Abstract
Black coral colonies belonging to Antipathella subpinnata (Myriopathidae), found in Adriatic Sea (off the Tremiti Islands) and in Tyrrhenian Sea (Mezzo Canale), were inspected with the aim of investigating their reproductive activity. The small colony (about 42 cm tall) from Adriatic Sea was sexually immature, whereas the larger (about 120 cm tall) five colonies from Tyrrhenian Sea were fertile. Four colonies were males and one was a female, thus confirming the gonochoric condition already known for the black coral. Semithin sections proved that most of the egg and sperm masses flow out through the pharynx, even though small gamete masses were also observed emerging from the tentacles. In the female polyps, egg masses were enveloped by parental tissue derived from the breakage of the mesenterial septa, as observed in the male polyps for the sperm cysts masses. Ultrastructural observations provided details on the characteristics of egg and sperm in this taxon. Sperm stood out for the presence of an acrosome-like structure derived from the merging of pro-acrosomal vesicles. On the whole, the fine organization of the sperm recalled previous descriptions reported for some tropical black coral taxa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In modular organisms, such as the reef-building corals, which grow through asexual reproduction, gamete differentiation is an important event, in that it gives rise to more rapid generation of genetic diversity, thus allowing adaptation to environmental changes. Nevertheless, most coral reef ecologists have emphasized the asexual capability of reef-building corals, while largely ignoring the role of sexual reproduction (Fadlallah and Pearse 1982). Gradually, however, the sexual reproduction of corals has received greater attention. Ultrastructural studies have significantly contributed to expanding our knowledge of gametogenesis, mature gamete structure, fertilization and ensuing development (Hinsch and Clark 1972; Hinsch 1974; Clark and Dewel 1974; Lyke and Robson 1975; Schmidt and Zissler 1979; Schmidt and Schäfer 1980; Steiner 1991, 1993; Goffredo et al. 2000; Gaino and Scoccia 2008).
Gamete spawning seems to be the most common mode of reproduction in hermaphrodite scleractinians (Bothwell 1982; Kojis and Quinn 1982; Harriot 1983; Harrison et al. 1984; Babcock et al. 1986), in which gamete release can occur synchronously, being triggered by lunar and daily cycles (Babcock et al. 1986). On account of this feature, it is often possible to predict the “mass spawning” of the male and female gametes, which are intermingled in compact spheres when released from the polyps (Kojis and Quinn 1982; Babcock and Heyward 1986; Babcock et al. 1986). A highly synchronized gametogenetic cycle has also been found in solitary gonochoric corals that reproduce only sexually and whose restricted spawning time may favor fertilization and larval survival (Fadlallah and Pearse 1982). The release of sperm clusters in spheres, resulting from the breakage of the mesentery walls, has recently been observed in reared colonies of the antipatharian Cupressopathes pumila (Brook 1889: Gaino and Scoccia 2009).
Most of these studies suggest that, in Anthozoa, gamete packages, which significantly reduce gamete dilution and maximize fertilization, could be much more frequent than expected (Olivier and Babcock 1992).
The black coral Antipathella subpinnata is a common component of the circalittoral environment of the Italian seas, where it lives on hard substrata in localities below 50 m depth, as reported by Bo et al. (2008). In that report, in addition to a detailed description of the species, the authors provided information on the habitat, population density and environmental conditions of the substrates colonized and the epibionts.
The specific objective of the present investigation was to study, by means of histological and ultrastructural techniques, the reproductive activity of A. subpinnata, so far unknown. This investigation was allowed by the finding out of several colonies with fertile polyps in a circalittoral area already suspected to be particularly suitable for black coral growth.
Materials and methods
Biogeographical information
Antipathella subpinnata (Ellis and Solander 1786) (Myriopathidae) has an Atlanto-Mediterranean distribution. According to Bo et al. (2008), A. subpinnata is one of the five species known to occur in the Mediterranean basin, where it represents the most widespread black coral taxon. Recent investigations along the Italian coasts added new recording of A. subpinnata mainly in Thyrrhenian and Ligurian Seas. The easternmost recording is situated in the Ionian Sea. The present record of A. subpinnata in the Adriatic Sea extends the area of its presence along the Italian coasts.
Study site
Diving surveys conducted by the “Laboratorio del Mare Marlin Tremiti” revealed the presence of a black coral colony in the Adriatic Sea (Secca di Punta Secca, Tremiti Islands, 48°8′28.84″N; 15°31′33.83″) (Fig. 1a), while the “Abissoblu” diving center discovered several colonies in the Tyrrhenian Sea (Mezzo Canale, 42°19′32.68″N; 11 2′49.75″E) (Fig. 1b).
Specimen collection
In April 2009, a small colony (about 42 cm tall) off the Tremiti Islands was identified by means of scuba diving at –51 m. Since then the colony has been monthly monitored up to September 2009. During each dive, a small fragment was cut off by scissors and placed in a sealed container. From April to September, the water temperatures were recorded during the samplings: 12, 14, 14, 14, 17 and 16°C. In August 2009, expert divers from the Abissoblu diving center found out a population of A. subpinnata at −70 m in the Tyrrhenian Sea (Mezzo Canale). Five colonies (about 120 cm tall) were randomly sampled by the divers by cutting off (with scissors) a fragment from each colony; the five fragments were brought to the surface after being placed in sealed containers. The water temperature was 16°C. No fertile colonies were observed from September to November when the water temperature slightly decreased up to 14°C.
Ultrastructural analysis
In the laboratory, the samples from the two sites were transferred from the sealed containers into Petri dishes and fixed for 12 h in 2.5% glutaraldehyde buffered with filtered sea-water (adjusted to a final pH of 7.5–7.8 with NaOH 0.1 N) and repeatedly rinsed in the same buffer. Specimens were photographed under a stereomicroscope (Leica MZ6, Holings GmbH, Wetzlar, Germany). For species identification, underwater photographs and examination of a part of the skeleton were used.
Five polyps from each of the fragments from the two sampling sites were processed for light and transmission electron microscopy investigations. For transmission electron microscopy, selected materials were postfixed in 1% osmium tetroxide in an artificial sea-water buffer for 1 h at 4°C, then repeatedly washed in the same buffer, dehydrated in an incremental alcohol series and propylene oxide, and finally embedded in an Epon–Araldite mixture. Semithin sections, 0.5–1.0 mm thick, were cut on a Leica DC 300 F Ultracut (Leica Microsystem AG, Rijswijk, The Netherlands), stained with toluidine blue and observed under a Leica microscope (Leica LSM Holings GmbH, Wetzlar, Germany). Ultrathin sections were cut with the same Ultracut device, collected on formvar-coated copper grids, stained with uranyl acetate and lead citrate, and examined with a Philips EM 208 electron microscope.
Results
The colonies of A. subpinnata had a branched configuration, consisting of numerous arms with simple elongated pseudopinnules arranged irregularly in one to four rows (Opresko 2001). Polyps showed the typical morphology: 6 tentacles encircling the mouth, four lateral tentacles shorter than the two sagittal ones (Fig. 2a).
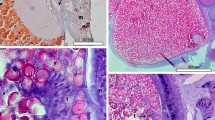
Fragments of the colonies of the black coral Antipathella subpinnata. Observations in toto (a–d), in semithin sections (e) and at the ultrastructural level (f–h). a a non-fertile colony showing the typical morphology of the black coral polyps characterized by the mouth (M) encircled by 6 tentacles, two sagittal (S) and four lateral (L ), b fertile female polyps showing egg masses emerging from the mouth (arrows), c detail of a fertile female polyp showing the just-released egg masses (arrows) encircled by the tentacles (T), d a small mass emerging from the apex of a tentacle (arrow), e just-extruded egg masses (arrows) along with internal eggs (E) filling up a mesenterial septum. In the inset, a mass showing eggs immersed in the mesogleal matrix, f egg surface with microvillar projections (arrow) associated to a fibrillar coat (FC), and inclusions with various morphologies and contents: membrane-bounded electron-lucent vesicles (V) occasionally including a fibrillar content (arrowhead); fairly round, electron-dense granules (G), and elongated granules with an electron-dense core (EC), g periphery of an egg mass showing the delimiting parental tissue (PT), the microvillar extensions of the egg surface (arrowheads) and the fibrillar layer (FL) in between, h detail of the parental tissue (PT) showing the flagellar specialization of the cells detailed in the inset. Note the flagellum (arrow) closely associated to microvilli (arrowheads)
The small colony living in the Adriatic Sea (Secca di Punta Secca, Tremiti Islands) did not display fertile polyps during the monitored months. The numerous gonochoric colonies living in the Tyrrhenian Sea (Mezzo Canale) were found fertile in August 2009: four were males and only one was a female. In both cases, some fertile polyps showed gamete masse extrusion, whereas others, without gamete masses, when sandwiched and squeezed between two glass plates revealed the presence of male or female gametes still included in the mesenterial filaments.
Female polyps with egg masses
The fragments of these colonies were characterized by polyps of which the column and tentacles were markedly broader and shorter. A large number of eggs were scattered on the polyp surface in such a way that the fragments assumed a lumpy, irregular appearance (Fig. 2b). In particular, the egg masses were typically observed emerging from the mouth opening (Fig. 2c), though they were also found extruded from the apical region of the tentacles (Fig. 2d). In this case, the masses were made up of fewer eggs than those emerging from the mouth (Fig. 2d).
Sections of fertile polyps showed mature eggs (maximum diameter 150 μm) inside the septa together with extruded egg masses (Fig. 2e). The eggs in the masses were separated from one another by the parental tissue, which ensures that the mature eggs are surrounded by the mesogleal matrix (Fig. 2e, inset). Ultrathin sections of the extruded egg masses showed that the egg surface had microvillar extensions protruding into a fibrillar coat (Fig. 2f). In the cortical region of the egg, several kinds of inclusions were seen: electron-lucent membrane-bounded bodies, some with a fibrillar content fairly round electron-dense granules with a homogeneous texture and elongated inclusions with a more electron-dense core (Fig. 2f).
Eggs located at the periphery of the clumps were delimited by an envelope of the maternal tissue in contact with the fibrillar coat (Fig. 2g). Many cells of this envelope had microvillar extensions (Fig. 2h) and exhibited flagellar specialization, consisting of a flagellum enveloped by a thick coat of fine, fibrillar material, which was closely associated to long microvilli (Fig. 2h, inset). Such organization is typical of the cells that form the gastrodermal layer.
Male polyps with spermatic cyst masses
The fragments of these colonies displayed large polyps with wide sagittal tentacles and extruded spermatic cyst masses overlapping the mouth (Fig. 3a). Some polyps extruded small masses from the apical region of the tentacles (Fig. 3b). Sections of fertile polyps showed spermatic cysts still included in the body, coexisting with elongated spermatic cysts emerging through the pharynx (Fig. 3c). Spermatic cysts included mature sperm arranged in rows (Fig. 3c inset). The spermatic cysts differed in size according to both location and shape. They were extruded together with a part of the mesenterial filaments and were separated from one another by the parental tissue, which enveloped them to form a large clump (Fig. 3d).
Fragments of male fertile colonies of the black coral Antipathella subpinnata showing extruded spermatic cyst masses. Observations in toto (a, b), in semithin sections (c, d) and at the ultrastructural level (e–i). a fertile polyps showing enlarged sagittal tentacles (S) and extruded sperm masses overlapping the mouth (arrow), b small sperm masses emerging from the apical region of the tentacles (arrows), c a polyp during sperm mass extrusion. Note the elongated shape of the spermatic cysts (SC) passing through the mouth. Note in the inset the linear arrangement of the sperm, d a sperm mass with spermatic cysts (SC) separated from one another by the parental tissue. A remnant of the mesenterial filament (MF) closely adheres to the mass, e detail of the periphery of a spermatic cyst (SC) delimited by the parental tissue (PT), which includes a spumous mucus cell (SMC), f organization of a sperm. Note the single mitochondrion (M), the electron-dense cup-like body (CB) encircling the centrioles from which the flagellum emerges (F), and the acrosomal-like structure (arrow), g proximal (P) and distal (D) centrioles located within the cup-like body (CB) of the sperm, h pericentriolar system showing the radial pattern anchoring it to the cup-like body (CP), i detail of the sperm pro-acrosomal vesicles (arrowheads) coalescing into an acrosome-like structure (AS)
On transmission electron microscopy, the external envelope showed the presence of spumous mucus cells resulting from numerous vacuoles with a dotted appearance (Fig. 3e). This layer was separated from the outermost cysts by a fibrillar coat (Fig. 3e).
Mature sperm within the cysts showed: a large, fairly round nucleus, with a diameter of 1–1.5 μm; an acrosome-like structure; a single mitochondrion partially encircling the nucleus; a wide electro-dense cup-like body (Fig. 3f) enveloping the proximal and distal centrioles (Fig. 3g). The distal centriole gave rise to the flagellar axonema consisting of 9 + 2 array of microtubules. The distal centriole had a pericentriolar system, consisting of nine arms arranged in a radial pattern (Fig. 3h). A group of pro-acrosomal vesicles merge into a more compact acrosome-like structure (Fig. 3i).
The divers did not observe the spawning event in situ. In contrast with the typical transparent color of the unfertile colonies of A. subpinnata, the fixed specimens we studied showed a brown-orange appearance due to the egg and sperm cyst masses extruded.
Discussion
In the congeneric species Antipathella fiordensis (Grange 1990), the colonies reach sexual maturity when their height is between 70 and 105 cm, which corresponds to a minimum age for sexual maturity (Parker et al. 1997). In this regard, in the small colony (42 cm tall) of A. subpinnata from Tremiti Islands, no fertile polyps were found (pers. observations), thus confirming that young colonies cannot reproduce. The dioecious condition, based on the presence of male and female colonies of A. subpinnata from the Tyrrhenian Sea, is in agreement with previous observations on A. fiordensis (Parker et al. 1997).
In the black coral Cirrhipathes sp., which afterward was tentatively attributed to C. anguina (Dana 1846), and reported as C. cf. anguina (Gaino and Scoccia 2008), mature sperm have been seen in the body cavity of the polyp, thus suggesting the direct spawning of discrete sperm through the pharynx (Gaino et al. 2008). By contrast, in Cupressopathes pumila, numerous spermatocysts are included in spheres and extruded from the polyps (Gaino and Scoccia 2009), a mechanism that parallels the formation of egg and spermatic clumps observed by us in A. subpinnata. In Anthozoa, this feature has also been observed in simultaneous hermaphroditic scleractinians, in which gametes are released as tight buoyant clusters of eggs and sperm (Babcock et al. 1986; Olivier and Willis 1987).
Simultaneous spawning is common in corals and this event is often triggered by environmental factors, including water temperature. Our data confirm previous observations on the black coral A. fiordensis, which, like the Tyrrhenian A. subpinnata, reproduces in summer (Miller 1996).
In C. pumila and A. subpinnata, extrusion of the large spheres takes place mainly through the mouth, though in A. subpinnata some masses flow out through the apical region of the tentacles. In both species, the breakage of the mesenterial septa seems to be the main modality of gamete spawning, as proved by the presence both of the irregular coat around the gamete masses and of the flagellate gastrodermal cells.
The spawning of numerous aggregated sperm (spermatozeugmata) has been described in some other modular organisms, namely Bryozoa, the zooids of which release and capture spermatozeugmata in order to promote cross-fertilization (Temkin 1994).
At the ultrastructural level, the sperm of A. subpinnata showed an organization very close to that already described in Cirrhipathes sp. (Gaino et al. 2008) and Cupressopathes pumila (Gaino and Scoccia 2009); both are characterized by: (1) almost coaxial proximal and distal centrioles; (2) a similar peri-centriolar system; (3) an electron-dense cup-like body to which the peri-centriolar system is linked. The main difference consisted in the confluence of the pro-acrosomal vesicles into a compact acrosome-like structure shown by the sperm of A. subpinnata. We have observed the same acrosome-like structure in Myriopathes species (pers. observations) but not in C. pumila. Therefore, an acrosome-like structure could represent a micro-characteristic that could be taken into account for phylogenetic considerations.
The predictability of synchronized spawning by the colonies of Antipathella subpinnata in the Tyrrhenian Sea could open up a new field of experimentation aimed at obtaining fertilization, cleavage and planula formation. The possibility of obtaining larvae from A. subpinnata could help us to understand their behavior, including dispersal ability, substrate selection and metamorphosis, which represent crucial unknown life-history characteristics for this species.
References
Babcock RC, Heyward AJ (1986) Larval development of certain gamete-spawning scleractinian corals. Coral Reefs 5:111–116
Babcock RC, Bull GD, Harrison PL, Heyward AJ, Oliver JK, Wallace CC, Willis BL (1986) Synchronous spawning of 105 scleractinian coral species on the Great Barrier Reef. Mar Biol 90:379–394
Bo M, Tazioli S, Spanò N, Bavestrello G (2008) Antipathella subpinnata (Antipatharia, Myriopathidae) in Italian seas. Ital J Zool 75:185–195
Bothwell AM (1982) Fragmentation, a means of asexual reproduction and dispersal in the coral genus Acropora (Scleractinia: Astrocoeniida: Acroporidae). In: Gomez ED, Birkeland CE, Buddemeier RW, Johannes RE, Marsh JA Jr, Tsuda RT (eds) Proceedings of the 4th international coral reef symposium, vol 2. Marine Science Center. University of the Philippines, Manila, Philippines, pp 137–144
Clark WH, Dewel WC (1974) The structure of the gonads, gametogenesis, and sperm-egg interactions in the Anthozoa. Am Zool 14:495–510
Fadlallah YH, Pearse JS (1982) Sexual reproduction in solitary corals: overlapping oogenic brooding cycles, and benthic planulas in Balanophyllia elegans. Mar Biol 71:223–231
Gaino E, Scoccia F (2008) Female gametes of the black coral Cirrhipathes cfr. anguina (Anthozoa, Antipataria) in the indonesian Marine Park of Bunaken. Invertebr Reprod Dev 51:119–126
Gaino E, Scoccia F (2009) Release of sperm clusters in spheres by the black coral Cupressopathes pumila (Anthozoa, Antipatharia). Coral Reefs 28:851–857
Gaino E, Bo M, Boyer M, Scoccia F (2008) Sperm morphology in the black coral Cirrhipathes sp. (Anthozoa, Antipatharia). Invertebr Biol 45:249–258
Goffredo S, Telo T, Scanabissi F (2000) Ultrastructural observations of the spermatogenesis of the hermaphroditic solitary coral Balanophyllia europaea (Anthozoa, Scleractinia). Zoomorphology 119:231–240
Harriot V (1983) Reproductive ecology of four scleractinian species at Lizard Island, Great Barrier Reef. Coral Reefs 2:9–18
Harrison PL, RC Babcock, GD Bull, Oliver JK, Wallace CC, Willis BL (1984) Mass spawning in tropical reef corals. Science 223:1186–1189
Hinsch GW (1974) Comparative ultrastructure of cnidarian sperm. Am Zool 14:457–465
Hinsch G, Clark WH (1972) Comparative fine structure of Cnidaria spermatozoa. Biol Reprod 8:62–73
Kojis BL, Quinn NJ (1981) Aspects of sexual reproduction and larval development in the shallow water hermatypic coral Goniastrea australiensis (Edwards and Haime, 1857). Bull Mar Sci 31:558–573
Kojis B, Quinn NJ (1982) Reproductive ecology of two Faviid coral (Coelenterata: Scleractinia). Mar Ecol 8:251–255
Lyke EB, Robson EA (1975) Spermatogenesis in Anthozoa: differentiation of spermatid. Cell Tissue Res 157:185–205
Miller K (1996) Piecing together the reproductive habits of New Zealand’s endemic black corals. Water Atmos 4:18–19
Olivier JK, Babcock RC (1992) Aspects of the fertilization ecology of broadcast spawning corals: sperm dilution effects and in situ measurements of fertilization. Biol Bull 183:409–417
Olivier JK, Willis BL (1987) Coral-spawn slicks in the Great Barrier Reef: preliminary observations. Mar Biol 94:521–529
Opresko DM (2001) Revision of the Antipatharia (Cnidaria: Anthozoa), Part I. Establishment of a new family, Myriopathidae. Zool Meded (Leiden) 75:343–370
Parker NR, Mladenov PV, Grange KR (1997) Reproduction biology of the antipatharian black coral Antipathes fiordensis in Doubtful Sound, Fiordland, New Zealand. Mar Biol 130:11–22
Schmidt H, Schäfer WG (1980) The anthozoan egg: trophic mechanisms and oocyte surfaces. In: Tardent P, Tardent R (eds) Developmental and cellular biology of Coelenterates. Elsevier, Amsterdam, pp 41–46
Schmidt H, Zissler D (1979) Die Spermien der Anthozoen und ihre phylogenetische Bedeutung. Zoologica 129:1–98
Steiner SCC (1991) Sperm morphology of scleractinians from the Caribbean. Hydrobiologia 216(217):131–135
Steiner SCC (1993) Comparative ultrastructural studies on scleractinian spermatozoa (Cnidaria, Anthozoa). Zoomorphology 113:129–136
Temkin MH (1994) Gamete spawning and fertilization in the Gymnolaemate Bryozoan Membranipora membranacea. Biol Bull 187:143–155
Acknowledgments
We are very grateful to the anonymous referees for their helpful advice and criticism, which markedly improved the content of the paper. Also, we express our gratitude to A. Sorci, C. Luciani, P. De Cecco and M. Tancredi (Laboratorio del Mare “Marlin Tremiti”) together with P. Adami and L. Paoles (Abissoblu diving center) for their technical support. Many thanks to Prof. G. Bavestrello and Dr. M. Bo (Dipartimento di Scienze del Mare -Università Politecnica delle Marche) for their help in species determination.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Bartolomaeus.
Rights and permissions
About this article
Cite this article
Gaino, E., Scoccia, F. Gamete spawning in Antipathella subpinnata (Anthozoa, Antipatharia): a structural and ultrastructural investigation. Zoomorphology 129, 213–219 (2010). https://doi.org/10.1007/s00435-010-0112-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-010-0112-x