse embryonic stem cells as a crucial factor leading cells from self-renewal state into differentiation (Fujita et al. 2008). We thereby suggest that activation of caspase-3 enzyme in oogonia could be a part of cellular differentiation process, when primary oogonia give rise to the secondary oogonia forming nests and entering meiosis afterward.
In conclusion, histological observations revealed that gonadal development of the parental species P. lessonae and P. ridibundus is regular and correlated with somatic development, whereas ovaries of P. esculentus are delayed taking into account presence of more advanced germ cell classes. In the ovaries of P. lessonae and P. ridibundus, degeneration of germ cells affected primary oogonia, secondary oogonia and diplotene oocytes and degenerating germ cells were directed to the apoptotic pathway. In the ovaries of P. esculentus, the only class of apoptotic germ cells was primary oogonia. The frequency of degenerating oogonia in hybrid ovaries was 10 times higher than in parental species, when estimated in tadpoles until the completion of metamorphosis, and increased together with the Gosner somatic developmental stage. Additionally, massive apoptosis of primary oogonia described in our study in P. esculentus strongly suggests imprecise removal of the parental genome.
References
Berger L (1983) Western palearctic water frogs (Amphibia, Ranidae): systematics, genetics and population compositions. Experientia 39:127–234
Berger L, Rybacki M, Hotz H (1994) Artificial fertilization of water frogs. Amphib Reptil 15:408–413
Chmielewska M, Symonowicz K, Pula B, Owczarek T, Podhorska-Okołów M, Ugorski M, Dzięgiel P (2015) Expression of metallothioneins I and II in kidney of doxorubicin-treated rats. Exp Toxicol Pathol 67:297–303. doi:10.1016/j.etp.2015.01.006
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516
Fujita J, Crane A, Souza M, Dejosez M, Kyba M, Flavell R, Thomson J, Zwaka T (2008) Caspase activity mediates the differentiation of embryonic stem cells. Cell Stem Cell 2:595–601. doi:10.1016/j.stem.2008.04.001
Gosner LK (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:513–543
Graf J-D, Polls-Pelaz M (1989) Evolutionary genetics of the Rana esculenta complex. In: Dawley R, Bogart J (eds) Evolution and ecology of unisexual vertebrates. New York State Museum, Albany, pp 289–302
Günther R, Plötner J (1990) Mating pattern in pure hybrid populations of water frogs, Rana kl. esculenta (Anura, Ranidae). Alytes 8:90–98
Hauswaldt S, Hoer M, Ogielska M, Christiansen D, Dziewulska-Szwajkowska D, Czernicka E, Vences M (2012) A simplified molecular method for distinguishing among species and ploidy levels in European water frogs (Pelophylax). Mol Ecol Resour 12:797–805
Hoffmann A, Plötner J, Pruvost NBM, Christiansen DG, Röthlisberger S, Choleva L, Mikulíček P, Cogălniceanu D, Sas-Kovács I, Shabanov D, Morozov-Leonov S, Reyer HU (2015) Genetic diversity and distribution patterns of diploid and polyploid hybrid water frog populations (Pelophylax esculentus complex) across Europe. Mol Ecol 24:4371–4391
Jakob C, Arioli M, Reyer HU (2010) Ploidy composition in all-hybrid frog populations in relation to ecological conditions. Evol Ecol Res 12(5):633–652
Joly P (2001) The future of the selfish hemiclone: a Neodarwinian approach to water frog evolution. Zoosystematics Evol 77:31–38
Lada GA, Borkin LJ, Vinogradov AE (1995) Distribution, population systems and reproductive behavior of green frogs (hybridogenetic Rana esculenta complex) in the central chernozem territory of Russia. Rus J Herpetol 2:46–57
Ogielska M (1994) Nucleus-like bodies in gonial cells of Rana esculenta [Amphibia, Anura] tadpoles—a putative way of chromosome elimination. Zool Pol 39:461–474
Ogielska M (2009) Development and reproduction of amphibian species, hybrids and polyploids. In: Ogielska M (ed) Reproduction of amphibians. Science Publisher, USA, pp 343–410
Ogielska M, Kotusz A (2004) Pattern and rate of ovary differentiation with reference to somatic development in anuran amphibians. J Morphol 259:41–54
Ogielska M, Wagner E (1990) Oogenesis and development of the ovary in European green frog, Rana ridibunda (Pallas). I. Tadpole stages until metamorphosis. Zool Jb Anat 120:211–221
Ogielska M, Wagner E (1993) Oogenesis and ovary development in the natural hybridogenetic water frog, Rana esculenta L. I tadpole stages until metamorphosis. Zool Jb Physiol 97:349–368
Ogielska M, Rozenblut B, Augustyńska R, Kotusz A (2010) Degeneration of germ line cells in amphibian ovary. Acta Zool Stockholm 91:319–327
Ogielska M, Kotusz A, Augustyńska R, Ihnatowicz J, Paśko Ł (2013) A stockpile of ova in the grass frog Rana temporaria is established once for the life span. Do ovaries in amphibians and in mammals follow the same evolutionary strategy? Anat Rec (Hoboken) 296:638–653. doi:10.1002/ar.22674
Rybacki M, Berger L (1994) Distribution and ecology of water frogs in Poland. Zool Pol 39:293–303
Rybacki M, Berger L (2001) Types of water frog populations (Rana esculenta complex) in Poland. Zool Reihe 77:51–57. doi:10.1002/mmnz.20010770109
Semlitsch RD, Schmiedehausen S et al (1996) Genetic compatibility between sexual and clonal genomes in local populations of the hybridogenetic Rana esculenta Complex. Evol Ecol 10:531–543
Socha M, Ogielska M (2010) Age structure, size and growth rate of water frogs from central European natural Pelophylax ridibundus—Pelophylax esculentus mixed populations estimated by skeletochronology. Amphib Reptil 31:239–250. doi:10.1163/156853810791069119
Tunner HG, Heppich-Tunner S (1991) Genome exclusion and two strategies of chromosome duplication in oogenesis of a hybrid frog. Naturwissenschaften 78:32–34
Wagner E, Ogielska M (1990) Oogenesis and development of the ovary in European green frog, Rana ridibunda (Pallas). II. Juvenile stages until adults. Zool Jb Anat (Jena) 120:223–231
Wagner E, Ogielska M (1993) Oogenesis and ovary development in the natural hybridogenetic water frog, Rana esculenta L. II. After metamorphosis until adults. Zool Jb Physiol (Jena) 97:369–382
Acknowledgements
This study was supported by the grant from Polish National Science Center No. 2012/07/B/NZ3/02563. Translation of publication was supported by Wrocław Centre of Biotechnology programme, the Leading National Research Centre (KNOW) for the years 2014–2018. We are grateful to Mrs. Ewa Serwa for help in immunohistochemical processing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed. To carry out the research, consent was obtained from the local ethical commission in Wroclaw No. 7/2013. The acquisition of specimens of the species was accepted by license number DOP-oz.6401.02.2.2013.JRO.
Additional information
Paweł Szydłowski and Magdalena Chmielewska have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Szydłowski, P., Chmielewska, M., Rozenblut-Kościsty, B. et al. The frequency of degenerating germ cells in the ovaries of water frogs (Pelophylax esculentus complex). Zoomorphology 136, 75–83 (2017). https://doi.org/10.1007/s00435-016-0337-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-016-0337-4