Abstract
Scent marking is widespread among individuals of Mammalia species, especially in resource defence social systems. Apart from urine and faeces that are used for claiming resource ownership, specialised scent glands are the main source of secretions in scent marking individuals. Most previous studies have described secretory epithelia macroscopically, since many glands are conspicuous. But macroscopically inconspicuous scent glands or morphological structures might then be overlooked. In Saccopteryx bilineata (greater sac-winged bat), behavioural observations suggest that both sexes have, apart from the conspicuous gular glands of males, specialised facial glands to display territorial marking. We investigated the facial glands of two males and one female S. bilineata histologically and found, first, that both sexes possess a bilateral symmetrically intermandibular gland, which is composed of a bed of modified apocrine sudoriferous cells. Second, we found lip glands consisting of modified apocrine sudoriferous cell units with pigmented ducts around the upper and the lower lip. Both gland types are probably involved during territorial marking.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The importance of olfactory signals in communication and orientation of Chiroptera has been recently acknowledged. The representatives of this group use odours to localise food (Kalko and Condon 1998; Thies et al. 1998), for kin or individual discrimination (Gustin and McCracken 1987; Safi and Kerth 2003), to distinguish between members of different colonies (Defanis and Jones 1995; Safi and Kerth 2003) and to mark territories (Horst 1966).
The specific position of glands on the body seems to be evolved in response to a particular habitat or behaviour (Schaffer 1940; Quay 1970; Albone 1984). Facial glands are well documented for a variety of microchiropteran species (Schaffer 1940; Dalquest et al. 1952; Starck 1958; Horst 1966; Mainoya and Howell 1976; Scully et al. 2000; Safi and Kerth 2003). The chin and cervical region is commonly the location of scent glands in chiropteran species that roost with their venter on the surface of their daytime roost (Quay 1970; Scully et al. 2000). In some Molossidae for example, males use the submandibular gular gland to mark the walls of their daytime roosts (Horst 1966) and probably also females of their social group (Heideman et al. 1990).
Saccopteryx bilineata Temminck, 1838, the greater sac-winged bat, is well known for their frequent use of chemical signals in courtship displays and for marking and defending their territory (Bradbury and Emmons 1974; Voigt and von Helversen 1999). S. bilineata forms colonies of up to 50 individuals that are divided into smaller social units, so-called harems, each including one territorial adult male and on average 2–3 females. Harems are stable throughout the year (Bradbury and Emmons 1974; Bradbury and Vehrencamp 1976) and the mating system of S. bilineata can best be described as harem-polygynous or single-male/multiple-female (McCracken and Wilkinson 2002). In contrast to females, males possess a gular gland, which is located in the throat area, and a sac-like pouch in their front wing membrane, which lacks any secreting cells (Starck 1958; Scully et al. 2000). The wing sacs are cleaned and refilled daily during a stereotypic time-consuming perfume blending behaviour, during which secretions from different body regions like saliva, urine and probably gular gland secretion are transferred into the wing-sacs (Voigt and von Helversen 1999; Voigt 2002). In S. bilineata, both sexes scent-mark their roosting territory by pressing their lower mandible onto the roosting substrate and by smearing secretion of a skin gland onto the wall substrate of their daytime roost, e.g. the bark of a tree (Voigt and von Helversen 1999, unpublished data). Male S. bilineata scent-mark most frequently the borders of their harem territory. By contrast, females scent-mark only their preferred roosting site within in a harem territory, and they do this less often than males (unpublished data). These behavioural observations suggest that both sexes have specialised glands in the submandibular and lip region used for territorial marking.
Previous histological studies already demonstrated that the dug-like gular gland of male S. bilineata consists of sebaceous cells and a large bed of sudoriferous cells caudal to the dug (Starck 1958; Scully et al. 2000). Both types of cells share a single duct that opens at the centre of the dug-like wart. Starck (1958) described a second pair of glands next to the large bed of sudoriferous cells, below the epithelial cells and the integument of the dug-like wart, suggesting that the two different glands end in the same duct. Based on Starck’s (1958) findings and the fact that females lack the dug-like wart, it follows that both glands are absent in females. However, females perform a similar scent marking behaviour to males, during which secretion is transferred from the submandibular region to the roosting site. It seems most likely that females possess glandular tissue at the submandibular region as well. To shed light on the different origins of glandular secretions that may be involved during scent marking, we examined the facial glands in two males and one female S. bilineata in greater detail.
Material and methods
Sample collection
We captured three specimens (1 female, 2 males) of S. bilineata Temminck, 1838 (Chiroptera, Emballonuridae) in the vicinity of “La Selva” biological station of the Organization for Tropical Studies (10°25′N, 84°00′W) in Costa Rica in December 2004 and in February 2005. The bats were sacrificed and the whole specimens were immediately preserved and stored in 10% buffered formalin until further investigation.
Sample processing
The formalin fixed heads were removed from the body and decalcified via electrolysis (Medax decalcification equipment, Medax Nagel GmbH, Kiel, Germany). Each head was submerged in a decalcification solution [8.5% formic acid (CH2O2), 2% hydrochloric acids (HCl), 417 ml Aqua dest.] and an electrical current was applied via two electrodes (12 V) for 6 h. Afterwards, the head of the female and one male were first dissected paramedian (longitudinally) into two halves. One of the two halves was also dissected transversally into slices of approximately 5 mm. All parts were decalcified for another 1–2 h. The head of the second male was dissected transversally and processed in the same way. We dissected the two male heads in different ways to get additional information about size and structure of the glands and the associated ducts. Routine procedure for paraffin embedding was performed for each part, which subsequently was sectioned serially at 3 μm. Tissue slides were stained with either haematoxylin–eosin (HE) for routine examination and with period-acid Schiff (PAS) to distinguish mucous glands.
Analysis and documentation
All slides were examined using a HUND H600 Phako microscope (Wetzlar, Germany) at a magnification factor of 40 and 200. Histological sections were documented with a digital camera (Leica DC 200; Solms, Germany) at a resolution of 2.6 megapixel.
Results
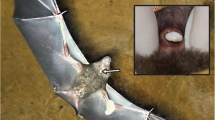
Macroscopically, the submandibular gular gland with the dug-like excretory duct, confined by the lower jaws and their symphysis with a caudal extension of approximately 7 mm, was only found in males and not in the female. The histological examination confirmed the macroscopic finding (Fig. 1 a, b) that the submandibular gular gland was only present in males. The wall of the dug consisted of a broad layer of tightly packed nests of holocrine cell units, forming the scaffolding for the excretory duct with its orifice at the very tip of the dug. The duct itself was lined by pigmented cuboidal epithelial cells and traversed the entire gland centrally (Fig. 1d). The gland tissue comprised a large bed of apocrine sudoriferous glands (SUD I in Fig. 1 d, g) with each alveola lined by a single layer of columnar cells and the whole gland was embedded in dermal collagen-rich connective tissue (Fig. 1d).
Picture of the facial glands of male and female Saccopteryx bilineata. Face and submandibular region of a an adult female and b an adult male. c Transversal section of the two intermandibular gland ducts in males (HE stain). d Transversal section of a male’s gular gland dug and intermandibular gland (HE stain). e Transversal section of a female intermandibular gland, showing modified apocrine sudoriferous cells (HE stain). f Longitudinal section of a male intermandibular gland, showing modified apocrine sudoriferous cells (HE stain). g Longitudinal section of a male gular gland, showing the bed of modified apocrine sudoriferous cells beneath the dug-like wart (HE stain). Abbreviations: GGD gular gland duct, IMD intermandibular gland duct, SUD I sudoriferous glands type I, SUD II sudoriferous glands type II, SEB sebaceous glands
Submandibular region
In both sexes, an area of hairless skin was located within the submandibular triangle between the lower lip and the gular gland (Fig. 1a, b). Histological sections of this area revealed two ducts lined by pigmented cuboidal epithelial cells in both sexes opening out caudally of the lower lip pads and rostral of the gular gland wart (Fig. 1c). The ducts were orientated in a bilateral symmetrical fashion to each other passing the gular gland laterally, while each duct originated from a bed of alveolar modified sudoriferous glands (SUD II in Fig. 1d–f), embedded in connective tissue. The male intermandibular gland had a length of 8.4 mm and width of 0.8 mm. In both males, the apocrine sudoriferous intermandibular gland cells (SUD II in Fig. 1f) show a large lumen (Ø 150 μm) with well-differentiated glandular epithelium. In the female, this gland (SUD II in Fig. 1e) was only rudimental (length 5 mm, width 0.5 mm, lumen of the gland alveolae Ø 10 μm).
Lip region
We found three different types of secreting cells adjacent to the lips in both males and the one female (Fig. 2): holocrine sebaceous glands which accompanied the normal hair follicles embedded in the dermis (Fig. 2a) and apocrine cells of modified sudoriferous type (SUD III in Fig. 2a). Each gland unit was demarcated by collagenous connective tissue. These units ranged from the lateral half of each lip to the lateral border of each lip. Each unit of the apocrine modified sudoriferous lip glands (SUD III in Fig. 2b) had pigmented ducts (LGD in Fig. 2a) dorsal to the upper and ventral to the lower lip and in the corner of the mouth. The third gland type was a mucous gland (MUC in Fig. 2b). This gland was located bilateral on the extreme lateral border of the lips within the connective tissue of the cheek and was embedded between sudoriferous glands.
Histological sections of a male (a) and female (b) S. bilineata, showing a the apocrine lip gland and its duct (HE stain), and b the mucous gland in between the apocrine lip gland (PAS stain). Abbreviations: SEB sebaceous gland, SUD III sudoriferous gland type III, LGD lip gland duct, MUC mucous gland
Discussion
Submandibular region
Our histological examinations confirmed the existence of an intermandibular gland in both sexes of S. bilineata. In males, this gland was morphologically not associated with the gular gland. Our findings are in contrast to former studies by Starck (1958) and Scully et al. (2000), in which the authors assume that the two different gland cell types, found in the submandibular region of males, release their secretion through one single duct embedded in the dug-like wart. The differences might be due to different dissection techniques, since we serial sectioned at about 3 μm and Scully et al. (2000) at about 7 μm.
Additionally, our study revealed that these glandular units occur in both males and also in the female (Fig. 3). This is also in contrast to Starck (1958), who did not find glandular cells in the submandibular region of females. The existence of an intermandibular gland, however, is indirectly supported by Starck (1958), who found ducts in both sexes, but not the associated glands. These differences might also be due to different dissection techniques.
We demonstrated the existence of two different types of glands in the submandibular region of males: first, a gular gland that consists of holocrine sebaceous and apocrine sudoriferous cells, releasing their secretion through a single duct orifice within the dug, and, second, bilateral symmetrical intermandibular glands. Sexual dimorphic scent glands are documented for a variety of other microchiropteran taxa, such as the frontal glandular organ of male Hipposideros ater Templeton, 1948 and H. fulvus Gray, 1938 (Scully et al. 2000). Gular glands exist also in male Tadarida brasiliensis Geoffroy, 1824 (Gutierrez and Aoki 1973), Taphozous nudiventris Cretzschmar, 1830 (Scully et al. 2000), or Molossus ater E. Geoffroy, 1805 and M. sinaloe Allen, 1906 (Horst 1966; Scully et al. 2000), suggesting that these glands are sexually selected and may play a role in mate attraction or male competition over females or resources. Gular glands consisting mainly of apocrine sudoriferous cells have already been found in other Emballonuridae, e.g. the gular glands of Peropteryx macrotis Wagner, 1843 and Taphozous nudiventris also consist of large beds of sudoriferous cells (Scully et al. 2000). By contrast, in some Molossidae, the gular glands consist mainly of sebaceous cells (Tadarida brasiliensis, Gutierrez and Aoki 1973; Dapson et al. 1977; Molossus bondae Allen 1904, Dapson et al. 1977; M. ater, M. sinaloe, Scully et al. 2000). The bilateral symmetrical intermandibular glands consisted of pairs of apocrine sudoriferous cells with their orifices in the middle of the hairless area between the gular gland and the lower lip (Figs. 1, 3). Sudoriferous intermandibular glands have also been described for female Taphozous flaviventris (Hall and Gordon 1982). Scully et al. (2000) described two small pores in the gular region of Saccopteryx leptura Schreber, 1774 indicating the existence of an intermandibular gland in the sister species of S. bilineata. In contrast to our findings, histological examination of the submandibular area of male T. flaviventris showed a large bed of sebaceous glands ventral to the gular pouch (Hall and Gordon 1982), but the authors assume that the pouch of male T. flaviventris was derived from sudoriferous glands, which have undergone atrophy. Given the diversity of glands in the submandibular region in the Emballonuridae studied so far, more detailed histological studies are required to compare the existence and the types of glands in other Emballonuridae.
Lip region
Units of apocrine sebaceous glands were found around the mouth within the upper and lower lip (Fig. 3). The medial borders of these glandular areas are located paramedian within the upper and the lower lip and they extend laterally into a large bed of gland cells within the cheek. Starck (1958) pointed out that the ducts of these glands open beneath the chin pad. In contrast to that, the present study revealed that the apocrine gland units have collecting ducts around the lower and the upper lip, and in the corner of the mouth (Fig. 3). Labial glands in Vespertilionidae and Phyllostomidae are primarily sebaceous (Quay 1970), but the function of these glands is still not known. Similar to the findings of Starck (1958), we proved the presence of salivary glands (MUC in Fig. 2) on the extreme lateral borders of the lips. Starck (1958) showed that these glands are opening in the Sulcus alveolinguali.
Scent marking and perfume blending behaviour
Behavioural observations showed that both sexes of S. bilineata scent-mark with their lip and submandibular region (Voigt and von Helversen 1999, unpublished data). Two types of glands, the apocrine lip glands and the pairs of modified sudoriferous glands occur in both sexes. We suggest that both, the modified sudoriferous glands and the apocrine lip glands are involved in scent marking of both sexes. We examined only one female and, therefore, we cannot deduce that females in general have a smaller intermandibular gland than males. Due to the small sample size, we can only speculate about potential sex-specific differences of gland size. Behavioural observations of the marking behaviour, however, might explain sex-specific differences. Males mark the boundaries of their territory, while females mark their roosting sites, suggesting that territorial marks are used in both sexes to demonstrate ownership. Males scent mark almost every day, while females mark less frequently (personal observation B. Caspers).
Behavioural observations suggest that the gular gland is most likely used during perfume blending (Voigt and von Helversen 1999), probably being the source of substances in the wing-sac odour used for species recognition (unpublished data). Individual differences found for the species-specific substances in the wing-sac odour (Caspers et al. 2008) might also be important information for territorial marking. Self-anointing with the same substances used for territorial marking is common in a wide range of species (reviewed in Gosling 1982). Thus, the male-specific gular gland might be involved in male territorial marking as well.
This present study reveals that both sexes of S. bilineata possess an intermandibular gland, which is most likely involved in territorial scent marking behaviour, and demonstrates the importance of histological studies for the understanding of olfactory communication in a mammal’s mating system.
References
Albone ES (1984) Mammalian semiochemistry. Wiley, New York
Bradbury JW, Emmons L (1974) Social organisation of some Trinidad bats. I. Emballonuridae. Z Tierpsychol 36:137–183
Bradbury JW, Vehrencamp SL (1976) Social organization and foraging in emballonurid bats I. Field studies. Behav Ecol Sociobiol 1:337–381. doi:10.1007/BF00299399
Caspers B, Franke S, Voigt CC (2008): The wing-sac odour of male greater sac-winged bats Saccopteryx bilineata (emballonuridae) as a composite trait: seasonal and individual differences, pp 151–160. In: Hurst JL, Beynon RJ, Roberts SC, Wyatt TD (eds) Chemical signals of vertebrates XI
Dalquest WW, Werner HJ, Roberts JH (1952) The facial glands of a fruit-eating bat, Artibeus jamaicensis Leach. J Mammal 33:102–103. doi:10.2307/1375650
Dapson RW, Studier EH, Buckingham MJ, Studier AL (1977) Histochemistry of odoriferous secretions from integumentary glands in three species of bats. J Mammal 58(4):531–535. doi:10.2307/1380001
DeFanis E, Jones G (1995) The role of odour in the discrimination of conspecifics by pipistrelle bats. Anim Behav 49:835–839. doi:10.1016/0003-3472(95)90057-8
Gosling LM (1982) A reassessment of the function of scent marking in territories. Z Tierpsychol 60:89–118
Gustin MK, McCracken GF (1987) Scent recognition between females and pups in the bat Tadarida brasiliensis mexicana. Anim Behav 35(1):13–19. doi:10.1016/S0003-3472(87)80205-1
Gutierrez M, Aoki A (1973) Fine structure of the gular gland of the free-tailed bat Tadarida brasiliensis. J Morphol 141:293–306. doi:10.1002/jmor.1051410305
Hall LS, Gordon G (1982) The throat-pouch of the yellow-bellied bat, Taphozous flaviventris. Mammalia 46(2):247–252
Heideman PD, Erickson KR, Bowles JB (1990) Notes on the breeding biology, gular gland and roost habits of Molossus sinaloae (Chiroptera, Molossidae). Z Saeuget 55:303–307
Horst R (1966) Observations of the gular gland of Molossus rufus. Anat Rec 154:465
Kalko EKV, Condon MS (1998) Echolocation, olfaction and fruit display: how bats find fruit of flagellichorous cucurbits. Funct Ecol 12:364–372. doi:10.1046/j.1365-2435.1998.00198.x
Mainoya JR, Howell KM (1976) Histology of the frontal sac in three species of leaf-nosed bats (Hipposideridae). Afr Wildl J 15:147–155
McCracken GF, Wilkinson GS (2002) Bat mating systems. In: Crichton EG, Krutzsch PH (eds) Reproductive biology of bats. Academic Press, San Diego, pp 321–362
Quay WB (1970) Integument and derivatives. In: Wimsatt WA (ed) Biology of bats, vol 2. Academic Press, New York, pp 1–56
Safi K, Kerth G (2003) Secretions of the interaural gland contain information about individuality and colony membership in the Bechstein’s Bat. Anim Behav 65:363–369. doi:10.1006/anbe.2003.2067
Schaffer J (1940) Die Hautdrüsenorgane der Säugetiere. Urban and Schwarzenberg, Berlin
Scully WMR, Fenton MB, Saleuddin ASM (2000) A histological examination of the holding sacs and glandular scent organs of some bat species (Emballonuridae, Hipposideridae, Phyllostominidae, Verspertillionidae, and Molossidae). Can J Zool 78:613–623. doi:10.1139/cjz-78-4-613
Starck D (1958) Beitrag zur Kenntnis der Armtaschen und anderer Hauptdrüsenorgane von Saccopteryx bilineata Temminck 1838 (Chiroptera, Emballonuridae). Gegenbaurs Morphol Jahrb 99:3–25
Thies W, Kalko EKV, Schnitzler H-U (1998) The roles of echolocation and olfaction in two neotropical fruit-eating bats, Carollia perspicillata and C. castanea feeding on Piper. Behav Ecol Sociobiol 42:397–409. doi:10.1007/s002650050454
Voigt CC (2002) Individual variation of perfume-blending in male sac-winged bats. Anim Behav 63:907–913. doi:10.1006/anbe.2001.1984
Voigt CC, von Helversen O (1999) Storage and display of odor in male Saccopteryx bilineata Emballonuridea. Behav Ecol Sociobiol 47:29–40. doi:10.1007/s002650050646
Acknowledgments
We specially thank Mrs. D. Krumnow for preparing the histological slides. Doris Fichte helped us taking most of the photographs. Figure 1a was taken by Janusz Hejduk. We thank two anonymous referees and T. Bartolomaeus for helpful comments on an earlier version of the manuscript. We also acknowledge the Costa Rican Authorities (MINAE), especially Javier Guevara, for research and export permits and the Organization for Tropical Studies (OTS) for allowing us to work on the field station. This study was supported by a grant from the Deutsche Forschungsgemeinschaft (Vo 890/3) and a grant from the Humboldt University (Berliner Chancengleichheitsprogramm für Frauen).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Caspers, B., Wibbelt, G. & Voigt, C.C. Histological examinations of facial glands in Saccopteryx bilineata (Chiroptera, Emballonuridae), and their potential use in territorial marking. Zoomorphology 128, 37–43 (2009). https://doi.org/10.1007/s00435-008-0072-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-008-0072-6