Abstract
Background
Exosomes are extracellular nanometric vesicles used by cells to communicate with each other. They are responsible for many pathological conditions, including tumors by transferring regulatory biomolecules that impact target cell activity. Because of their high concentration in exosomes compared with parental cells and the rest of exosomal content, specificity to the cell of origin, and their well-organized sorting mechanism, microRNAs (miRNAs) are thought to be the most potent exosomes cargo and used by scientists to track exosomes and to detect cell activity changes and prognosis in cancer early.
Purpose
In this review, the results of studies examining the role of exosomes in cancer pathophysiology and their clinical potential are discussed in detail.
Summary of the Findings
Tumor-derived exosomes (TDEs) mediate the dynamic changes of cancer growth and invasion, including local microenvironment remodeling, distance metastasis, angiogenesis, and tumor-associated immunosuppression. They also contribute to hypoxia-induced tumor progression and cancer cell drug resistance. As a result of exosomes being present in all body fluids, it is possible to have early accessible and less-invasive diagnostic and prognostic measures by forming a table for each cancer type and its matched specific miRNAs. Under testing, available therapeutic uses of exosomes include interference of exosomes biogenesis, secretion, or uptake, and recruitment of exosomes as target-specific drug delivery vehicles, and immunostimulatory agents for both cancer patients and healthy population to avoid cancer development from the start.
Conclusion
These data suggest that exosomes and exosomal microRNA are directly related to cancer progression mechanisms, and could be used in cancer early diagnosis, prognosis, and therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malignancies are complex structures that are formed of cancer cells and the surrounding tumor stroma (Sund and Kalluri 2009). Effective tumor growth and systemic dissemination entail a continuous cross-talk between cancer cells and the local or distant host environment (Kalluri and Zeisberg 2006).
The exosome is a member of the extracellular vesicles and it is a recent popular research object in the tumor research study. Exosomes are vesicles derived from a multivesicular body (MVB) with a diameter of 30–150 nm, which contain lipids, proteins, and nucleic acids. Since exosomes had been discovered for the first time in 1983 in serum, they were seen as transportation for cellular waste with little potential research values (Balaj et al. 2011; Valadi et al. 2007).
These vesicles with lipid bilayer are found to contain coding and non-coding RNA including long non-coding RNA (lncRNA), microRNAs (miRNAs), messenger RNAs (mRNA), DNA fragments, and proteins (Tang et al. 2020).
It is well documented that exosomal miRNAs are the effective component of exosomes by which cancer cells influence nearby and distant cells, causing cell proliferation, differentiation and migration, and disease initiation and progression (Zhang et al. 2015).
Tumor-derived exosome (TDE) can be captured efficiently with a wide variety of novel isolation techniques. TDE reflects ample oncogenic information. Interestingly, exosomes exist in serum, urine, saliva, or any other body fluids. TDE fuses with the cell membrane to exert pathological changes (Tang et al. 2020).
There is huge stress being placed on understating cancer mechanisms of action and improving cancer therapy with no focus on having a better understanding of cancer-related changes and having more effective diagnostic measures. As TDEs provide a better understanding of the behavior of cancer cells, and consequently the root of the problem, the need for TDEs’ studies is arising to have a complete picture of the mechanisms and tracking the dynamic changes associated with cancer in addition to a superior diagnostic and prognostic tool and tailored treatment with fewer side effects (Shepard 2017).
Exosomes’ potential biocompatibilities could help fight against cancers, suppressing tumor local and distant spread, detecting cancer early, inhibiting drug resistance, and treating different types of cancer (Tang et al. 2020).
The main contribution of this work is to shed light on the significant studies on exosomes and exosomal miRNA’s different roles in carcinogenesis and their clinical applications as a diagnostic and therapeutic target.
Biogenesis of exosomes
Exosomes from different cell types contain a core set of identical proteins including members of the tetraspanin family (CD9, CD63, CD81, and CD82), members of the ESCRT complex [tumor susceptibility gene 101(TSG101), ALG‐2-interacting protein X (Alix)], and heat shock proteins (Hsp 60, Hsp70, Hsp90) Fig. 1 (McAndrews and Kalluri 2019).
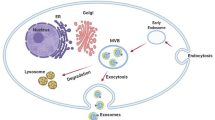
Exosomes are derived from the intracellular endosomal compartment Fig. 2, compared with larger microvesicles, which are a direct shedding from the plasma membrane. They are initially formed by inward budding from the limiting membrane of late endosomes through a ceramide-triggered process in the presence of sphingomyelinase (converts sphingomyelin to ceramide), encapsulating cytoplasmic RNA molecules and functional proteins into exosomes (Kahlert and Kalluri 2013).
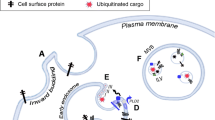
A concise illustration of exosomes biogenesis and secretion important steps. (1) Inward invagination of the cell wall mediated by either ESCRT complex with the help of ubiquitin (ubiquitinated ESCRT-dependent way) or ceramide-triggered inward budding (ESCRT-independent way) in the presence of CD63. (2) Early endosome is formed, carrying cell surface proteins. (3) RNAs and cytosolic proteins invade the endosome, forming small exosomes inside an MVB. (4) MVB may undergo degradation by lysosome for recycling its content. (5) Exosomes’ secretion is regulated by Rab 27 small GTPase
In a second step, the fusion of multivesicular endosomes with the cell plasma membrane releases exosomes into the extracellular space. Endosomal sorting complexes required for transport (ESCRT) are thought to be another mechanism of the inward budding and also responsible for the regulation of multivesicular endosome biogenesis, nevertheless, alternative pathways may also exist along with a ceramide-triggered way (Kahlert and Kalluri 2013). They recognize ubiquitinated membrane proteins and promote their internalization into the multivesicular endosome (Peinado et al. 2012).
Docking of MVBs at the cell membrane for the secretion of their content (exosomes) is regulated by Rab 27 small GTPase. The mechanism associated with the packaging of exosomes content remains elusive; however, a comparison of the exosomes composition with their parental cells indicates a selective enrichment process within the exosomes (Kahlert and Kalluri 2013).
Aside from these proteins, exosomes contain some specific proteins reflective of the parental cell. Examples include: epithelial cell adhesion molecule (ECAM) secreted with epithelial tumor cells-derived exosomes (Hessvik et al. 2021; Pavlyukov et al. 2018), melanoma-derived exosomes contain the tumor-associated antigen melanoma antigen recognized by T cells 1 (Mart-1), and cancer members of the human epidermal receptor (HER) family (which is expressed by gastric cancer, breast cancer, and pancreatic cancer secreted exosomes) (Chen et al. 2018; Zhao et al. 2016). Exosomes biogenesis and secretion include many well-organized steps (Figs. 2, 3, 4). Interruption of any of these steps could affect exosome-related physiological and pathological actions.
Exosomes uptake
Exosomes uptake relies on transmembrane proteins (Fig. 2). It is recently indicated that the tetraspanin–integrin complex is considerably responsible for targeting, allowing the binding of exosomes to focus on cells (Rana et al. 2012). Besides, the expression of receptor molecules like intercellular adhesion molecule (ICAM-1) on the membrane surface is enhanced by the pro-inflammatory environment, which facilitates exosomal adhesion to target cells (McAndrews and Kalluri 2019).
Despite the exosomal internalization mechanism is not completely clear, the presence of the T-cell receptor/CD3 complex and chemokine receptors on exosomes of T cells suggests juxtacrine signaling through receptor–ligand interactions Fig. 3a (Montecalvo et al. 2012).
Exosomes may also fuse with the cell membrane of target cells, which results in a direct release of their cargo into the cytoplasm Fig. 3b (Montecalvo et al. 2012). Another mechanism is phagocytosis in the actin cytoskeleton and phosphatidylinositol 3-kinase-dependent manner Fig. 3c (Du 2010).
Exosomal miRNA
Importance of exosomal miRNAs
After the exosome release in the extracellular environment, exosomal miRNAs can reach near and distant cells (Montecalvo et al. 2012). Although it is difficult to exclude the role of other exosomal cargo, miRNAs are the key functional element, facilitating a wide variety of changes in both the donor and the target cells (Montecalvo et al. 2012; Zhang et al. 2015; Zhou et al. 2014).
There are two mechanisms by which exosomal miRNAs carry their functions have been detected. First, the regular function is a negative regulation and conferring characteristic changes in expression levels of target genes Fig. 3 (Zhang et al. 2015). For instance, in many studies, exosomal miR-105 from breast cancer cells promoted metastases to the lung and brain (Zhou et al. 2014). Migration and angiogenesis in neighboring microvascular endothelial cell line (HMEC-1) cells were induced by exosomal miR-214 from (HMEC-1) (Zhang et al. 2015).
The second mechanism is a novel one identified in some miRNAs. Exosomal miRNAs were discovered to activate immune cells by acting like ligands that bind to Toll-like receptors (TLRs) (Hu et al. 2018).
The expression profile of microRNAs between exosomes and their parent cancer cells displays a different pattern in many cancers including bladder cancer and gastric cancer (Hessvik et al. 2021). Studies emphasize the assumption that it is not just a random event but a specific sorting of mRNAs and non-coding RNA into exosomes takes place. The specific packing of exosomes may occur in different body cells to discard tumor-suppressive microRNAs, thus increasing their oncogenicity (Peinado et al. 2012; Hessvik et al. 2021).
Exosomal miRNAs’ sorting mechanism
Recently, exosomal miRNA drew much attention due to their regulatory roles in gene expression. The proportion of miRNA is greater in exosomes than in primary cells compared with other exosome cargo (Goldie et al. 2014).
The incorporation of exosomal miRNA is not random. In a variety of cell lines and their respective derived exosomes, subsets of miRNAs (miR-150, miR-142-3p, and miR-451) are preferentially loaded in exosomes. Parent cells have a sorting mechanism, guiding specific intracellular miRNAs to enter exosomes (Hu et al. 2018). These features of exosome-related miRNAs made it the focus of the studies on cancer early diagnosis and treatment.
Tumor-derived exosomes and cancer development
Tumor microenvironment remodeling
The tumor microenvironment, which surrounds cancer cells, is abundantly filled with mesenchymal cells, such as fibroblasts, endothelial cells, and hematopoietic cells, both from the lymphoid and myeloid origin, within the extracellular matrix (ECM) which is important for cancer support (Pattabiraman and Weinberg 2014).
The presence of growth factors or the release of exosomes emphasized the bidirectional relationship between cancer and stromal cells. Cancer exosomes were found to modulate the surrounding cells which support tumor growth and dissemination. Different myofibroblast phenotypes and activated fibroblasts were found to be formed by tumor growth factor β (TGFβ) loaded in exosomes, showing the role of intercellular communication (Ruivo et al. 2017).
Stromal cells also communicate back with cancer cells, serving their growth and invasion. Activated fibroblasts are responsible for tumor progression by secreting growth factors, chemokines, and by the deposition of ECM constituents, easing tumor growth and invasion (Ruivo et al. 2017). Unregulated expression of IL6, which promotes the inflammatory reaction, was observed in vitro after treatment of cancer cells with tumor microenvironment exosomes (Baglio et al. 2017). In summary, the bidirectional exchange of exosomes between stromal and cancer cells provides a suitable environment for tumor progression.
Cancer metastasis
Multistep cancer metastasis is the commonest cause of cancer-related death (Milane et al. 2015). Remodeling of extracellular matrix architecture and reprogramming of the contributing cells in distant organ sites, such as bone marrow progenitor cells, cancer-associated fibroblast (CAF), tumor-associated macrophages (TAM), and tumor-associated neutrophils, toward establishing suitable premetastatic niches in advance of cancer metastasis was found to be induced by metastatic cell secreted exosomes (Tai et al. 2018).
Hart and Fidler elaborated Paget’s “seed and soil” hypothesis: the non-random pattern of cancer metastasis, observing the impact of metastatic colonization by the interaction with the target organ tumor microenvironment. However, regulations of organ-specific metastasis are still poorly interpreted. Until recently, it was discovered that TDEs help priming premetastatic niches, activating bone marrow-derived vascular endothelial growth factor receptor 1 (VEGFR1) and hematopoietic progenitor (Hoshino et al. 2015).
It was further indicated that integrin expression profiles of TDEs function as “ZIP codes”, leading to metastatic organotropism. Exosomal integrins α6β4 and α6β1 caused lung metastasis, while exosome integrin αvβ5 was correlated with liver metastasis. Additionally, upregulation of pro-inflammatory S100 genes in cells of target organs was found to be induced by TDEs, resulting in the establishment of premetastatic niches (Chowdhury et al. 2015).
Many studies have also elucidated that ECM remodeling enzymes such as matrix metallopeptidases (MMP2 or MMP9) are delivered by TDEs, causing degradation of ECM and contributing to cancer invasion and metastasis (Milane et al. 2015). Recruitment of bone marrow-derived macrophages is further induced by the remodeled microenvironment, encouraging premetastatic niche formation and providing suitable conditions for metastasis (Costa-Silva et al. 2015).
TDEs have a positive impact on tumor invasiveness, and consequently metastasis due to their contribution to invadopodia formation. Rab27a knockdown significantly decreases TDEs secretion, resulting in the suppression of mature invadopodia formation and digestion of the extracellular matrix. Interestingly, invadopodia are critical sites for exosome secretion, suggesting that exosomes synergistically regulate invasive activity (Re et al. 2017). Also, the change of gene expression facilitating metastasis within less metastatic cells occurs by miRNA transferred via TDEs from metastatic to less metastatic cells (Zhou et al. 2014).
Recent studies have shown that metastatic cancer cells can invade the central nervous system (CNS) despite the presence of the blood–brain barrier (BBB) as exosomes derived from metastatic cancer cells disrupt the structure and function of the BBB (Zhou et al. 2014; Banks et al. 2020; Lu et al. 2020). Vascular endothelial cells take up miRNAs such as miR-105 and miR-181c within cancer exosomes and destruct vascular endothelial barriers. They target the tight junction proteins and induce abnormal cytoskeleton localization; thus, metastatic cells could reach the brain. Some exosomes showed the ability to cross BBB, namely endothelial cells of brain–blood vessel-derived exosomes (Zhou et al. 2014; Lu et al. 2020).
Together, these studies explain how exosomes mediate metastasis by inducing vascular basement membrane and local destructive pathways to invade nearby blood vessels and stimulate new vessels formation. A premetastatic niche, which is a microenvironment remodeling in a secondary organ, is a well-organized organotropic process regulated by TDEs that carry specific integrins subtypes that selectively direct the site of the distant metastasis.
Effect of hypoxia, angiogenesis, and immunosuppression
Hypoxia
Hypoxia is associated with glycolysis increment, leading to lactate accumulation in the EC microenvironment and decrement of the pH. Physiological acidic pH (6.5–6.9) showed an increment in exosome secretion and their cargo (Casazza et al. 2014). Therefore, proton pump inhibition and alkaline pH cause exosome secretion and exosomal cargo reduction. Additionally, acidic pH alters integrin activation which is the main regulator for exosome uptake. However, storage in extreme acidic solutions (pH < 4.0) leads to exosomal protein degradation (Sahebi et al. 2020). Thus, hypoxia exaggerates cancer development induced by TDEs.
Studies have demonstrated that, by increasing exosomes secretion, hypoxia prompts HIF‐1α (an induced factor in hypoxia conditions) activity, producing aggressive cell phenotype. In another study, in malignant glioma, it was seen that hypoxia promoted angiogenic pathways by secreting exosomes, created a suitable tumor growth microenvironment, and facilitated cancer metastasis as a result (Svensson et al. 2011).
Angiogenesis
Angiogenesis is crucial for the progression of cancer, tumor growth, and metastasis and also essential for cancer progression (Mashreghi et al. 2018). TDEs contain vascular endothelial growth factor (VEGF) and interleukin-6 (IL6), which cause proliferation, migration, and increased permeability of endothelial cells. Hypoxia increases the secretion of exosomes, prompting the process of angiogenesis (Goradel et al. 2019). Normal endothelial cells catch tumor-derived exosomes proteins, stimulating their migration and new vessel formation that nourish cancer cells (Huang and Deng 2019).
Immunosuppression
Myeloid-derived suppressor cells (MDSCs) regulate the immunosuppressive microenvironment and assist the tumor to escape immune response (mainly by inhibiting T-cell activation). A study of glioma TDEs showed that exosomal miR-29a and miR-92a prompted differentiation of MDSCs. Hypoxia aggravates exosomes related to MDSCs activation by increasing exosome secretion and new blood vessel formation (Guo et al. 2019).
Overexpression of heat shock protein (HSP) on exosomes activates MDSCs via the Toll‐like receptor 2 (TLR2) (Guo et al. 2019). After examining various samples, exosomes showed increased HSP70 expression to activate MDSCs via HSP70/TLR2 in breast cancer, lung cancer, and ovarian cancer (Li et al. 2019).
Displaying programmed death-ligand 1 (PD-L1), TDEs facilitate circulating cancer cells to escape immunity. Exosomal PD-L1 triggered silence of T-cell activation, while antitumor immune response induction and remissions were achieved by exosomal PD-L1 blockade in tumor patients (Poggio et al. 2019). Via miR-23a, endoplasmic reticulum stress in hepatic cell carcinoma (HCC) urges secretion of exosomes activating PD‐L1 in macrophages, and as a result, suppressing T-cell function. Indirect T-cell apoptosis could occur by TDEs carrying Fas ligand (Liu et al. 2019). These findings could help to clarify cancer-related immunosuppression.
However, major histocompatibility complex class I and class II molecules are expressed by dendritic cell-derived exosomes along with T-cell co-stimulatory molecules for antigen presentation. B-cell-derived exosomes present tumor antigens to macrophages which are responsible for T-cell priming (Li et al. 2019), emphasizing that exosomes are involved in both immunostimulation and immunosuppression, but the immunosuppressive outweighs the immunostimulatory effect.
Collectively, these findings suggest the importance of exosomes for tumor growth and invasion of the surrounding and distant cells by manipulating both the local and systemic environments.
Drug resistance
It is well known that cancer cells can mask the cytotoxic and cytostatic effects of cancer therapeutic drugs by various mechanisms. One of them is by the ATP-binding cartridge membrane protein which removes toxic materials out of the cell (Avan et al. 2018). Other causes include the CYP gene family genetic variation among cancer cells as it gives different cytochrome P450 activity. As a result, drugs are effectively metabolized with low-to-almost no effect. Moreover, repair of DNA damage is considered efficient in many tumors (Rasouli et al. 2018).
Recent studies have demonstrated that exosomes play a key role in drug resistance by which cancer cells escape their detrimental effect (Sany et al. 2015; Ciravolo et al. 2012; Xiao et al. 2014). That was confirmed by studying the effect of cisplatin on ovarian cancer cells. It was noted that chemotherapy injection to the tumor cells increased their secretion rate of exosomes which are loaded with the drug, releasing it outside the cell. They attracted the drug toward them, as well (Sany et al. 2015).
It is confirmed that drugs bind to proteins expressed on exosomes derived from cancer cells, limiting the effect of the therapy. On the other hand, target cells show increased proliferation when they take up human epithelial receptor (HER) positive exosomes. It is also proved that these exosomes tend to be more enriched with the therapeutic drug in advanced stages compared with early stages (Ciravolo et al. 2012).
Another detailed study was done by Xiao et al. to demonstrate the role of exosomes in drug resistance to cisplatin in the lung cancer cell line (A549). Untreated cells secrete fewer exosomes compared with the treated ones. An increase in survival and proliferation was documented when examining the effect of exosomes containing cisplatin on the target cells and the primary cancer cells exposed to the drug only (Xiao et al. 2014).
MiRNAs and DNA repair gene expression related to drug resistance are prompted in response to cisplatin injection. A study conducted by Chen et al. revealed that breast cancer cells do transmit drug resistance to their cell population via exosome transmission of gene expression modulating miRNAs, in addition to the reduction of apoptosis in sensitive tumor cells (Chen et al. 2014). Mechanisms by which exosomes could help cancer cells resist therapy are summarized in Fig. 4.
Summary of mechanisms of therapy resistance induced by exosomes. a Cancer cell with normal exosomes secretion rate. b Adaptive mechanisms by which cancer cell escapes the therapeutic effect. b1 Drugs attack exosomes expressing target proteins, leaving the cancer cell. b2 Increased rate of exosomes secretion loaded with the therapy. b3 Increased rate of exosomes secretion loaded with regulatory proteins and RNAs responsible for the inheritance of drug resistance techniques
Tumor-derived exosomes and exosomal miRNA clinical potential
Tumor markers for early cancer diagnosis and prognosis
Exosomes are stable at 4 °C for 4 days and at − 70 °C for a longer time, qualifying them to be suitable biomarkers for early cancer diagnosis (Sahebi et al. 2020).
MiRNAs are the most characteristic, potent, and effective protein of exosomal cargo, known to play many physiological processes such as cell differentiation, cell division, apoptosis, and morphogenesis of various organs. At present, tissue-specific panels for miRNA exosomes are being prepared which are important for the prognosis, diagnosis, and evaluation of cancer aggressiveness and treatment response Table 1 (McAndrews and Kalluri 2019).
Early detection of malignant tumors could be achieved with more than 270 exosomal miRNA have been identified, and with time, a complete panel, which includes almost all types of cancers, can be formed (Tamkovich et al. 2016).
CircRNA and lncRNA are stable exosomal proteins that are found to have a role in cancer development and can also be included as a tumor biomarker. It was discovered that bladder cancer TDEs loaded with long non-coding RNA‐UCA1 influenced tumor microenvironment remodeling. That is why, it is proposed that exosomal long non-coding RNAs‐UCA1 could be used in future studies (Fani et al. 2019).
Unfortunately, there are some limitations to the current studies of exosomal miRNAs. First, diversity of exosome isolation methods as they can be enriched from cell culture media by ultracentrifugation, density gradient separation, immunoaffinity capture, size exclusion chromatography, and ExoQuick™ Precipitation (System Biosciences, USA). Different strategies partially affect exosomal contents assessing accuracy, including miRNAs (Rezayi et al. 2018).
Second, different signaling pathways are carried out by a large number of exosomal miRNAs, generating integral impacts on the target cells, and thus, it is difficult to have a complete understanding of their function. However, portions of their sequences are currently used for the classification, and the functions of each group can be distinguished separately. Third, in the rare cases of the low abundance of miRNA exosomes, it is complicated to get a satisfying result. More sophisticated techniques and methods development could overcome most of these hurdles (Ikoma et al. 2018).
Tumor exosomes as therapeutic targets
Interference with exosomes biogenesis and uptake
TDE cargoes are the first attributor to cancer development. Therefore, therapeutic strategies, such as blockage of exosome production, secretion, and exosome-mediated cell–cell communication and ablation of specific active exosomal cargos, are suggested as novel cancer treatments Table 2 (Tai et al. 2018). ESCRT-dependent (such as syndecan/syntenin/ALIX signaling) or ESCRT-independent (like sphingomyelinases) mechanism-mediated exosome biogenesis inhibition showed significant suppression of cancer invasion and progression. Rab27, which controls MVB size and exosomes secretion, greatly stopped both cancer proliferation and metastasis. Knockdown of Rab27a and Rab27b resulted in the accumulation of tumor-suppressive miRNA within bladder cancer cells, preventing further tumor growth (Tian et al. 2014).
Specific glycosylation patterns are displayed by surface proteins of TDE which are responsible for exosome uptake regulation. Alteration of exosomal proteins glycosylation could be effective repression of cancer progression (Tian et al. 2014). In recent studies, extracorporeal hemofiltration of exosomes from the circulation by an affinity plasmapheresis platform to remove exosomes from the circulation is an additional suggested therapeutic strategy (Marleau et al. 2012).
One of the causes of increased exosomes secretion is the increased intracellular calcium concentration. Therefore, blocking the function of Na+/H+ and Na+/Ca+2 channels by D-methyl amyloid reduces exosomes release efficiently in CT26 mice in which colon cancer was induced (Xu et al. 2015). Sphingomyelinase 2 inhibitor (GW4869) was found to suppress lung cancer progression and metastasis in mice due to the reduction of TDEs’ formation (Le et al. 2017).
Immunotherapy
Studies showed that exosomes loaded with interferon-γ from dendritic cells facilitate anticancer immune responses in NK and T cells. That explains the abnormal increase in NK-cell activity and survival rate in advanced tumors (Tai et al. 2018). In other studies, novel cell-free vaccines could be provided using exosomes in immunotherapy. Autologous dendritic cell-derived exosomes enriched with cancer antigens induce anticancer immune responses, such as induced natural killer (NK) cell effector functions (Besse et al. 2016).
Cultivation of immunostimulatory exosomes secreting cells, such as dendritic cells, is a new way to tackle cancer progression Table 2. These exosomes can be concentrated and injected into cancer patients to reactivate their immunity or to act as cell-free vaccines if injected into a healthy population, preventing cancer growth from the very beginning as a result.
Therapeutic agent delivery
Similar to liposomes, attention is growing toward the usage of the naturally secreted exosomes as drug delivery vehicles. However, exosomes represent many features that make them the most effective drug delivery vehicle for the delivery of anticancer drugs and selected small interfering RNAs (siRNAs) and miRNAs, responsible for tumor suppression (Ha et al. 2016; Hoshino et al. 2015). First, the size of exosomes is suitable for their transfer between cells. Second, the structure of exosomes including the lipid bilayer of the cell membrane provides a protective medium and stability for exosomal bioactive cargoes. Third, compared with other drug delivery strategies, exosomes show low immunogenicity and toxicity (Ha et al. 2016). Fourth, exosomes are selective as they target specific cells according to specific surface proteins, such as integrins, making it possible to attack specific cells of an organ (Hoshino et al. 2015).
These findings indicate that, despite staying for a short period in circulation compared with the PEGlyted liposomes, exosomes are better used as a drug delivery vehicle because of their high organotropic properties and being natural with low-to-almost no immune stimulation.
Surprisingly, it is proven that exosomes have the potential to cross BBB. That is why, exosomes are the first suspicious to be the cause of brain metastasis. Knowing that, exosomes loaded with anticancer drugs from autologous cancers were injected into these patients led to increased cytotoxicity in parental cells (Saari et al. 2015). The use of exosomes as a therapeutic vehicle could make it possible to avoid all threats of brain surgeries. Drug delivery using nasal sprays containing therapeutic exosomes may draw the future for non-invasive strategies for brain-related pathologies (Kang et al. 2019).
Regarding selectivity and targeting specificity, αv integrin-specific RGD (Arg–Gly–Asp) peptide was engineered on exosomes enriched with an anticancer drug (doxorubicin) to preferentially improve the uptake of exosomes by αv integrin-positive cancer cells, selectively inhibiting cancer cells function with no apparent effect on the healthy ones (Saari et al. 2015). This suggests that, with the addition of synthetic RGD peptide to increase exosomes selectivity, exosomes could be efficient therapeutic agent delivery vehicles Fig. 5.
Different modalities of the use of exosomes for therapeutic agent delivery. Introduction of regulatory proteins, RNAs (miRNAs and siRNAs), and hormonal/chemotherapy into exosomes for target therapy, increasing therapy efficacy and decreasing its systemic side effects. Synthetic RGD is added for better organotropism
Conclusion
TDEs are the main contributors to the pathophysiological changes which facilitate cancer progression. The bidirectional exchange of exosomes gives rise to local microenvironmental changes important for tumor support and favors its growth. Exosomes perform a crucial role for metastasis main steps; local ECM and nearby blood vessels basement membrane destruction (by transferring matrix metallopeptidases), angiogenesis (by carrying VEGF, IL6, and miRNAs), and premetastatic niche formation (by recruitment of bone marrow progenitor cells and upregulation of expression of inflammatory genes). Sites of premetastatic niches are predetermined according to integrin subtypes expressed on exosomes surface. Because of its ability to cross BBB or to cause its destruction, exosomes can mediate CNS invasion. Hypoxia, which is often encountered in tumors, promotes and triggers the secretion of exosomes.
After the exposure to the chemotherapeutic and hormonal drugs, an increase in the rate of exosomes secretion loaded with target proteins and the drugs out of the cancer cell is always detected. Inheritance of resistance takes place by the transfer of biomolecules regulating resistance mechanisms.
The use of exosomes as a biomarker could be an effective and less-invasive method to diagnose and get information about the prognosis of tumors simply by tracking them in various body fluids. TDEs can be a therapeutic target by suppressing their activity, interfering with their formation and secretion, or by changing their properties to interrupt their uptake; thus, tumor progression can be put under control. By harnessing immunostimulatory exosomes, immunotherapy might help to boost immune response in cancer patients and can be used as novel cell-free vaccines in cancer-free people.
Compared with liposomes, employing exosomes as a vehicle for drug delivery is proved to be an efficient strategy because of the natural characteristics of exosomes, having low immunogenicity, in addition to their ability to reach specific target cells at any site in the body, including the brain. In the future, nasal sprays containing therapeutic exosomes could be used to reach the brain with no need for invasive surgeries. Unresolved issues should be the focus of future studies, such as sensitive unified methods for exosomes and exosomal miRNA purification, separation, and categorization.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Avan A, Tavakoly Sany SB, Ghayour-Mobarhan M, Rahimi HR, Tajfard M, Ferns G (2018) Serum C-reactive protein in the prediction of cardiovascular diseases: overview of the latest clinical studies and public health practice. J Cell Physiol 233(11):8508–8525
Baglio SR, Lagerweij T, Pérez-Lanzón M, Ho XD, Léveillé N, Melo SA, Cleton-Jansen AM, Jordanova ES, Roncuzzi L, Greco M, Van Eijndhoven MA (2017) Blocking tumor-educated MSC paracrine activity halts osteosarcoma progression. Clin Cancer Res 23(14):3721–3733
Balaj L, Lessard R, Dai L, Cho YJ, Pomeroy SL, Breakefield XO, Skog J (2011) Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat Commun 2(1):1–9
Banks WA, Sharma P, Bullock KM, Hansen KM, Ludwig N, Whiteside TL (2020) Transport of extracellular vesicles across the blood-brain barrier: brain pharmacokinetics and effects of inflammation. Int J Mol Sci 21(12):4407
Besse B, Charrier M, Lapierre V, Dansin E, Lantz O, Planchard D, Le Chevalier T, Livartoski A, Barlesi F, Laplanche A, Ploix S (2016) Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology 5(4):e1071008
Casazza A, Di Conza G, Wenes M, Finisguerra V, Deschoemaeker S, Mazzone M (2014) Tumor stroma: a complexity dictated by the hypoxic tumor microenvironment. Oncogene 33(14):1743–1754
Chen WX, Liu XM, Lv MM, Chen L, Zhao JH, Zhong SL, Ji MH, Hu Q, Luo Z, Wu JZ, Tang JH (2014) Exosomes from drug-resistant breast cancer cells transmit chemoresistance by a horizontal transfer of microRNAs. PLoS ONE 9(4):e95240
Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, Yu Z, Yang J, Wang B, Sun H, Xia H (2018) Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 560(7718):382–386
Chowdhury R, Webber JP, Gurney M, Mason MD, Tabi Z, Clayton A (2015) Cancer exosomes trigger mesenchymal stem cell differentiation into pro-angiogenic and pro-invasive myofibroblasts. Oncotarget 6(2):715
Ciravolo V, Huber V, Ghedini GC, Venturelli E, Bianchi F, Campiglio M, Morelli D, Villa A, Mina PD, Menard S, Filipazzi P (2012) Potential role of HER2-overexpressing exosomes in countering trastuzumab-based therapy. J Cell Physiol 227(2):658–667
Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, Xiang J (2015) Pancreatic cancer exosomes initiate premetastatic niche formation in the liver. Nat Cell Biol 17(6):816–826
Del Re M, Biasco E, Crucitta S, Derosa L, Rofi E, Orlandini C, Miccoli M, Galli L, Falcone A, Jenster GW, van Schaik RH (2017) The detection of androgen receptor splice variant 7 in plasma-derived exosomal RNA strongly predicts resistance to hormonal therapy in metastatic prostate cancer patients. Eur Urol 71(4):680–687
Du FENG et al (2010) Cellular internalization of exosomes occurs through phagocytosis. Traffic 11.5:675–687
Fani M, Rezayi M, Meshkat Z, Rezaee SA, Makvandi M, Abouzari-Lotf E, Ferns GA (2019) Current approaches for detection of human T-lymphotropic virus Type 1: a systematic review. J Cell Physiol 234(8):12433–12441
Goldie BJ, Dun MD, Lin M, Smith ND, Verrills NM, Dayas CV, Cairns MJ (2014) Activity-associated miRNA are packaged in Map1b-enriched exosomes released from depolarized neurons. Nucleic Acids Res 42(14):9195–9208
Goradel NH, Mohammadi N, Haghi-Aminjan H, Farhood B, Negahdari B, Sahebkar A (2019) Regulation of tumor angiogenesis by microRNAs: state of the art. J Cell Physiol 234(2):1099–1110
Guo X, Qiu W, Wang J, Liu Q, Qian M, Wang S, Zhang Z, Gao X, Chen Z, Guo Q, Xu J (2019) Glioma exosomes mediate the expansion and function of myeloid-derived suppressor cells through microRNA-29a/Hbp1 and microRNA-92a/Prkar1a pathways. Int J Cancer 144(12):3111–3126
Ha D, Yang N, Nadithe V (2016) Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharmaceutica Sinica B 6(4):287–296
Hessvik NP, Phuyal S, Brech A, Sandvig K, Llorente A (2021) Profiling of microRNAs in exosomes released from PC-3 prostate cancer cells. Biochimica et Biophysica Acta (BBA) 1819(11–12):1154–1163
Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Mark MT, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S (2015) Tumour exosome integrins determine organotropic metastasis. Nature 527(7578):329–335
Hu Y, Rao SS, Wang ZX, Cao J, Tan YJ, Luo J, Li HM, Zhang WS, Chen CY, Xie H (2018) Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics 8(1):169
Huang T, Deng CX (2019) Current progresses of exosomes as cancer diagnostic and prognostic biomarkers. Int J Biol Sci 15(1):1
Ikoma M, Gantt S, Casper C, Ogata Y, Zhang Q, Basom R, Dyen MR, Rose TM, Barcy S (2018) KSHV oral shedding and plasma viremia result in significant changes in the extracellular tumorigenic miRNA expression profile in individuals infected with the malaria parasite. PLoS ONE 13(2):e0192659
Kahlert C, Kalluri R (2013) Exosomes in tumor microenvironment influence cancer progression and metastasis. J Mol Med 91(4):431–437
Kalluri R, Zeisberg M (2006) Fibroblasts in cancer. Nat Rev Cancer 6(5):392–401
Kang X, Zuo Z, Hong W, Tang H, Geng W (2019) Progress of research on exosomes in the protection against ischemic brain injury. Front Neurosci 13:1149
Le M, Fernandez-Palomo C, McNeill FE, Seymour CB, Rainbow AJ, Mothersill CE (2017) Exosomes are released by bystander cells exposed to radiation-induced biophoton signals: reconciling the mechanisms mediating the bystander effect. PLoS ONE 12(3):e0173685
Li L, Cao B, Liang X, Lu S, Luo H, Wang Z, Wang S, Jiang J, Lang J, Zhu G (2019) Microenvironmental oxygen pressure orchestrates an anti-and pro-tumoral γδ T cell equilibrium via tumor-derived exosomes. Oncogene 38(15):2830–2843
Liu J, Fan L, Yu H, Zhang J, He Y, Feng D, Wang F, Li X, Liu Q, Li Y, Guo Z (2019) Endoplasmic reticulum stress causes liver cancer cells to release exosomal miR-23a-3p and up-regulate programmed death ligand 1 expression in macrophages. Hepatology 70(1):241–258
Lu Y, Chen L, Li L, Cao Y (2020) Exosomes derived from brain metastatic breast cancer cells destroy the blood-brain barrier by carrying lncRNA GS1–600G8.5. Bio Med Res Int 2020
Marleau AM, Chen CS, Joyce JA, Tullis RH (2012) Exosome removal as a therapeutic adjuvant in cancer. J Transl Med 10(1):1–2
Mashreghi M, Azarpara H, Bazaz MR, Jafari A, Masoudifar A, Mirzaei H, Jaafari MR (2018) Angiogenesis biomarkers and their targeting ligands as potential targets for tumor angiogenesis. J Cell Physiol 233(4):2949–2965
McAndrews KM, Kalluri R (2019) Mechanisms associated with biogenesis of exosomes in cancer. Mol Cancer 18(1):52
Milane L, Singh A, Mattheolabakis G, Suresh M, Amiji MM (2015) Exosome mediated communication within the tumor microenvironment. J Control Release 10(219):278–294
Montecalvo A, Larregina AT, Shufesky WJ, Beer Stolz D, Sullivan ML, Karlsson JM, Baty CJ, Gibson GA, Erdos G, Wang Z, Milosevic J (2012) Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood 119(3):756–766
Pattabiraman DR, Weinberg RA (2014) Tackling the cancer stem cells—what challenges do they pose? Nat Rev Drug Discov 13(7):497–512
Pavlyukov MS, Yu H, Bastola S, Minata M, Shender VO, Lee Y, Zhang S, Wang J, Komarova S, Wang J, Yamaguchi S (2018) Apoptotic cell-derived extracellular vesicles promote malignancy of glioblastoma via intercellular transfer of splicing factors. Cancer Cell 34(1):119–135
Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, García-Santos G, Ghajar CM, Nitadori-Hoshino A (2012) Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 18(6):883–891
Poggio M, Hu T, Pai CC, Chu B, Belair CD, Chang A, Montabana E, Lang UE, Fu Q, Fong L, Blelloch R (2019) Suppression of exosomal PD-L1 induces systemic antitumor immunity and memory. Cell 177(2):414–427
Rana S, Yue S, Stadel D, Zöller M (2012) Toward tailored exosomes: the exosomal tetraspanin web contributes to target cell selection. Int J Biochem Cell Biol 44(9):1574–1584
Rasouli E, Basirun WJ, Rezayi M, Shameli K, Nourmohammadi E, Khandanlou R, Izadiyan Z, Sarkarizi HK (2018) Ultrasmall superparamagnetic Fe3O4 nanoparticles: honey-based green and facile synthesis and in vitro viability assay. Int J Nanomed 13:6903
Rezayi M, Farjami Z, Hosseini ZS, Ebrahimi N, Abouzari-Lotf E (2018) MicroRNA-based biosensors for early detection of cancers. Curr Pharm Des 24(39):4675–4680
Ruivo CF, Adem B, Silva M, Melo SA (2017) The biology of cancer exosomes: insights and new perspectives. Can Res 77(23):6480–6488
Saari H, Lázaro-Ibáñez E, Viitala T, Vuorimaa-Laukkanen E, Siljander P, Yliperttula M (2015) Microvesicle-and exosome-mediated drug delivery enhances the cytotoxicity of Paclitaxel in autologous prostate cancer cells. J Control Release 28(220):727–737
Sahebi R, Langari H, Fathinezhad Z, Bahari Sani Z, Avan A, Ghayour Mobarhan M, Rezayi M (2020) Exosomes: new insights into cancer mechanisms. J Cell Biochem 121(1):7–16
Sany SB, Hashim R, Rezayi M, Rahman MA, Razavizadeh BB, Abouzari-lotf E, Karlen DJ (2015) Integrated ecological risk assessment of dioxin compounds. Environ Sci Pollut Res 22(15):11193–11208
Shepard A (2017) Vesicles of the future: exosomes and cancer research | Fulbright EndCap 2017, from https://www.youtube.com/watch?v=wcuSysYNcaI
Sinha S, Hoshino D, Hong NH, Kirkbride KC, Grega-Larson NE, Seiki M, Tyska MJ, Weaver AM (2016) Cortactin promotes exosome secretion by controlling branched actin dynamics. J Cell Biol 214(2):197–213
Sund M, Kalluri R (2009) Tumor stroma derived biomarkers in cancer. Cancer Metastasis Rev 28(1–2):177–183
Svensson KJ, Kucharzewska P, Christianson HC, Sköld S, Löfstedt T, Johansson MC, Mörgelin M, Bengzon J, Ruf W, Belting M (2011) Hypoxia triggers a pro-angiogenic pathway involving cancer cell microvesicles and PAR-2–mediated heparin-binding EGF signaling in endothelial cells. Proc Natl Acad Sci 108(32):13147–13152
Tai YL, Chen KC, Hsieh JT, Shen TL (2018) Exosomes in cancer development and clinical applications. Cancer Sci 109(8):2364–2374
Tamkovich SN, Tutanov OS, Laktionov PP (2016) Exosomes: Generation, structure, transport, biological activity, and diagnostic application. Biochem (Moscow) Supplement Ser A 10(3):163–173
Tang Z, Li D, Hou S, Zhu X (2020) The cancer exosomes: clinical implications, applications and challenges. Int J Cancer 146(11):2946–2959
Tian T, Zhu YL, Zhou YY, Liang GF, Wang YY, Hu FH, Xiao ZD (2014) Exosome uptake through clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 delivery. J Biol Chem 289(32):22258–22267
Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 9(6):654–659
Xiao X, Yu S, Li S, Wu J, Ma R, Cao H, Zhu Y, Feng J (2014) Exosomes: decreased sensitivity of lung cancer A549 cells to cisplatin. PLoS ONE 9(2):e89534
Xu S, Wang J, Ding N, Hu W, Zhang X, Wang B, Hua J, Wei W, Zhu Q (2015) Exosome-mediated microRNA transfer plays a role in radiation-induced bystander effect. RNA Biol 12(12):1355–1363
Zhang J, Li S, Li L, Li M, Guo C, Yao J, Mi S (2015) Exosome and exosomal microRNA: trafficking, sorting, and function. Genom Proteom Bioinf 13(1):17–24
Zhao H, Yang L, Baddour J, Achreja A, Bernard V, Moss T, Marini JC, Tudawe T, Seviour EG, San Lucas FA, Alvarez H (2016) Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. elife 5:e10250
Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, Yu Y, Chow A, O’Connor ST, Chin AR, Yen Y (2014) Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 25(4):501–515
Acknowledgements
“Not applicable.” This review is a collection of information and a result of individual’s work and not related to any institution.
Funding
This work has no funding agency.
Author information
Authors and Affiliations
Contributions
Both ME and AS chose the article’s title. ME collected the data, then he wrote the manuscript and created the figures. He also made the modifications as suggested. AS supervised and scientifically guided ME.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Ethical approval is not applicable for this article. This article does not contain any studies with human or animal subjects.
Consent for publication
There are no human subjects in this article and informed consent is not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Elewaily, M.I., Elsergany, A.R. Emerging role of exosomes and exosomal microRNA in cancer: pathophysiology and clinical potential. J Cancer Res Clin Oncol 147, 637–648 (2021). https://doi.org/10.1007/s00432-021-03534-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-021-03534-5