Abstract
The Australian thorny devil, Moloch horridus Gray, 1841, and the Texas horned lizard, Phrynosoma cornutum Harlan, 1825, have the remarkable ability to rapidly move water through interscalar spaces on their skin’s surface to their mouth for drinking. The morphology of these scale hinges has not been studied. We used histological and SEM techniques to examine and compare the scale hinges of both species. Additional taxa in their respective lineages were examined in order to evaluate the potential that convergent evolution has occurred. In the two species that transport water, each scale hinge has a basally expanded and semi-enclosed channel formed by the hinge joint that is interconnected with all scale hinges on the body. We hypothesize that it is within this semi-tubular channel system of hinge joints, where the β-layer keratin of the integument is very thin, that water is transported. Hinge joint walls are covered by a complex topography of fractured surfaces that greatly expand the channel’s surface area and probably enhance capillary transport of water. In addition, we note differing morphology of scale surfaces at the rear of the jaws of both species. We hypothesize that capillary forces fill the scale-hinge system and additional forces, generated within the mouth by observed motions during drinking, depress local water-pressure to pull water through the channels of the hinge-joint system. We conclude that the combined features in the two species, semi-tubular hinge-joint channels with convoluted walls and a jaw-buccal cavity pumping-mechanism, have convergently evolved for capture, transport, and drinking of water from sporadic rainfall.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Both Moloch horridus (Agamidae) and the unrelated Phrynosoma cornutum (Iguanidae) have the remarkable ability to transport water over their skin’s surface to the mouth where drinking occurs (Bentley and Blumer 1962; Sherbrooke 1990). Apparently, capillary forces that move water over the skin are generated in scale hinges, or channels, located between scales (Gans et al. 1982). This system is so effective that M. horridus individuals appear capable of removing water from damp sand by rubbing their ventral scales into it after rain (Sherbrooke 1993; Withers 1993). Drinking, using pan-cutaneous-surface transport, has also been noted in a few other species of the Agamidae (Schwenk and Greene 1987; Vesely and Modry 2002) and Iguanidae (Peterson 1998; Sherbrooke 2002). Previous analyses used scanning electron microscopy (SEM) (Gans et al. 1982; Sherbrooke 1990; Withers 1993) to examine skin-surface structure, but did not examine the internal architecture of scale hinges.
Here, we present the first descriptions of the morphology of scale hinges in the skin of M. horridus and P. cornutum. These data reveal previously unreported features that help to explain the movement of water over skin surfaces during “rain harvesting” (Sherbrooke 1990, 1993). We use these morphological features, focusing on ventral scales, to account for the surface-tension forces realized by the interconnected scale-hinge systems. In addition, we report differences between the labial and associated scales of the posterior jaw regions of M. horridus and P. cornutum, and discuss their potential significance for water ingestion. We hypothesize a complete water-movement scenario from the points of rainwater capture on the external surfaces to transfer at the jaws to the internal surfaces.
Several authors have discussed possible convergence between morphological and ecological features in M. horridus and Phrynosoma spp., inhabitants of arid and semi-arid regions (Pianka and Pianka 1970; Mayhew and Wright 1971; Pianka and Parker 1975; Gans et al. 1982; Schwenk and Greene 1987; Sherbrooke 1990, 1993, 1999, 2003, 2004; Meyers and Herrel 2005), but details of potential morphological convergence of their integumental water-collection systems are lacking. We compare architectural features of scale hinges in M. horridus and P. cornutum, both rain-harvesting lizards (Sherbrooke 1990, 1993), with those of respective related species. And then, we use these comparisons as a means of evaluating the potential for convergent evolution having been the mechanism for the observed morphological similarities in the scale hinges of these two phylogenetically distinct taxa.
Materials and methods
Specimens
All M. horridus Gray, 1841, (n = 7) were compared with Australian agamid taxa: Ctenophorus caudicinctus Günther, 1875, Physignathus lesueurii Gray, 1831, Pogona barbata Cuvier, 1829, Pogona henrylawsoni Wells and Wellington, 1985. All P. cornutum Harlan, 1825, (n = 5) were compared with closely related Callisaurus draconoides Blainville, 1835, and more distantly related North American iguanids, Crotaphytus collaris Say in James, 1823, and Gambelia wislizenii Baird and Girard, 1852. Specimens were formalin fixed and preserved in ethyl alcohol, and loaned from collections at the Queensland Museum (Australia), except P. cornutum from the Southwestern Research Station (American Museum of Natural History) and P. henrylawsoni (natural shed skin only).
Throughout the study, our examinations were focused on ventral scales because of their relative uniformity in size and shape. Figures are arranged with agamids on the left and iguanids on the right to facilitate comparison.
Methods
Standard histological techniques were employed in preparation of 5-μm thick cross-sections of skin that were stained with Mayer’s hematoxylin and Young’s eosin. Sections were examined (100–1,000 ×) and photographed using a Leica DMLB Stereomicroscope with Leica DC 300 Photolink and Leica IM50 Image Manager (version 1.2) program.
Intact patches of loose epidermal layers of skin were removed from externally-dried alcoholic specimens by means of application of blue Polyvinlsiloxane Impression Material (Type 3: Low Consistency-Light Bonded, Twin-pack Item# 28418, Kerr Corporation, 2820 Wick Road, Romulus, MI 48174–2600, USA). Once the liquid set, the skin layer remained attached to the hardened material as it was removed from the specimen. This allowed dissection microscopic and SEM examination of inner surfaces of the thick β-layer keratin of the epidermis and the interior structural features of scale hinges. In addition, inadvertent fractures and induced breaks (by flexing) opened holes in the inner surfaces of scale-hinge walls. These openings allowed SEM examination of the outer-surface β-layer Oberhäutchen as folded into scale hinges. Microscopic examinations and photographs were made of the external surfaces of scales from various areas of the lizards with a Leica MZ 12.5 dissection microscope (8–100 ×).
Whole pieces of skin were excised from various areas of the lizard’s body for SEM examination. These were mounted, epidermis up, and sputter coated with platinum. Some body wall pieces were cross-sectioned with a razor and similarly prepared. The loose outer-epidermal β-layer of scales (alcoholic specimens; see Irish et al. 1988) was removed with forceps for SEM examination of distal and medial surfaces. Medial (inner) and distal (outer) refer to the positions of two surfaces of a tissue or cell layer, or segments of a structure, in relation to the body core of the animal. Additionally, microtome cut cross-sections (5 μm-thick) were cleared of paraffin and mounted on cover slips for platinum coating and SEM examination. SEM examinations were done with a JEOL JSM–5410LV Scanning Microscope and images were recorded with JEOL Semafore Properties (version 4.02).
Terminology
Over the years a terminology for description of epidermal and dermal layers in lepidosaurian integument has developed around the events of their skin-shedding cycles and the morphology of the external surface. We follow Maderson (1985), Maderson et al. (1998), and Alibardi and Maderson (2003). The epidermis has an outer β-layer (β-keratin), of variable thickness (thickest at scale keels/spines, thinnest around sense organs and in scale-hinge joints), and an inner α-layer (α-keratin), the two layers separated by a mesos layer. The β-layer is a syncytium, with the external surface often covered by superficial Oberhäutchen (sensu Maderson 1985; Harvey 1993) and micro-ornamentation (of variable form; Peterson 1984), composed of structurally rigid proteins that guard against abrasion (Mittal and Singh 1987a). The patterned ornamentation of the outer surface of the β-layer is sometimes termed “honeycomb” (Peterson 1984), but is perhaps better designated Oberhäutchen, not implying cell border structures (Maderson 1985; Harvey 1993; Harvey and Gutberlet 1995; Maderson et al. 1998; Alibardi 1999, 2001), as is followed here. The α-layer is composed of more flexible proteins. The two layers of keratin differ in the diameter of their cytoplasmic filaments and chemical bonding (Maderson 1985; Alibardi 2001). Splitting of the α- and β-layers, at the mesos layer, is often seen in alcoholic specimens (Lillywhite and Maderson 1982; Irish et al. 1988; Maderson et al. 1998). The mesos layer contains extracellular lipids that serve as a water impermeable barrier for retention of the body’s aqueous fluids. This function may be compromised by its state of hydration (Zucker 1980; Lillywhite and Maderson 1982). Below the α-layer, during the epidermal renewal phase prior to shedding, there are lacunar and clear cell layers. The ultrastructure of scale mechanoreceptors has been described in Phrynosoma modestum Girard in Baird and Girard, 1852, (Sherbrooke and Nagle 1996), and in an agamid (Maclean 1980). Scale-hinge morphology of lepidosaurians has received little study (Spearman and Riley 1969; During and Miller 1979; Mittal and Singh 1987b).
Each scale is defined by its surrounding perimeter, interscalar channel or scale hinge. Because scales may overlap in an anterior–posterior orientation, and laterally, the areas of intersection of adjacent scales and their hinges are subject to transitioning structure between distinct scales. When scale surfaces are folded so that an edge of one covers the upper surface of another a covered and enclosed region is formed. This may have significance in functional aspects of scale hinges. Thus, the scale hinge is three-dimensional and variable in conformation. At their deeper points both walls of scale hinges abruptly change structure with a thinning of the β-layer. The epidermis then bridges between the two scales by means of a hinge joint. Scale hinges are flexible and distensible due to the reduced thickness of β-keratin in the hinge joint.
Results
Morphology of scale surfaces and hinges of M. horridus (Agamidae)
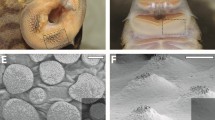
The scales of M. horridus are highly varied in size and form, but might be considered as belonging to two types, large spines and smaller scales. Spines are scattered over the head, body, legs, and tail surfaces with notably enlarged forms dorsally, above eyes, on the head, on the neck boss, and down the back and tail. Ventral spines are reduced in size (1–2 mm) and surrounded by polygonal scales of varied shape and size (<0.5 mm), often with overlapping edges (Fig. 1a). Both spine and scale surfaces may be fully or partially covered by Oberhäutchen ornamentation (Gans et al. 1982).
a, b Moloch horridus (Agamidae), c, d Phrynosoma cornutum (Iguanidae). a Overlapping edges of ventral scales. b Medial surface of β-layer epidermis of ventral scales and spines showing the interconnected network of scale hinges (white). c Overlapping of ventral scales. d Medial surface of β-layer epidermis of ventral scales showing the interconnected network of scale hinges (white), and vacuous tissue (pitting) in the hinge joint. Insert: higher magnification of the hinge joint pitting
Scale hinges form an interconnecting lace-like network seen on the inner epidermal surface of removed β-layer keratin (Figs. 1b, 2a). This internal surface also reveals dendritic-like patterns of supportive sculpturing in the β-layer of individual scales (Fig. 2a). Visualized in three dimensions, each scale is constricted basally by the surrounding hinge joints as they widen (to 100–250 μm; Fig. 2a). The widened expanse at the bottom of scale hinges and their interconnecting links between scales form a continuous hinge-joint network throughout the integument. Breaks in hinge walls (Fig. 2b) show that the Oberhäutchen on β-layer keratin covering the outer surfaces of scales is also found on the scale-hinge walls of the inwardly-folded scale surfaces.
a, b Moloch horridus (Agamidae), c, d Phrynosoma cornutum (Iguanidae). a SEM of medial surface of ventral scale epidermis (β-layer) showing the interior dentritic bracing-support of each scale (s), and the scale defining interconnections of scale hinges. Deep portions of scale hinges spread laterally, as hinge joints (hj), forming a continuously-connected surface. b SEM of ruptured epidermal wall (medial side of β-layer) of a scale hinge at the hinge joint (hj), illustrating Oberhäutchen (ob) cover on the walls of the scale hinge (distal side of β-layer). Scale-hinge walls are continuous with the external epidermal scale surface. (Smooth area at lower center is adhesive material used to remove β-layer epidermis). c SEM of medial surface of ventral-scale epidermis, β-layer. Note smooth interior of each scale (s) and walls of scale hinges (sh), and expanded and rounded hinge joints (hj) with abundant surface pitting. d SEM of medial surface of ventral scales (s), β-layer epidermis. Note different levels at hinge joint (hj) interceptions and their surface pitting (viewed at 70° angle from vertical)
Examination of SEM and histological cross-sections of the hinge area (Figs. 3a–c, 4a, b) confirms that the outer Oberhäutchen extends from outer scale surfaces down the interior walls of the scale hinge. In an abrupt transition, the thickness of the β-layer is reduced on both sides of the hinge walls, thereafter becoming the hinge joint (Figs. 3a–c, 4a, b). The hinge joint is notably wider than the scale-hinge opening leading down between adjacent scales (Figs. 3a–c, 4a, b), with the hinge joint, a semi-tubular channel, having a diameter of roughly 100–150 μm. Histological sections show that hinge joints are composed of multiple layers of eosinophilic tissues (Fig. 4a, b), the α-layer epidermis folded into contorted relief that lies on apparently vacuous tissue (Fig. 3a–c). On its external surface this hinge-joint folding appears as fractured “islands,” not ridges, on exposed hinge joints (Figs. 3b, 5a). These island protrusions into the semi-tubular hinge-joint channels are separated by narrow spaces of variable depth, about 50 μm or less. At higher SEM magnifications, hinge-joint surfaces are highly sculptured but smooth in surface texture, with no micro-ornamentation (Fig. 5b). A thin β-layer of epidermis extends over hinge-joint islands and appears to have Oberhäutchen surface structure (Fig. 4b).
a–c Moloch horridus (Agamidae), d–f Phrynosoma cornutum (Iguanidae). a Cross-section SEM of scale hinge (dark space) defining adjacent scales (s). Note Oberhäutchen on scale hinge walls and the folded multi-layers of epidermis in the widened (>100 μm) hinge joint (hj). The hinge joint begins as the thickened β-layer thins abruptly (white arrows). b Cross-section SEM of scales showing hinge in longitudinal section, extending around side of one (middle) scale (s) from its continuation with scale hinges defining scales to the left and right (with hinge joint, hj). This scale hinge view shows several upwardly-protruding island-like structures (black arrow), part of hinge-joint surface morphology. c Cross-section SEM of 5 μm-thick integument illustrating two scale hinges (sh), with well-developed Oberhäutchen (ob, with adhering soil particles) on the outer scale surfaces. The α- and β-layers of epidermis are artificially (a) separated. Note regions of abrupt thinning of the β-layer in the scale hinge (white arrows), as it transitions into the hinge joint (to the right). d Cross-section SEM of scale hinge (sh) extending between two scales (s) to a proximally expanded hinge joint (hj) cavity, part of the integumental semi-tubular, water-transport channel network. Note the transition from thick to thin β-layer epidermis (white arrows) at the beginning of the hinge joint and the protruding “islands” of tissue (black arrow) forming the hinge joint walls. e Cross-section SEM of scale hinge extending between two scales into a hinge joint which is compress by a shifted relationship between the two scales. Thus, the two thinning transition areas of β-layer epidermis (white arrows) are not opposite one another. The hinge joint (hj) exhibits surface bulging, increasing its internal surface area. f Cross-section SEM of 5 μm-thick integument illustrating a scale hinge (sh) leading into an expanded hinge joint (hj), with “islands” of bulging tissue (black arrow). Weak Oberhäutchen covers the outer surface of the β-layer epidermis of the two adjacent scales. The α- and β-layers of epidermis are artificially separated. White arrows point to transition areas of thick to thin β-layer epidermis in the walls of the scale hinge
a, b Moloch horridus (Agamidae), c, d Phrynosoma cornutum (Iguanidae). a Light microscopy cross-section of scale hinge showing continuation of Oberhäutchen β-layer epidermis from outer scale to scale hinge (sh) surfaces and the hinge joint (hj). Note pronounced widening of the hinge joint, forming a semi-tubular channel, the abrupt transition from thick to thin β-layer keratin (white arrows), and multiple folded layers of eosinophilic tissue forming protrusions on the hinge joint walls. Portions of the α- and β-layers of epidermis are artificially (a) separated. b Light microscopy cross-section of scale hinge showing continuation of Oberhäutchen β-layer epidermis (ob, with adhering soil particles) extending into the scale hinge and hinge joint to cover raised hinge-joint topographic features as a thin layer, of Oberhäutchen (black arrows). Note pronounced widening of the hinge joint, forming a semi-tubular channel, the abrupt transition from thick to thin β-layer keratin (above upper black arrow point; only one transition in view), and multiple folded layers of eosinophilic tissue forming protrusions into the hinge joint. c Light microscopy cross-section of scale hinge showing continuation of Oberhäutchen β-layer epidermis (ob) from outer scale to scale hinge (sh) surfaces, and the hinge joint (hj). Note pronounced widening of the hinge joint, forming a semi-tubular channel, the abrupt transition from thick to thin β-layer keratin (white arrows), and multiple folded layers of eosinophilic tissue forming protrusions into the hinge-joint walls. Portions of the α- and β-layers of epidermis are artificially (a) separated. d Light microscopy cross-section of hinge joint illustrating folded multiple-layers of eosinophilic tissues, forming protrusions into the hinge joint (hj), covered by a thin epidermal β-layer (black arrow; soil particles imbedded in surface)
a–b Moloch horridus (Agamidae), c–d Phrynosoma cornutum (Iguanidae). a SEM of torn edges of hinge joints (above) within the hinge-joint continuum (hj), illustrating the topographic complexity resulting from the bulging outward of the epidermis as island-like protrusions (white arrows). Note the Oberhäutchen (ob) covering of three ventral scales separated by scale hinges (sh). b Higher magnification SEM of the hinge-joint surfaces, illustrating lack of micro-ornamentation. c SEM of torn edges of hinge joints (above) within the hinge-joint continuum (hj), illustrating the topographic complexity resulting from the bulging outward of the epidermis as island-like protrusions (white arrows). Note the low-relief Oberhäutchen (ob) covering of two ventral scales (s) separated by a scale hinge (sh). d Higher magnification SEM illustrating the pitted micro-ornamentation covering surfaces of bulging island-like structures in the hinge joint
Morphology of scale surfaces and hinges of P. cornutum (Iguanidae)
The dorsal skin of P. cornutum is covered with small scales (<1 mm) and larger spined scales (>2 mm), of varied form and size. Spines cover portions of the head (including lower jaws), all of the back and upper surfaces of the legs and tail, and form a linear line extending laterally down each side between dorsal and ventral surfaces (lateral fringe scales) of this typically flat-bodied genus (Sherbrooke 2003). Cranial ossified-horns are covered by integument. The ventral surface is covered with smooth scales, uniformly sized (1 mm) and shaped, that overlap at scale hinges (Fig. 1c).
The inner surface of the β-layer of the epidermis illustrates the hinge-joint channel system connecting scale hinges (Figs. 1d, 2c, d). The inner surface of the β-layer epidermis of scales is rather smooth. This texture extends down the scale-hinge walls to the broadened hinge joint. The inner surface of the expanded and rounded medial termination of the hinge joint (100–150 μm) is highly pitted with open spaces (Figs. 1d, 2c, d). Hinge-joint intersections are not evenly connected across hinge joints, in P. cornutum, apparently so that the joints accommodate depth changes of scale hinges due to the overlapping of layered scales (Fig. 2c, d).
SEM and histological cross-sections of the inner scale-hinge surfaces show only low-relief Oberhäutchen (Figs. 3d–f, 4c, d). An abrupt transition of thickness in the β-layer keratin occurs deep within the hinge as it transitions into the hinge joint (Figs. 3d–f, 4c, d). The hinge joints form a semi-tubular channel with an internal diameter of approximately 100–150 μm. Within the hinge joint, multi-layered eosinophilic-tissues (α-layer of epidermis) are folded so as to form numerous structures protruding into the expanded space of the hinge joint (Figs. 3d–f, 4c, d). They appear to rest on vacuous tissues (Figs. 3d–f, 4c, d). The depth of spaces separating the protruding islands of tissue is approximately 20–30 μm. The β-layer keratin continues into the hinge joint as a very thin layer covering the folded surfaces (Fig. 4c, d). These protruding structures, seen in torn hinge joints (Fig. 5c), reveal their fractured island-like nature as protrusions into the hinge joint from under-lying eosinophilic tissues (Fig. 4c, d). At higher SEM magnification these folded tissues are covered by a pattern of tiny indentations in a surface matrix, a micro-ornamentation (Peterson 1984) (Fig. 5d).
Morphology of scale surfaces and hinges of other species of Agamidae and Iguanidae
Ventral scales of one species, C. caudicintus, are used to illustrate the major features of scale surfaces and hinge morphology noted in agamids (Fig. 6a–c). Other species examined (P. lesueurii, P. barbata, and P. henerylawsoni) showed similar features except as indicated below.
a–c Ctenophorus caudicinctus (Agamidae), d–f Callisaurus draconoides (Iguanidae). a Overlapping ventral-body scales (s). Insert: SEM of medial surface of β-layer epidermis of scales (s) and interconnecting hinge joints (hj). Scale bar = 100 μm. b SEM cross-section of overlapping scales (s) with broad openly-exposed scale hinge (black arrow). c SEM showing smooth area in hinge joint (hj), illustrating the lack of prominent topographic structures, and lack of semi-tubular channel. Note low-relief Oberhäutchen (ob) on scale (s) to the right. d Ventral-body scales (s), non-overlapping, with exposed scale-hinge continuum between scales. Insert: SEM of medial surface of β-layer epidermis of scale (s) with interconnecting hinge joints. Scale bar = 100 μm. e SEM cross-section of ventral body wall and scales (s), with scale hinges (black arrows) lacking semi-tubular hinge joints. Note artificial (a) splitting of the α- and β-layers of the epidermis. f SEM of scale hinge-joint between two scales (s), illustrating the smooth, slightly folded, distal surface of the non-tubular hinge joint (hj). Low-relief Oberhäutchen (ob) on scales. Insert: high magnification SEM of pitted micro-ornamentation on smooth surfaces of hinge joint. Scale bar = 1 μm
In C. caudicintus, belly scales are flat and uniform in size (1–2 mm), with wide entry openings into the interscalar channels, scale hinges (Fig. 6b, c). Oberhäutchen covers some scale surfaces leading into scale hinges (apparently all scale surfaces in P. barbata), continuing down the inner scale surfaces to the region of transition to the hinge joint, where the β-keratin layer abruptly thins. The hinge joints exhibit few folds, which are not obviously underlain by vacuous pockets to form island-like structures in the hinge joint (Fig. 6b, c). The inner surface of the β-layer keratin (insert, Fig. 6a) shows that the hinge joints are broad (100–200 μm), interconnected, and lack pitting. The externally exposed surface of the hinge joint, between spread scales, is broad (100–200 μm, Fig. 6b), uniformly flat, and devoid of island-like protruding structures (Fig. 6c). At higher SEM magnification, this surface lacks micro-ornamentation. One agamid species, P. barbata (not illustrated), deviated from the C. caudicintus hinge-joint pattern by having some β-layer pitting and folding in the medial (inner) surface of the hinge joints of separated epidermis. Similar to C. caudicintus, it also lacks scale hinge and hinge-joint formation into semi-tubular channels.
Ventral scales of an iguanid species, C. draconoides, are used to illustrate the major features of scale surfaces and scale-hinge morphology noted in this and other iguanids (Fig. 6d–f). The additional iguanid species examined (C. collaris, G. wislizenii) showed similar features, except as indicated below.
The ventral scales of C. draconoides are uniform in shape and size (1 mm), and fairly smooth on the outer scale surface (Fig. 6d, e), although flattened outlines of Oberhäutchen may appear in the β-layer keratin (Fig. 6f). The medial side of the β-layer shows the interior of the narrow scale hinges, with narrow unpitted hinge joints (insert, Fig. 6d). Cross-sections illustrate that the scale hinges are narrow (Fig. 6e) and that the thick epidermal β-layer thins rapidly towards the bottom of the hinge (not figured), where the hinge joint begins. Histological examination shows that the distal epidermal surface of the hinge joint, covered by a thin β-layer, is not extensively folded (not figured). External SEM views seen between the scales of the hinge joints show that the surface of the hinge joint is not highly fractured into multiple island-like structures (Fig. 6f). Hinge joints are covered by micro-ornamentation (insert, Fig. 6f).
SEM examination of epidermal layers of groups of scales from the belly of C. collaris and G. wislizenii (not figured) showed scale-hinge structure different from C. draconoides. In both species, the distal surfaces of the hinge joints, within scale hinges, showed island-like protrusions (very similar to those of P. cornutum in Fig. 5d) and on medial β-layer surfaces of separated hinge joints, vacuous structures occur that are similar in appearance to those seen in P. cornutum (Fig. 1d). Scale-hinge joints are widely open, not forming enclosed semi-tubular channels. At higher SEM resolution micro-ornamentation is seen on hinge-joint islands, as in P. cornutum (Fig. 5f).
Morphology of labial scales at the jaw angle in M. horridus and P. cornutum
At low magnification SEM, the posterior labial scales, both upper and lower, of M. horridus exhibit a pronounced spine and numerous mechanoreceptors, crowded around each spine (Fig. 7a). Other mechanoreceptors are abundant on adjacent scales (Fig. 7a). The surface of each labial scale has two faces, one oriented toward the exterior of the jaws (Fig. 7a) and a second, at an angle of almost 90°, leading into the mouth (Fig. 7b). The inward directed face forms a wide (>0.5 mm) lip-like surface (Fig. 7b, c). This broad lip-like portion of adjacent labial scales is crossed by a series of ridges (20–50 μm wide) and furrows (50–100 μm wide) (Fig. 7b–d). The faces of these labial scales, both outer-facing and lip-like, are covered with deeply-indented Oberhäutchen (Fig. 7c, d). No similar labial structures were seen in the other agamids. There is no development of scale-covered rictal plate tissue between the upper and lower jaws in the posterior jaw angle of M. horridus. But interestingly, and previously unreported, within the buccal cavity and running adjacent to the posterior upper-labial scales in M. horridus, there is a ragged curtain of tissue hanging from the roof of the mouth (Fig. 7b). These dangling tissues, apparently non-glandular, shorten in length anteriorly and terminate before the region of the maxillary teeth (Hocknull 2002).
a–d Moloch horridus (Agamidae), e–h Phrynosoma cornutum (Iguanidae). a SEM of lower and upper labial scales (lls and uls) of left-rear jaw (anterior to right) showing well-developed opposing spines (tooth-like in appearance), surrounded by numerous circular mechanoreceptors (mechanoreceptors also seen on adjacent scales; black arrow). b Dissecting microscope view of “lip” surface transition zone, at rear of upper jaw, extending from external (above) to buccal (below) sides. Note the series of white labial scale surfaces that extend inward from their darker-pigmented portions on the face, above. Below the labial scales, a groove separates them from a fringe-curtain of soft tissue that extends anteriorly (left), where it shortens and terminates. Lower edge of curtain is unattached. Raised lines of white tissue, apparently supportive, run from the top to the bottom of the curtain. c SEM of lower labial scale, the lip-like face oriented upward, and the external face (below) with spine (black arrow) and several mechanoreceptors (white arrow). The lip-surface face is covered by Oberhäutchen extending over interrupted-ridges and furrows running from the outside of the scale to the inside of the mouth. d SEM of lip-like transition-zone surface (Oberhäutchen covered) of a lower rear labial scale with ridges (r) and furrows (f) running between the outer and inner edges of the jaw. e SEM of upper labial scales, at the rear (left) of the jaw, with indented edges of nearly-smooth (low-relief Oberhäutchen) scales and single circular mechanoreceptors (black arrow) on each labial and adjacent scale. f SEM of lip edge (above) of rear-jaw lower-labial scale showing no or low-relief Oberhäutchen (ob) surfaces. g SEM of scales (s) covering the surface of a rictal plate, in the rear jaw angle and medial to the labial scales. Note Oberhäutchen (ob) on these scales. h SEM of scale-hinge surfaces between scales on rictal plate, illustrating presence of pitted micro-ornamentation and complex structural topography
The surfaces of posterior labial scales of P. cornutum are smooth, with only minor development of Oberhäutchen (Fig. 7e, f). The lip edges of the individual labial scales are acutely rounded, forming a narrow “lip” transition area between outer and inner edges of the jaws. Labial scales are characterized by the presence of a single mechanoreceptor and no spines.
In the angle of the jaw a fold of skin, the rictal plate, stretches between the upper and lower jaws, folding inward on a median crease when the jaws are closed (Sherbrooke 2004). The surface of this plate is covered with small scales of various sizes, covered by Oberhäutchen (Fig. 7g). At higher SEM magnification, the surfaces of tissues folded between rictal plate scales, as well as the scales, exhibit micro-ornamentation (Fig. 7h). This micro-ornamentation is similar to that also found in hinge joints of this species (Fig. 5f).
Discussion
Literature descriptions of structures pertaining to cutaneous water movement
Bentley and Blumer (1962) attributed water transport to scale surface “channels” between “keratin ridges” on scale surfaces and figured a “skin fold” with two opposing surfaces. We reinterpret their figure as representing two scale surfaces (overlapping scale above), not a skin fold, and note that the ridges they illustrate are cross-sections of Oberhäutchen, not channels. Gans et al. (1982) corrected the interpretation that water flows over scale surfaces in such channels to the mouth, noting rather that it moves between scales. They attributed a surface-wetting role to Oberhäutchen, an issue discussed without resolution by others (Schwenk and Greene 1987; Sherbrooke 1993; Vesley and Modry 2002). Both M. horridus and P. cornutum exhibit Oberhäutchen covering on scale surfaces, with its depth more pronounced in M. horridus. Related taxa in both lineages also exhibit Oberhäutchen (Peterson 1984), so they are unlikely to be a specialized feature solely for promoting cutaneous water transport. Micro-ornamentation was lacking in all agamid species and was present in all iguanid species examined, leaving its role in water transport, if any, in doubt. Hydrophobic characteristics have been attributed to the presence of surface microtexture in other squamata (Gans and Baic 1977). The chemical compounds in scale surfaces, and their hydrophobic or hydrophilic properties, are unknown. Their potential role in water transport over squamate skin should be investigated.
Differences and similarities in hinge-joint structures between M. horridus and P. cornutum
Although there is remarkable similarity, in terms of hinge-joint structure, between the two species studied that show cutaneous water movement, there are also some differences. The hinge joint in both species widens laterally. But it is much flatter basally in M. horridus, whereas it is rounder basally in P. cornutum. In addition, there are differences in arrangement of the tissues supporting the “islands” or convolutions that protrude into the hinge-joint expansion. The inner β-layer epidermis surface differs in the two species, unpitted (M. horridus) versus pitted (P. cornutum), as does their layering of folded eosinophilic tissues.
Scale-hinge depth in both M. horridus and P. cornutum varies with position on their body and with scale-hinge channel conformation around individual scales. In general, the depth varies between 50 and 200 μm, with ventral-surface scale hinges of P. cornutum deeper than M. horridus. Also, scale-hinge openings vary in width, but typically are <50 μm.
We propose that for cutaneous water movement to be effective, a semi-tubular expansion is required at the base of the scale hinge, at the hinge joint, possibly accompanied by convolutions of the skin within the channel and with narrow interscalar spaces in distal portions of the scale hinge. Thus, to evolve cutaneous water movement, M. horridus and P. cornutum both needed to evolve the semi-tubular expansions at the base of the scale hinge, accompanied by a narrowing of the outer interscalar space. Both M. horridus and P. cornutum may have had to derive convolutions within the semi-tubular hinge-joint channel as well, since convolutions in the hinge joint appear to be absent in some related species. This depends on the specific ancestral condition of this character in the groups from which the water-transporting species were derived.
Jaw structures used in drinking
Apparently, water ingestion in P. cornutum occurs via the rictal plate and jaw/tongue actions (Sherbrooke 2004). We provide new data on rictal plate surface morphology, Oberhäutchen and micro-ornamentation. No comparable connecting structure between the jaws was found in M. horridus, but the rear labial scales have apparently unique surface features. Tooth morphology, hyobranchial musculature, and sublingual gland hypertrophy are unusual in this animal as well (Meyers et al. 2001; Hocknull 2002). Deep Oberhäutchen covers the labial scales and crosses a widened lip-like area with furrows leading from the exterior integumental surface to the interior edge of the jaws. Additionally, a screen of tissue is suspended from the posterior upper jaws in M. horridus, adjacent to the specialized labial scales. The surfaces of these soft tissues may play a role in water ingestion. Chemical factors in oral mucus have been hypothesized to facilitate water ingestion, but this has not been demonstrated (Bentley and Blumer 1962; Withers 1993). As with P. cornutum, M. horridus is known to exhibit jaw motions during drinking that are likely associated with movements of the tongue and hyobranchial apparatus (Sherbrooke 1993, 2004; Withers 1993), and are an essential component of the rain-harvesting systems of the two species.
Convergent evolution between M. horridus and P. cornutum
Both M. horridus and P. cornutum are able to transport water rapidly over the skin, whereas observations of water movement on living and preserved specimens of other species of both agamids and iguanids (Agamidae: P. barbata, C. caudicintus, P. lesueurii; Iguanidae: C. collaris, G. wislizenii, C. draconoides) demonstrate that they do not transport water, or can only move water slowly (e.g., P. barbata) compared to M. horridus and P. cornutum (Sherbrooke et al. unpublished data).
In order to further explore which components of the scale hinge and hinge joint are most likely to facilitate the transport of water in M. horridus and P. cornutum, we examined these structures in a number of non-water transporting species of their separate phylogenetic lineages (Frost and Etheridge 1989; Baverstock and Donnellan 1990; Losos and Miles 2002). In most agamid species examined, the morphology of the hinge joint differed noticeably from that of M. horridus. All of the agamid species examined lacked semi-tubular expanded channels, and most lacked topographic complexity of the hinge joints, although both Pogona species had some folding (much less than M. horridus). Similarly, none of the iguanid species studied had semi-tubular hinge-joint channels, and two of four iguanid species lacked topographic complexity in hinge joints, whereas the other two species had convolutions.
Both M. horridus and P. cornutum possess a series of apparently unusual morphological features of the integument that function within a drinking-water-acquisition system. These features include, (1) interscalar spaces that are deep and narrow enough to partially close off an expanded semi-tubular network of interconnected hinge joints, (2) convoluted features of the hinge-joint walls that greatly increase the internal surface area of the semi-tubular network, and (3) modification of scales and integumental structures at the rear of the jaws that facilitate, along with buccal action, oral ingestion of water from the scale-hinge network. These combined similarities of the two species, and their absence as consistent multiple-occurring components in related taxa, that apparently do not exhibit a similar ability to capture, move, and consume integumentally-captured water, leads us to the hypothesis that the co-occurrence of these features in the two species is due to evolutionary convergence to facilitate water capture and transport for drinking. A detailed examination of scale-hinge structure in additional phylogenetically-relevant species and study of potential movement of water by these species is required to further test this hypothesis.
Movement of water during cutaneous transport
Our study allows us to better interpret the movement of water within integumental scale-hinge systems of M. horridus and P. cornutum during rain-harvesting (Fig. 8a, b). Oberhäutchen may enhance surface forces on the inner walls of the scale hinges to carry water present at the scale surfaces into the hinges and to their deeper levels, the hinge joints (Fig. 8c). Here water enters an expanded cavity and comes into contact with convoluted and fragmented surfaces of the interior of the semi-tubular, hinge-joint system (Fig. 8c). As capillary action draws water down scale-hinge openings, it fills the hinge-joint channels where attractive forces between the surfaces and water are enhanced by the large surface area of the expanded and convoluted hinge joint.
a, b Schematic representations of two rain-harvesting lizards, Moloch horridus (Agamidae) and Phrynosoma cornutum (Iguanidae). M. horridus (a, left) illustrates uptake of water from a water puddle on the substratum via capillary forces generated in limb scale hinge-joint channels and its transport over all integumental surfaces leading to the rear angle of the jaws for drinking. P. cornutum (b, right), similarly, illustrates the capture of raindrops on dorsal integumental surfaces and their transport within scale hinge-joint channels to the jaws for drinking. Both species rain harvest; arrows suggest patterns of water movement over external surfaces of the integument. c–e Composite (M. horridus and P. cornutum), schematic reconstructions of anatomical and functional aspects of scale hinge-joint channels and their role in water transport across cutaneous surfaces during rain-harvest drinking. c Cross-sections through two scales (outer surface, stippled keratin, above, and dermis/muscle tissue, shaded/lined, below). Scale hinges have narrow elongated entry from scale surfaces to expanded scale-hinge joints below. The scale hinge fills as raindrops falling on the surface of the skin (above) are pulled, by adhesive forces, into the scale hinge (left) and down to fill the scale hinge-joint channels (right). Arrows suggest flow patterns of water. Folds into the lumina of hinge-joint channels represent cross-sections through islands of tissue that protrude into the channels from their wall and increase internal surface area. Black scale bar = 100 μm. d Three-dimensional (two-surface; outer skin scales, above, and cross-section cut through scales, below) reconstruction of a scale hinge-joint channel system. This illustrates channel connections below scale surfaces to a continuous semi-tubular network. For clarity in presentation, the scale hinge openings at the surface are represented as open, and for the upper portion of the diagram (below the scale surfaces) scale hinge opening connections to the semi-tubular system (represented as tubular) below have been omitted (see Figs. 1d and 2c for SEM views from below of these connections surrounding the base of each scale. Black scale bar = 100 μm. e Schematic horizontal section of skin below scales (above outlined white spaces), illustrating continuous floor of semi-tubular, scale-hinge-joint channel system. Arrows illustrate the flow patterns of water pulled by multiple menisci spreading throughout the interconnected system. Black scale bar = 200 μm
We hypothesize that the directional, pan-cutaneous flow of water within the system is mainly accomplished in the hinge-joint channels (Fig. 8d), not in portions of scale hinges external to the hinge joints. The speed with which water is carried may be enhanced by the effect of multiple menisci spreading water throughout the entire branching network of semi-tubular hinge-joint channels (Fig. 8e). Capillary forces may move water, against gravitational force, from body surfaces in contact with the substratum to the head (Fig. 8a) (Bentley and Blumer 1962; Gans et al. 1982; Sherbrooke 2004). In M. horridus, Withers (1993) estimated the capillary forces generated based on an interscalar channel width of 5–50 μm, and found the actual capillary-lift force to be considerably greater. Our findings on hinge-joint channel diameter and surface morphology appear to explain this discrepancy. We propose that the spaces between scales should be restrictively termed “scale hinges” (not “interscalar channels”, e.g., Bentley and Blumer 1962) and that the portion associated with the hinge joint, at least in water transporting species where it is semi-tubular, should be termed the “hinge-joint channel.”
Once the water-holding areas in the hinges are saturated (for water-holding volumes, see, Withers 1993; Sherbrooke 2004), water must proceed to the mouth for effective drinking. Capillary forces (Withers 1993; Adamson and Gast 1997), do not move water once the scale-hinge system is filled to capacity, thus, a negative pressure, generated at the jaws and in the mouth by jaw and tongue movements (Sherbrooke 2004), is required to promote water flow. This negative pressure, transmitted throughout the entire water-filled system, moves water against the drag of hinge-joint-channel walls. Such a negative pressure may also facilitate movement of new rainwater into scale hinges, and hinge joints as it continues to fall onto the lizards’ backs during rain harvesting (Fig. 8a, b).
References
Adamson AW, Gast AP (1997) Physical chemistry of surfaces, 6th edn. Wiley, New York
Alibardi L (1999) Formation of large micro-ornamentations in developing scales of agamine lizards. J Morphol 240:251–266
Alibardi L (2001) Keratohyalin-like granules in lizard epidermis: evidence from cytochemical, autoradiographic, and microanalytic studies. J Morphol 248:64–79
Alibardi L, Maderson PFA (2003) Observations on the histochemistry and ultrastructure of the epidermis of the tuatara, Sphenodon punctatus (Sphenodontia, Lepidosauria, Reptilia): a contribution to an understanding of the lepidosaurian epidermal generation and the evolutionary origin of the squamate shedding complex. J Morphol 256:111–133
Baverstock PR, Donnellan SC (1990) Molecular evolution in Australian dragons and skinks: a progress report. Mem Queensl Mus 29:323–331
Bentley PJ, Blumer WFC (1962) Uptake of water by the lizard, Moloch horridus. Nature 194:699–700
During M von, Miller MR (1979) Sensory nerve endings of the skin and deeper structures. In: Gans C, Northcutt RG, Ulinski P (eds) Biology of the Reptilia, vol 9. Academic, London, pp 407–441
Frost DR, Etheridge R. (1989) A phylogenetic analysis and taxonomy of iguanian lizards (Reptilia: Squamata). Univ Kansas Mus Nat Hist Misc Publ 81:1–65
Gans C, Baic D (1977) Regional specialization of reptilian scale surfaces: relation to texture and biological role. Science 195:1348–1350
Gans C, Merlin R, Blumer WFC (1982) The water-collecting mechanism of Moloch horridus re-examined. Amphib-Reptil 3:57–64
Harvey MB (1993) Microstructure, ontogeny, and evolution of scale surfaces in xenosaurid lizards. J Morphol 216:161–177
Harvey MB, Gutberlet RL Jr (1995) Microstructure, evolution, and ontogeny of scale surfaces in cordylid and gerrhosaurid lizards. J Morphol 226:121–139
Hocknull SA (2002) Comparative maxillary and dentary morphology of the Australian dragons (Agamidae: Squamata): a framework for fossil identification. Mem Queensl Mus 48:125–145
Irish FJ, Williams EE, Seling E (1988) Scanning electron microscopy of changes in epidermal structure occurring during the shedding cycle in squamate reptiles. J Morphol 197:105–126
Lillywhite HB, Maderson PFA (1982) Skin structure and permeability. In: Gans C, Pough FH (eds) Biology of the Reptilia, vol 12. Academic, New York, pp 397–442
Losos JB, Miles DB (2002) Testing the hypothesis that a clade has adaptively radiated: iguanid lizard clades as a case study. Am Nat 160:147–157
Maclean S (1980) Ultrastructure of epidermal sensory receptors in Amphibolurus barbatus (Lacertilis [sic]: Agamidae). Cell Tissue Res 210:435–445
Maderson PFA (1985) Some developmental problems of the reptilian integument. In: Gans C, Billett F, Maderson PFA (eds) Biology of the Reptilia, vol 14. Wiley, New York, pp 523–598
Maderson PFA, Rabinowitz T, Tandler B, Alibardi L (1998) Ultrastructural contributions to the understanding of the cellular mechanisms involved in lizard skin shedding with comments on the function and evolution of a unique lepidosaurian phenomenon. J Morphol 236:1–24
Mayhew WW, Wright SJ (1971) Water impermeable skin of the lizard Phrynosoma m’calli. Herpetologica 27:8–11
Meyers JJ, Herrel A (2005) Prey capture kinematics of ant-eating lizards. J Exp Biol 208:113–127
Meyers JJ, Herrel A, Mead J (2001) Morphology of the feeding apparatus of the Australian thorny devil (Moloch horridus). Am Zool 41:1526
Mittal AK, Singh JPN (1987a) Scale epidermis of Natrix piscator during its sloughing cycle—structural organization and protein histochemistry (Reptilia: Colubridae). J Zool, Lond 213:545–568
Mittal AK, Singh JPN (1987b) Hinge epidermis of Natrix piscator during its sloughing cycle – structural organization and protein histochemistry. J Zool, Lond 213:685–695
Peterson CC (1998) Rain-harvesting behavior by a free-ranging desert horned lizard (Phrynosoma platyrhinos). Southwest Nat 43:391–394
Peterson JA (1984) The microstucture of the scale surface in iguanid lizards. J Herpetol 18:437–467
Pianka ER, Pianka HD (1970) The ecology of Moloch horridus (Lacertilia: Agamidae) in Western Australia. Copeia 1970:90–103
Pianka ER, Parker WS (1975) Ecology of horned lizards: a review with special reference to Phrynosoma platyrhinos. Copeia 1975:141–162
Schwenk K, Greene HW (1987) Water collection and drinking in Phrynocephalus helioscopus: a possible condensation mechanism. J Herpetol 21:134–139
Sherbrooke WC (1990) Rain-harvesting in the lizard, Phrynosoma cornutum: behavior and integumental morphology. J Herpetol 24:302–308
Sherbrooke WC (1993) Rain-drinking behaviors of the Australian thorny devil (Sauria: Agamidae). J Herpetol 27:270–275
Sherbrooke WC (1999) Thorny devils and horny toads. Nat Aust 26(6):54–63
Sherbrooke WC (2002) Phrynosoma modestum (round-tailed horned lizard). Rain-harvest drinking behavior. Herpetol Rev 33:310–312
Sherbrooke WC (2003) Introduction to horned lizards of North America. University of California Press, Berkeley
Sherbrooke WC (2004) Integumental water movement and rate of water ingestion during rain harvesting in the Texas horned lizard, Phrynosoma cornutum. Amphib-Reptil 25:29–39
Sherbrooke WC, Nagle RB (1996) A dorsal intraepidermal mechanoreceptor in horned lizards (Phrynosoma; Phrynosomatidae; Reptilia). J Morphol 228:145–154
Spearman RIC, Riley PA (1969) A comparison of the epidermis and pigment cells of the crocodile with those in two lizard species. Zool J Linn Soc 48:453–466
Vesely M, Modry D (2002) Rain-harvesting behavior in agamid lizards (Trapelus). J Herpetol 36:311–314
Withers P (1993) Cutaneous water acquisition by the thorny devil (Moloch horridus; Agamidae). J Herpetol 27:265–270
Zucker A (1980) Procedural and anatomical considerations of the determination of cutaneous water loss in squamate reptiles. Copeia 1980:425–439
Acknowledgement
For assistance in providing specimens, we thank P. Couper (Queensland Museum), D. Wilson (AMNH), and V.N. Sherbrooke. K. Koopman drew the water-flow diagrams (Figure 8). EPA Queensland Parks and Wildlife Service provided collecting and transfer permits, and the STB Animal Ethics Sub-Committee approved protocols. K. Blake (Advanced Analytical Centre, JCU) facilitated SEM use and S. Reilly (STB, JCU) prepared histological slides. Financial support was provided by the Australian Defence Science and Technology Organization—Biomimetic Fouling Control Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sherbrooke, W.C., Scardino, A.J., de Nys, R. et al. Functional morphology of scale hinges used to transport water: convergent drinking adaptations in desert lizards (Moloch horridus and Phrynosoma cornutum). Zoomorphology 126, 89–102 (2007). https://doi.org/10.1007/s00435-007-0031-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-007-0031-7











