Abstract
Purpose
The expression of cytochrome B561 (CYB561) and its role in breast cancer (BC) prognosis remain unclear. We analyzed the differential expression and prognostic value of CYB561 using online databases and a clinical cohort through bioinformatics and immunohistochemistry.
Methods
The differential expression of CYB561 and its association with BC were analyzed using the tumor immune estimation resource (TIMER), gene expression profiling interaction analysis2 (GEPIA2), Human Protein Atlas, Cancer Cell Line Encyclopedia, and Kaplan–Meier Plotter website. Important pathways of CYB561 enrichment were explored using gene set enrichment analysis. Immunohistochemistry detected CYB561 expression in normal breast, breast hyperplasia, ductal carcinoma in situ (DCIS), para-cancer, and invasive BC groups. Association between CYB561 expression and BC prognosis was analyzed using Kaplan–Meier and Cox regression analyses.
Results
CYB561 mRNA expression was higher in GEPIA and TIMER BC patients than in para-cancer tissues. CYB561 was expressed in the glandular epithelium and myoepithelium, with positive localization in the cytoplasm and cell membrane. CYB561 protein expression significantly differed among the groups. CYB561 expression was correlated with ERBB2/HER2 and infiltrating CD4+ T cells in GEPIA and TIMER BC patients and associated with HER2 status, histological grade, and molecular subtypes in the clinical cohort but not related to tumor-infiltrating lymphocytes. CYB561 mRNA overexpression predicted reduced recurrence-free survival and overall survival in BC. Patients with CYB561 expression had significantly reduced overall survival and increased risk of death.
Conclusion
CYB561 can serve as an effective clinical prognostic biomarker for BC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer (BC) is one of the most common malignant tumors and the leading cause of cancer-related deaths among women (Torre et al. 2015). Along with population growth and aging, the cancer burden of women is increasing in various countries, regardless of income level, and the incidence rate is rising by 3.1% annually. In 2018, approximately 2.1 million women were newly diagnosed with BC (Bray et al. 2018). Although the application of many therapeutic methods (such as surgery, neoadjuvant or adjuvant chemoradiotherapy, endocrine therapy, targeted therapy (Balmus et al. 2019), and immunotherapy (Gobbini et al. 2018) proposed in recent years) has significantly improved BC patient prognoses, many patients are threatened with recurrence and death. Biomarkers are pathological and physiological status indicators and can predict disease outcomes, especially cancer. Currently, biomarkers are used in the early detection, prognosis, and therapeutic targeting of BC, including CA153, human epidermal growth factor receptor 2 (HER2), circulating tumor cells, programmed death 1/Programmed death—ligand 1 (PD-1/PD-L1) (Gobbini et al. 2018), and infiltrating immune cells (Sui et al. 2020). However, as a group of complex and highly heterogeneous tumors (Liang et al. 2020), the prognosis of BC differs and is affected by ethnicity/race, genetic factors, regional environment, molecular typing, pathological features, and treatment methods. For example, Asians have lower morbidity but higher mortality than non-Hispanic white women (Ghoncheh et al. 2015). Therefore, exploring novel biomarkers involved in BC progression and prognosis would contribute to identifying therapeutic targets and predicting prognosis more accurately.
Cytochrome B561 (CYB561) is a conserved transmembrane transporter specific to secretory vesicles of neuroendocrine substances (catecholamines and neuropeptides) (Srivastava et al. 1994) with chelate reductase activity (Asard et al. 2013). It is widely expressed in human tissues, such as neuroendocrine tissues) (Pruss and Shepard 1987), and promotes anti-castration neuroendocrine prostate cancer growth and metastatic potential (Willis et al. 2016). The low expression level of CYB561 mRNA in ovarian cancer, which is associated with poor prognosis, may become a single-gene prognostic biomarker (Olarte et al. 2020). In addition, the regions of chromosome 17q23 were amplified in BC cells by gene sequencing, suggesting that CYB561 may be the driving gene that regulates their proliferation and survival (Mahmood et al. 2014). Cyb561d2 (also known as tscytb /101F6), a member of the CYB561family, can inhibit the growth of lung cancer cells (Ohtani et al. 2007) and the Duodenal cytochrome b (Dcytb), another member of the CYB561 family, is an important predictor of prognosis in BC patients (Lemler et al. 2017). Therefore, we speculated that CYB561 is involved in BC development.
However, there are few studies on CYB561 expression, as well as its correlation with clinicopathological factors and its potential predictive value in BC. Our study analyzed the differential expression and prognostic value of CYB561 using online databases and a clinical cohort through bioinformatics and immunohistochemistry.
Materials and methods
Online data acquisition and analyses
The expression of CYB561mRNA in human tissues and cell lines was detected using the Human Protein Atlas (HPA), TIMER (https://cistrome.ShinYapps.io/timer/), and the Cancer Cell Line Encyclopedia (CCLE). The differential expression of CYB561 mRNA between carcinomas and para-cancerous samples was verified using TIMER and GEPIA2 (http://gepia2.cancer-pkucn/), which include RNA sequencing data of 9736 tumor tissues and 8587 normal tissues from The Cancer Genome Atlas (TCGA) and The Genotype Tissue Expression (GTEX) databases (Tang et al. 2017). The correlation between CYB561 and BC prognosis (overall survival and recurrence-free survival) was explored using the Kaplan–Meier Plotter website (https://kmplot.com/analysis/). The correlation between CYB561 and immune cells and Erb-B2 receptor tyrosine kinase 2 (ERBB2) was investigated in the TIMER database, which contains 10,897 samples across 32 cancer types from TCGA, to allow the evaluation of the abundance of immune infiltration. A statistical deconvolution method was applied to infer the abundance of tumor-infiltrating immune cells from gene expression profiles (Li et al. 2017). The important pathway for the possible enrichment of CYB561 was explored using gene set enrichment analysis (GSEA; GSEA4.0.3). Taking “h.all. V.7.1. Symbols. GMT” as the reference gene set, 1,000 genome substitutions were performed to obtain the standardized enrichment scores for each analysis. Gene sets with standardized P < 0.05 and a false discovery rate P < 0.05 were defined as positive gene sets. The flow chart was shown in Fig. 1.
Clinical cohort acquisition
Five groups of tissue wax blocks were collected, including normal breast tissue, breast hyperplasia tissue, para-cancer tissue, ductal carcinoma in situ (DCIS) tissue, and invasive BC, with clear pathological diagnosis and complete clinical data that underwent radical mastectomy or modified radical mastectomy at the Affiliated Hospital of Qinghai University from 2011 to 2020 (Fig. 1). The median age of BC patients was 48 years (range 29–90 years). According to the 2019 version of the Chinese Breast Cancer Human Epidermal Growth Factor Receptor 2 (HER2) testing guidelines, HER2 was classified as positive by IHC 3+ or FISH amplification. Based on the 13th St. Gallen International Breast Cancer Conference 2013, BC was divided into four subtypes as follows: luminal A, luminal B, HER2 over-expression, and triple-negative.
CYB561 immunohistochemistry and interpretation
According to established methods (Rimm et al. 2001), collected wax blocks were re-embedded into a tissue microarray containing 11 tissue chip arrays. The primary antiCYB561 (Sigma-Aldrich, HPA014753) antibody (dilution ratio 1:50) was incubated at 4 °C overnight. After washing, the sections were incubated with the appropriate horseradish peroxidase (HRP)-labeled secondary antibody for 1 h, DAB staining, and hematoxylin re-staining. Phosphate-buffered saline instead of the primary antibody was used as a negative control, and paraffin-embedded tissue sections of healthy pancreas were used as positive controls.
The staining intensity of CYB561 was divided into the following: “0” for staining without any intensity, “1 + ” (weak) for light yellow, clearly visible under 10 × microscopy, “2 + ” (medium)for a color depth between “1 + ” and “3 + ,” visible under 4 × microscopy, “3 + ” (strong) for dark brown or brownish-yellow, visible to the naked eye. Staining was located in the cytoplasm and cell membrane. For the staining range, the proportion of stained tumor cells/all tumor cells or stained breast cells/all breast cells ≥ 1% was judged as “1; “otherwise, it was “0.” The product of staining intensity and range was judged as the result of immunohistochemical staining: “score 0” and “score 1” were interpreted as negative, and “score 2” and “score 3” were interpreted as positive.
Interpretation of tumor-infiltrating lymphocytes (TILs)
Paraffin blocks of invasive breast carcinoma tissue were selected, and conventional hematoxylin and eosin staining was performed. Two pathologists with senior professional titles read the slides under a microscope using a single-blind method. TILs were evaluated, and the percentage of stromal TILs was recorded on a continuous scale in an approximate semi-quantitative manner. The specific interpretation steps and evaluation methods were based on the 2014 recommendations of the international TILs working group (Salgado et al. 2015).
Follow-up
Follow-up reviews for all patients occurred by telephone or in-person at the hospital. The follow-up endpoint was January 31, 2021, or death. Disease-free survival was defined as the time from the date of initial pathological diagnosis to the date of breast cancer-related events, last follow-up date, or date of death (Huo et al. 2017). BC-related events included local recurrence, distant metastasis, new primary breast tumor, and death from BC. Overall survival was defined as the time from the initial tumor diagnosis to death due to various causes or to the date of the last follow-up.
Statistical methods
The results of Kaplan–Meier plots and GEPIA are presented with HR and P values from a log-rank test. Spearman correlation coefficient was used to measure the expression correlation among the genes. Clinical data were analyzed using SPSS24.0 (IBM, Chicago, USA). Statistical data were described by relative numbers and compared using the χ2 test. Data conforming to a normal distribution were expressed as the mean ± standard deviation and compared using the t test. Kaplan–Meier curves and log-rank tests were used to evaluate the prognostic value of CYB561. Univariate analysis was performed using the χ2 test and the Fisher exact probability test. Variable assignments are shown in Online Resource 1. Factors with statistical significance in Cox regression univariate analysis were included in the multivariate analysis, and the entry method was adopted. The hazard ratio (HR) and 95% confidence interval (CI) were analyzed using Cox regression. The statistical significance of the two-tailed test was set at P < 0.05.
Results
CYB561 expression in GEPIA and TIMER BC patients
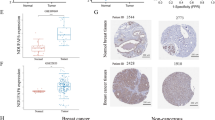
CYB561 mRNA is expressed in many human systems, including the nervous, digestive, female reproductive, and urinary systems. Its expression at the protein level was consistent with the results at the mRNA level. The differential expression of CYB561 between the tumor and adjacent normal tissues from TIMER is shown in Fig. 2A. CYB561 was upregulated in BC, bladder urothelial carcinoma, serous ovarian carcinoma, and uterine corpus endometrial carcinoma. In contrast, it had lower expression levels in adrenocortical carcinoma and renal chromophobe, and prostate adenocarcinoma. CYB561 was highly expressed in BC by analyzing 1376 samples from GEPIA. As shown in Fig. 2B and C, CYB561 mRNA was significantly upregulated in BC tissues (1085 cases) compared with that in normal tissues (291 cases) (P < 0.05, Fig. 2C), which was consistent with the TIMER data (Fig. 2A). In addition, CYB561 mRNA expression levels increased in BC cell lines (Fig. 2D and E).
Expression of CYB561 mRNA in BC and cell lines. a. Determination of CYB561 expression levels in different tumor types using TIMER (*P < 0.05, **P < 0.01, ***P < 0.001). b. CYB561 is expressed in various cancers and is increased in breast cancer by GEPIA. c. The difference in CYB561 mRNA in the invasive breast carcinoma (BRCA) group and the normal group was significant by GEPIA (P < 0.05). d. The expression level of CYB561 mRNA was elevated in CCLE BC cell lines. e. The expression of CYB561RNA in the HPA database was enhanced in all BC cell lines
Moreover, CYB561 was associated with the prognosis of BC, glioblastoma multiforme, lower-grade brain glioma, and uterine corpus endometrial carcinoma (Fig. 3D), and high CYB561 expression was correlated with worse overall survival of breast cancer patients with GEPIA (P = 0.00025, n = 700, Fig. 3A). Kaplan–Meier survival curve analysis indicated that CYB561 expression significantly affected BC prognosis. Patients with CYB561 mRNA over-expression had significantly reduced overall survival [HR = 1.38 (1.10–1.73), P = 0.006] and recurrence-free survival [HR = 1.57 (1.39–1.76), P = 2.70e-14] compared with those with low CYB561 expression (Fig. 3B and C). In the BC subgroup analysis, CYB561 was not associated with the prognosis of the HER2 negative group (Fig. 3E), but was closely correlated with prognosis in the HER2 positive group [HR = 2.89 (1.43–5.85), P = 0.002, Fig. 3E).
Association with CYB561 and prognosis of BC. a. In GEPIA, CYB561 was associated with the overall survival of BC patients, and the survival rate of the high expression group was poor (P < 0.001). b, c. In the Kaplan–Meier drawing platform, CYB561 was associated with OS and RFS, and the prognosis of the high expression group was poor (P < 0.05). d. CYB561 is related to the prognosis of major tumors in GEPIA, such as BRCA, GNM, and LGG. e. CYB561 was associated with prognosis in the HER2 positive BC group (P < 0.05). f. BC patients with CYB561 expression had reduced overall survival (log-rank P = 0.017)
CYB561 expression is significantly correlated with BC
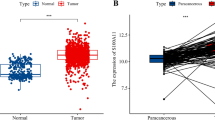
Immunohistochemical staining of CYB561 expression was performed in 24 healthy breast samples, 28 hyperplastic breast samples, 30 DCIS samples, 31 para-cancer samples, and 232 invasive BC samples. CYB561 was expressed in glandular and myoepithelial cells of mammary tissue, positively localized in the cytoplasm and cell membrane, and showed no expression in fibrocytes of the mammary stroma. The CYB561 protein was expressed at different levels in these five groups. The expression was weak in the acinar epithelium, ductal epithelium, and myoepithelial cells of the healthy breast (4.17%, 1/24) and para-cancer tissues (3.23%, 1/31, Fig. 4A and E), enhanced in invasive BC cases (19.83%, 46/232, Fig. 4D and E), and significantly elevated in proliferative acinar and ductal epithelial cells (35.71%, 10/28, Fig. 4B) and DCIS (46.67%, 14/30, Fig. 4C). The difference was statistically significant among the five groups (χ2 = 25.88, p < 0.001), as shown in Table 1. The difference in CYB561 expression between invasive BC and para-cancer was also significant (χ2 = 5.14, P = 0.023).
CYB561 protein expression in different breast tissues by immunohistochemical staining. “Score 0” and “score 1” were interpreted as negative, “score 2” and “score 3” were interpreted as positive. a. CYB561 protein expression was weak in para-cancerous tissue and not detected “score 3” (magnification × 100). b. CYB561 protein expression was elevated in breast hyperplasia tissue (magnification × 100). c. CYB561 protein expression was elevated in ductal carcinoma in situ (magnification × 100). d. CYB561 protein expression was enhanced in invasive BC tissues (magnification × 100). e. The normal terminal duct-lobular unit shows weak CYB561 immunoreactivity in the cytoplasm and membranes (left, magnification × 100). High expression of CYB561 in invasive BC (right magnification × 100). f. Control for CYB561 staining. Left, negative control (magnification × 200); right, pancreatic tissue as a positive control (magnification × 200)
Association of CYB561 and ERBB2/HER2 in invasive BC
A strong correlation was observed between CYB561 and ERBB2 in both major cancers and BC in TCGA (Fig. 5A). CYB561expression and ERBB2 expression were closely related in most tumors in the TIMER database (Fig. 5E). CYB561 showed a significant positive correlation with ERBB2 in pan-cancers, the common cancer species as a whole, did not distinguish the types of cancer (γ = 0.44, P < 0.01, Fig. 5B), and in invasive BC tissues in TCGA (γ = 0.42, P < 0.01, Fig. 5C). We also observed that the expression of CYB561 had a positive linear correlation with ERBB2 expression in BC in TIMER (cor = 0.38, P = 1.51e-39, Fig. 5D).
Association with CYB561 and ERBB2 in BC and major tumors. a. There was an association with CYB561 and ERBB2 in major tumors in TCGA. b. There was a positive correlation between CYB561 and ERBB2 in para-cancers in TCGA (P < 0.001). c. CYB561 was positively correlated with ERBB2 expression in invasive BC in TCGA (P < 0.001). d. The expression of CYB561 had a positive linear correlation with ERBB2 expression in BC in TIMER (cor = 0.38, P = 1.51e-39). e. CYB561expression and ERBB2 expression were closely related in most tumors in TIMER
In the clinical cohort, CYB561expression was enhanced in HER2 positive BC than in HER2 negative patients (χ2 = 9.31, P = 0.002, Table 2). A low degree of linear correlation was observed between CYB561 expression and HER2 status (Spearman’s rho = 0.20, P = 0.002). Moreover, the difference between CYB561 and HER2 immunohistochemical expression was statistically significant (χ2 = 21.46, P < 0.001). Surprisingly, there was a low linear positive correlation between CYB561 immunohistochemical results and HER2 expression (Spearman’s rho = 0.26, P < 0.001).
Correlation of CYB561 with TILs in invasive BC
We analyzed CYB561 expression based on the abundance of all six types of immune infiltrating cells, including B cells, CD4+ T cells, CD8+ T cells, neutrophils, macrophages, and dendritic cells. Our findings demonstrated that CYB561 expression had a significant negative correlation with the infiltration level of CD4+ T cells, neutrophils, and dendritic cells in BC (Fig. 6A), and the correlation with CD4+ T cells was the strongest (cor = − 0.184, P = 9.30e-09). The relationship between the expression level of CYB561 and tumor purity was also determined. CYB561 expression was significantly positively correlated with tumor purity (cor = 0.184, P = 5.18e-09, Fig. 6A), indicating its comparative enrichment in tumor cells. We found that CYB561 expression was closely related to CD4 + T cells in BC and different BC subtypes after adjustment for tumor purity (Fig. 6B) and closely related to the CD4+ T cell naive subtype by visually analyzing the correlation between the expression of CYB561 and CD4+ T cell subtypes infiltrating levels in various cancer types using the Gene module in TIMER2.0. Similarly, the gene copy number of CYB561 was closely related to CD4+ T cells (Fig. 6C), the infiltrating levels of CD 4+ T cells in BC group with CYB561 arm-level gain and in BC group with high amplification were lower than that in healthy group. Compared with the healthy group, the infiltrating levels of neutrophils, macrophages, and dendritic cells in BC with an arm-level gain of CYB561 declined.
Association with CYB561 and immune cell infiltration in BC in TIMER and GSEA. a. The level of CYB561 expression had a positive correlation with tumor purity and a negative correlation with the infiltration level of CD4+ T cells, neutrophils, and dendritic cells in BC in TIMER. b. The association between CYB561expression and CD4+ T cell subtype infiltration levels in various cancer types in TIMER2.0. CYB561 was associated with CD4+ T cells and closely related to the CD4+ T cell naive subtype in BC. c. Relationship between CYB561 copy numbers and levels of immune cell infiltration in BC. The infiltration level of CD 4+ T cells in BC with arm-level gain and high amplification of CYB561 was lower than the healthy group (P < 0.001), P value Significant Codes: 0 ≤ *** < 0.001 ≤ ** < 0.01 ≤ * < 0.05 ≤ . < 0.1. d. GSEA showed that the enrichment pathways of CYB561 included peroxisomes, Wnt/β-catenin signaling, and the PI3K/Akt /mTOR signaling pathways
The average degree of TILs in clinically invasive BC tissue was 12.60 ± 20.58%, ranging from 0 to 90%, with a median of 3%. TILs < 10% were considered low-level infiltration, and TILs ≥ 10% were considered high-level infiltration. Based on association analysis, the difference between CYB561expression and TILs infiltration was not statistically significant (χ2 = 1.68, P = 0.194, Table 2).
GSEA of CYB561
The results of single gene GSEA of CYB561 showed that the high CYB561 expression group was mainly enriched in Wnt/β-catenin signaling, peroxisomes, PI3K/ Akt /mTOR signaling, and other pathways (Fig. 6D).
Association between CYB561 expression and other clinicopathological characteristics in invasive BC
Based on the association analysis, the difference in CYB561 expression among different histological grades and molecular subtypes was statistically significant (χ2 = 6.62, P = 0.037; χ2 = 8.14, P = 0.043), as shown in Table 2. A low linear positive correlation was observed between CYB561 positivity and histological grade (Spearman’s rho = 0.17, P = 0.011).
CYB561 expression is correlated with decreased survival among BC patients
The follow-up period of 232 patients with BC ranged from 1 to 113 months, with a median time of 67 months. The 3 year and 5 year disease-free survival rates were 84.52% and 76.63%, respectively, and the overall survival rates were 87.77% and 79.88%, respectively. At the endpoint, 43 patients died, and 189 were alive. Kaplan–Meier survival analysis indicated that patients with positive CYB561 expression had significantly reduced overall survival compared to those who were negative (log-rank χ2 = 5.67, P = 0.017, Fig. 3F). Based on the univariate analysis using the Cox regression model, CYB561 expression [HR = 1.46, 95% CI (1.06–2.01); P = 0.020] was considered a strong prognostic factor for poor overall survival (Table 3). In multivariate analysis, CYB561 expression was not independent of lymph node metastasis, PR status, and nationality for overall survival (P = 0.088, Table 4). In addition, high infiltration of TILs (≥ 10%) was associated with longer disease-free survival (log-rank χ2 = 6.11, P = 0.013) and overall survival (log-rank χ2 = 4.47, P = 0.034) (Table 3).
Discussion
CYB561 is a single-copy protein-coding gene that is highly conserved throughout phylogeny. We found that CYB561 mRNA expression was increased in many malignant tumors and significantly elevated in BC subtypes. We confirmed CYB561 expression and cellular localization in breast tissue. We found that CYB561 expression in DCIS and invasive BC was higher than that in para-cancer tissue, suggesting that it may play an important role in the occurrence and development of BC. Therefore, we further analyzed the relationship between CYB561 and BC prognosis and discovered that CYB561 mRNA and protein expression were closely related to reduced overall survival, indicating that CYB561 may become an important prognostic biomarker for BC. To our knowledge, this is the first study to identify an association between CYB561 and BC prognosis.
We further investigated the relationship between CYB561 expression and the clinicopathological characteristics of BC. BC with HER2 overexpression has a high degree of malignancy, is prone to early recurrence and metastasis, and has a poor overall survival prognosis (Zeng et al. 2015). Most of the advanced HER2-positive BC patients were resistant to trastuzumab treatment within 1 year, and the patients who survived for more than 5 years accounted for only 25–30% (Yardley et al. 2014). Therefore, it is of great significance to explore the factors influencing poor prognosis in BC patients with HER2 positivity for the screening and diagnosis of patients at high risk of recurrence. Our clinical cohort showed that the positive expression of CYB561 was positively correlated with HER2 expression. We showed that CYB561 expression was increased in HER2-positive BC, correlated with HER2 and molecular subtypes, and closely associated with the prognosis of HER2-positive BC. Unfortunately, there was no correlation between CYB561 expression and the prognosis of HER2-positive BC in the clinical cohort, which may be related to the number of specimens. Therefore, the correlation between CYB561 and HER2-positive BC and its specific mechanism deserves further study.
In addition, we observed that CYB561 expression in clinically invasive breast carcinoma specimens was positively correlated with histological grade. In other words, the higher the histological grade, the higher the expression of CYB561. Histological grading reflects the degree of tumor cell differentiation and is a clinicopathological factor associated with poor prognosis. This finding implied that the measurement of CYB561 could provide additional prognostic information and further reflects that CYB561 is related to cell proliferation and differentiation.
To further study the possible mechanism of action of CYB561 in BC, we investigated its relationship with cell metabolism, proliferation, iron metabolism, and TILs. CYB561 members are involved in cell metabolism and mitochondrial activation (Polak et al. 2014). They can affect heart rate and blood pressure by regulating the adrenaline pathway and participating in iron uptake, ascorbic acid regeneration of neuroendocrine tissue, and tumor growth. A pathogenic homozygous mutation of the CYB561 gene can stop norepinephrine synthesis and cause a new autosomal recessive postural hypotension syndrome (van den Berg et al. 2018). Notably, CYB561 expression was stronger in breast hyperplasia tissue and DCIS, and its expression was higher than that in invasive carcinoma. The performance of CYB561 was similar to that of HER2. This finding indicated that CYB561 is closely related to the proliferation of mammary ductal epithelial cells. This difference may be related to different genetic manifestations at various stages of BC development.
As shown above, the association between CYB561 and HER2 is very close. HER2 amplification can promote cell proliferation, protein synthesis, apoptosis resistance, and cell cycle progression, promoting tumor initiation and progression. Both CYB561 and HER2 are located on human chromosome 17q, which may affect each other and amplify simultaneously. In addition, CYB561 and HER2 may interact and influence each other through their joint participation in the PI3K/Akt/mTOR signaling pathway. We found that CYB561 expression was enriched in the PI3K/Akt/mTOR signaling pathway. Over-activated gene changes in the PI3K/Akt/mTOR signaling pathway occur in approximately 60% of BC cases (Engelman 2009). Activation of this signaling pathway is also one of the causes of anti-HER2 drug resistance (André et al. 2016). Hence, further studies are needed to explore the possible modes of action of CYB561 and HER2 in this signaling pathway.
Iron plays an important role in mammalian cell replication, metabolism, and growth (Crichton 2016) and can produce important iron-containing enzymes (including mitochondrial enzymes and DNA synthase involved in the respiratory complex) and blood-containing enzymes (peroxidase and catalase). As a potentially toxic substance, iron is also involved in cancer, participating in free radical generation reactions by acquiring and losing electrons via the Fenton reaction. The reaction between ferrous iron and H2O2 produces hydroxyl radical reactive oxygen species (Akatsuka et al. 2012). The CYB561 family has an electron transfer function owing to its unique molecular structure. Dcytb can reduce trivalent iron in the diet to ferrous iron, allowing iron to be absorbed by divalent metal transporter 1 (DMT1) and mediating the reduction and absorption of duodenal dietary iron (Latunde-Dada et al. 2002; Wyman et al. 2008). Therefore, we speculated that CYB561 in mammary epithelial cells may also be involved in iron metabolism owing to its similar molecular structure, thus participating in the proliferation of mammary cells and the occurrence of BC. CYB561 may be one of the initiating factors of BC, or it may be a passive amplification caused by the excessive proliferation of BC cells. Thus, an increase in iron ions is required, resulting in the passive enhancement of CYB561 mRNA synthesis and protein translation. As shown in our experiment, CYB561 expression was increased in breast hyperplasia and DCIS, and the expression level of CYB561 in BC was higher than that in adjacent cancer tissues. The specific mechanism is worth exploring further.
TILs are the main components of the tumor immune microenvironment and are closely related to BC prognosis and chemotherapy efficacy. The TIL content was significantly correlated with the histological grade of HER2-positive BC (Raphael et al. 2016). TILs are a positive prognostic marker of TNBC, and the prognostic value of TILs in TNBC was considered grade I evidence (Simon et al. 2009). The online data analysis showed that CYB561 was correlated with CD4+ T lymphocytes, neutrophils, and dendritic cells. Among them, the expression of CYB561mRNA and gene copy number were most related to CD4+ T lymphocytes, which promoted tumor growth (Dushyanthen et al. 2015). Our clinical cohort analysis confirmed a correlation between TILs and BC prognosis. However, there was no correlation between the expression of CYB561 and the degree of TIL infiltration in invasive BC. Given the inconsistency between online data and clinical cohorts, more clinical data are required for further study.
Conclusions
This study showed CYB561mRNA and protein were upregulated in BC tissues and associated with ERBB2/HER2, immune cells, histological grade, and molecular subtypes. CYB561 expression is associated with poor prognosis in patients with BC and could be used as a clinical biomarker to predict prognosis.
Data availability
Three public datasets were obtained from TIMER (https://cistrome.ShinYapps.io/timer/), GEPIA 2(http://gepia2.cancer-pkucn/), and Kaplan–Meier Plotter websites (https://kmplot.com/analysis/).
References
Akatsuka S, Yamashita Y, Ohara H et al (2012) Fenton reaction induced cancer in wild type rats recapitulates genomic alterations observed in human cancer. PLoS One 7:e43403. https://doi.org/10.1371/journal.pone.0043403
André F, Hurvitz S, Fasolo A et al (2016) Molecular alterations and everolimus efficacy in human epidermal growth factor receptor 2-overexpressing metastatic breast cancers: combined exploratory biomarker analysis from BOLERO-1 and BOLERO-3. J Clin Oncol 34:2115–2124. https://doi.org/10.1200/JCO.2015.63.9161
Asard H, Barbaro R, Trost P et al (2013) Cytochromes b561: ascorbate-mediated trans-membrane electron transport. Antioxid Redox Signal 19:1026–1035. https://doi.org/10.1089/ars.2012.5065
Balmus G, Pilger D, Coates J et al (2019) ATM orchestrates the DNA-damage response to counter toxic non-homologous end-joining at broken replication forks. Nat Commun 10:87. https://doi.org/10.1038/s41467-018-07729-2
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. https://doi.org/10.3322/caac.21492
Crichton R (2016) Iron metabolism: from molecular mechanisms to clinical consequences, 4th edn. Wiley. https://doi.org/10.1002/9781118925645
Dushyanthen S, Beavis PA, Savas P et al (2015) Relevance of tumor-infiltrating lymphocytes in breast cancer. BMC Med 13:202. https://doi.org/10.1186/s12916-015-0431-3
Engelman JA (2009) Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer 9:550–962. https://doi.org/10.1038/nrc2664
Ghoncheh M, Mohammadian-Hafshejani A, Salehiniya H (2015) Incidence and mortality of breast cancer and their relationship to development in Asia. Asian Pac J Cancer Prev 16:6081–6087. https://doi.org/10.7314/apjcp.2015.16.14.6081
Gobbini E, Ezzalfani M, Dieras V et al (2018) Time trends of overall survival among metastatic breast cancer patients in the real-life ESME cohort. Eur J Cancer 96:17–24. https://doi.org/10.1016/j.ejca.2018.03.015
Huo D, Hu H, Rhie SK et al (2017) Comparison of breast cancer molecular features and survival by African and European ancestry in the cancer genome atlas. JAMA Oncol 3:1654–1662. https://doi.org/10.1001/jamaoncol.2017.0595
Latunde-Dada GO, Van der Westhuizen J, Vulpe CD et al (2002) Molecular and functional roles of duodenal cytochrome B (Dcytb) in iron metabolism. Blood Cells Mol Dis 29:356–360. https://doi.org/10.1006/bcmd.2002.0574
Lemler DJ, Lynch ML, Tesfay L et al (2017) DCYTB is a predictor of outcome in breast cancer that functions via iron-independent mechanisms. Breast Cancer Res 19:25. https://doi.org/10.1186/s13058-017-0814-9
Li TW, Fan JY, Wang BB et al (2017) TIMER: a web server for comprehensive analysis of tumor-infiltrating immune cells. Can Res 77:e108–e110. https://doi.org/10.1158/0008-5472.CAN-17-0307
Liang Y, Zhang H, Song X et al (2020) Metastatic heterogeneity of breast cancer: molecular mechanism and potential therapeutic targets. Semin Cancer Biol 60:14–27. https://doi.org/10.1016/j.semcancer.2019.08.012
Mahmood SF, Gruel N, Chapeaublanc E et al (2014) A siRNA screen identifies RAD21, EIF3H, CHRAC1 and TANC2 as driver genes within the 8q23, 8q24.3 and 17q23 amplicons in breast cancer with effects on cell growth, survival and transformation. Carcinogenesis 35:670–682. https://doi.org/10.1093/carcin/bgt351
Ohtani S, Iwamaru A, Deng W et al (2007) Tumor suppressor 101F6 and ascorbate synergistically and selectively inhibit non-small cell lung cancer growth by caspase-independent apoptosis and autophagy. Cancer Res 67:6293–6303. https://doi.org/10.1158/0008-5472.CAN-06-3884
Olarte KCV, Bagamasbad PD (2020) SAT-132 the secretory vesicle membrane protein, CYB561, promotes the growth and metastatic potential of castration-resistant neuroendocrine prostate cancer. J Endocr Soc. https://doi.org/10.1210/jendso/bvaa046.1194
Polak ME, Thirdborough SM, Ung CY et al (2014) Distinct molecular signature of human skin langerhans cells denotes critical differences in cutaneous dendritic cell immune regulation. J Investig Dermatol 134:695–703. https://doi.org/10.1038/jid.2013.375
Pruss RM, Shepard EA (1987) Cytochrome b561 can be detected in many neuroendocrine tissues using a specific monoclonal antibody. Neuroscience 22:149–157. https://doi.org/10.1016/0306-4522(87)90205-3
Raphael J, Gong IY, Nofech-Mozes S et al (2016) Tumour infiltrating lymphocytes and stromal CD68 in early stage HER2 positive breast cancer. J Clin Pathol 69:552–555. https://doi.org/10.1136/jclinpath-2015-203493
Rimm DL, Camp RL, Charette LA et al (2001) Tissue microarray: a new technology for amplification of tissue resources. Cancer J 7:24–31
Salgado R, Denkert C, Demaria S et al (2015) International TILs working group 2014. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs working group 2014. Ann Oncol 26:259–271. https://doi.org/10.1093/annonc/mdu450
Simon RM, Paik S, Hayes DF (2009) Use of archived specimens in evaluation of prognostic and predictive biomarkers. J Natl Cancer Inst 101:1446–1452. https://doi.org/10.1093/jnci/djp335
Srivastava M, Gibson KR, Pollard HB et al (1994) Human cytochrome b561: a revised hypothesis for conformation in membranes which reconciles sequence and functional information. Biochem J 303(Pt 3):915–921. https://doi.org/10.1042/bj3030915
Sui S, An X, Xu C et al (2020) An immune cell infiltration-based immune score model predicts prognosis and chemotherapy effects in breast cancer. Theranostics 10:11938–11949. https://doi.org/10.7150/thno.49451
Tang Z, Li C, Kang B et al (2017) GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res 45:W98-102. https://doi.org/10.1093/nar/gkx247
Torre LA, Bray F, Siegel RL et al (2015) Global cancer statistics, 2012. Cancer J Clin 65:87–108. https://doi.org/10.3322/caac.21262
van den Berg MP, Almomani R, Biaggioni I et al (2018) Mutations in CYB561 causing a novel orthostatic hypotension syndrome. Circ Res 122:846–854. https://doi.org/10.1161/CIRCRESAHA.117.311949
Willis S, Villalobos VM, Gevaert O et al (2016) Single gene prognostic biomarkers in ovarian cancer: a meta-analysis. PLoS One 11:e0149183. https://doi.org/10.1371/journal.pone.0149183
Wyman S, Simpson RJ, McKie AT et al (2008) Dcytb (Cybrd1) functions as both a ferric and a cupric reductase in vitro. FEBS Lett 582:1901–1906. https://doi.org/10.1016/j.febslet.2008.05.010
Yardley DA, Tripathy D, Brufsky AM et al (2014) Long-term survivor characteristics in HER2-positive metastatic breast cancer from regist HER. Br J Cancer 110:2756–2764. https://doi.org/10.1038/bjc.2014.174
Zeng H, Zheng R, Guo Y et al (2015) Cancer survival in China, 2003–2005: a population based study. Int J Cancer 136:1921–1930. https://doi.org/10.1002/ijc.29227
Acknowledgements
We would like to thank Project of Basic Research for application of Science and Technology Department of Qinghai Province for its funding support and Editage (www.editage.cn) for English language editing.
Funding
This work was supported by Project of Basic Research for application of Science and Technology Department of Qinghai Province (2019-ZJ-7003).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by [XZ], [GS], [DR], [XG], [JH] and [JZ]. The first draft of the manuscript was written by [XZ] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This article was approved by the ethics committee of Affiliated Hospital of Qinghai University.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
Written informed consent was obtained from all participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhou, X., Shen, G., Ren, D. et al. Expression and clinical prognostic value of CYB561 in breast cancer. J Cancer Res Clin Oncol 148, 1879–1892 (2022). https://doi.org/10.1007/s00432-022-03928-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-022-03928-z