Abstract
Purpose
Pembrolizumab is an effective front-line treatment for advanced non-small cell lung cancer (NSCLC) in patients expressing high levels of programmed death-ligand 1 (PD-L1). However, it is unclear whether first-line pembrolizumab has similar efficacy among elderly (aged ≥ 75 years) and younger patients. This study aimed to investigate the safety and efficacy of front-line pembrolizumab monotherapy in older adults with NSCLC expressing high PD-L1.
Methods
A total of 128 patients with advanced NSCLC expressing high PD-L1, including 47 older adults, received first-line pembrolizumab monotherapy at ten institutions in Japan, between February 2017 and February 2018. Data related to patient characteristics, efficacy of pembrolizumab therapy, and the type and severity of adverse events were recorded.
Results
Overall, 47 patients [40 men and 7 women; median age 79 (range 75–88) years] were included in our analysis. In patients who received first-line pembrolizumab monotherapy, overall response, disease control rates, median progression-free survival (PFS), and median overall survival (OS) were 53.1%, 74.4%, 7.0 months, and not reached, respectively. Common adverse events included anorexia, fatigue, skin rash, and hypothyroidism. Two treatment-related deaths were noted, due to pneumonitis and infection. First-line pembrolizumab monotherapy was associated with improved PFS in patients with non-progressive disease (PD). In patients with non-PD and good performance status (PS), pembrolizumab monotherapy improved OS.
Conclusions
Elderly patients with NSCLC expressing high PD-L1 tolerated front-line pembrolizumab monotherapy well. Their survival outcomes were equivalent to those of younger patients. In patients with non-PD, first-line pembrolizumab monotherapy may improve PFS; in conjunction with good PS, it additionally improves OS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the most common cancer worldwide, with 1.8 million new cases and approximately 1.6 million deaths reported globally in 2012. This made it the leading cause of cancer-related mortality in males, and the second-leading cause in females, after breast cancer (Ferlay et al. 2012). Owing to the aging population and advances in cancer therapy, the aged population with advanced lung cancer is increasing globally (Miller et al. 2016). More than half of all patients with lung cancer are older than 65 years; in epidemiological research, this age is the cut-off for defining elderly populations (Davidoff et al. 2010). Non-small cell lung cancer (NSCLC) accounts for 85% of all lung cancer cases among adults, including elderly people (Owonikoko et al. 2007). Despite the increasing incidence and prevalence of cancer among older adults, those older than 75 years of age account for < 10% of patients enrolled in National Cancer Institute cooperative group studies. Similarly, despite the high incidence of NSCLC among older adults, this subpopulation is frequently under-represented in clinical studies (Pang et al. 2012; Sacher et al. 2013). This lack of trial registration has been attributed to various factors, including advanced age, poor performance status (PS), lack of adequate social support, and presence of multiple comorbid conditions. Notably, three-quarters of patients older than 70 years expressed willingness to participate in clinical studies (Townsley et al. 2005, 2006).
Remarkably, a recent meta-analysis which included 21 immune checkpoint inhibitor treatment [programmed death-ligand 1 (PD-L1) inhibitors and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4)] phase II or III clinical trials indicated no obvious differences in overall survival (OS), progression-free survival (PFS), or adverse events in the setting of an age subgroup analysis (age < 65 vs ≥ 65 years) among patients with metastatic melanoma, renal cell carcinoma, and NSCLC (Poropatich et al. 2017).
A previous open-label phase III clinical trial (the KEYNOTE-024 study) demonstrated the efficacy of pembrolizumab monotherapy as first-line treatment in patients with NSCLC and PD-L1 expression measurable in at least 50% of tumor cells (Reck 2016). However, it is unclear whether the efficacy of first-line pembrolizumab monotherapy in elderly patients (aged ≥ 75 years) is similar to that of younger patients expressing high PD-L1.
The development of pembrolizumab offers potentially promising therapy for the treatment of advanced NSCLC. However, the efficacy and safety of first-line pembrolizumab in elderly patients have not been fully evaluated. In this study, we retrospectively assessed the efficacy and safety of pembrolizumab monotherapy as first-line treatment in elderly patients with advanced NSCLC expressing high PD-L1.
Patients and methods
Patients
A total of 128 patients with advanced NSCLC and high PD-L1 expression (≥ 50%) treated at ten Japanese institutions between February 2017 and February 2018 were retrospectively evaluated. The study protocol was approved by the individual institutional review boards, and the requirement for written informed consent was waived owing to the retrospective study design.
All patients received pembrolizumab monotherapy (200 mg/3-weekly) as first-line treatment. Among 128 cases, 47 elderly (aged ≥ 75 years) patients were included. Pathological diagnosis and staging were based on the World Health Organization classification, 2015, and the TNM staging system, version 8, respectively. Patients with histologically confirmed NSCLC, unresectable stage III/IV or postoperative recurrent disease, and measurable PD-L1 expression in ≥ 50% of cells were included. All patients underwent pre-treatment physical examination, chest radiographs, thoracic and abdominal computed tomography (CT), bone scintigraphy or 18F-fluorodeoxyglucose positron emission tomography (FDG-PET), and brain magnetic resonance imaging (MRI) or CT for staging. Data regarding baseline characteristics, responses to first-line pembrolizumab monotherapy, status of administration of second-line chemotherapy, and the reasons for withholding second-line treatment were obtained from medical records.
PD-L1 expression was evaluated using PD-L1 immunohistochemistry (IHC) 22C3 pharmDx assay (Dako) on formalin-fixed tumor samples (Roach 2016). Archival biopsy specimens collected at cancer diagnosis or before pembrolizumab monotherapy, were obtained. PD-L1 membrane staining was identified in the viable tumor cells, and expression was evaluated as the percentage of positive cells.
All patients received first-line pembrolizumab (200 mg intravenously, every 3 weeks), which was continued until disease progression, intolerable toxicity, or withdrawal of consent. After disease progression, patients received various subsequent treatments.
Response evaluation
The best overall response and maximum tumor shrinkage were recorded as tumor responses. Radiographic responses were evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1 (Eisenhauer 2009) as follows: complete response (CR): disappearance of all target lesions, partial response (PR): decrease in the sum of the target lesion diameters by ≥ 30% compared to baseline diameters, progressive disease (PD): increase of ≥ 20% in the sum of the target lesion diameters compared to the smallest sum during the study, and stable disease (SD): insufficient shrinkage or enlargement to qualify as PR or PD, respectively.
Statistical analyses
Fisher’s exact test was used to evaluate the differences in categorical variables. PFS was measured from the initiation of therapy until PD or death from any cause, and OS was measured from the first day of therapy until death or was censored on the date of the last follow-up. Survival curves were plotted using the Kaplan–Meier method. Cox proportional hazards models with stepwise regression were employed to evaluate the prognostic factors for PFS and OS, and to assess the hazard ratios with 95% confidence intervals. p values < 0.05 were considered statistically significant for both, one- and two-tailed tests. Adverse events were assessed according to the Common Terminology Criteria for Adverse Events (version 4.0). All statistical analyses were performed using the JMP version 11.0 for Windows (SAS Institute, Cary, NC, USA) software package.
Results
Patient characteristics
A total of 47 eligible elderly patients (comprising 40 and 7 male and female individuals, respectively) with NSCLC expressing PD-L1 underwent first-line pembrolizumab monotherapy between February 2017 and February 2018. The patient characteristics have been presented in Table 1. The median age of the cohort was 79 (range 75–88) years. Overall, 37 (78.7%) and 10 (21.3%) had a performance status (PS) of 0–1 and 2–3, respectively, and 43 (91.5%) were current or former smokers; 17 (36.1%) and 23 (48.9%) had adeno- and squamous cell carcinomas, respectively. No patients exhibited targetable EGFR mutations or ALK translocations. A total of 30 (63.8%) patients had stage IV disease, and 7 (15%) had postoperative recurrences. At the point of data cut-off for analysis (December 2018), 13 patients were either receiving pembrolizumab therapy, or were undergoing follow-up.
Efficacy and survival of treatment
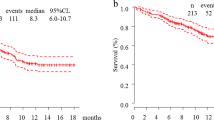
Table 2 shows the objective tumor response to front-line pembrolizumab monotherapy. During follow-up, 2 patients achieved CR, while 23, 10, and 8 patients had PR, SD, and PD, respectively. In patients with PS 0–1 and 2–3, the rates of controlled disease were 86.5% and 30.0%, respectively; this difference was statistically significant (p = 0.03). Conversely, on stratification based on histology (adeno- or squamous cell carcinoma) or PD-L1 tumor proportion score (TPS) (of either 50–74% or 75–100%), there were no significant differences across groups with respect to objective response rate (ORR) or disease control. The overall ORR and disease control rate were 53.1% (95% CI 38.9–67.4) and 74.4% (95% CI 62.0–86.9), respectively. The durations of median follow-up and PFS were 10.1 and 7.0 months (95% CI 5.4–10.6), respectively (Fig. 1a). Among the 47 patients, 17 (36.1%) died during follow-up, and the median OS was not reached (95% CI 10.3–not reached; Fig. 1b).
a Kaplan–Meier analysis of progression-free survival among 47 patients. The median progression-free survival after the initiation of first-line pembrolizumab monotherapy was 7.0 months (95% confidence interval, 5.4–10.6). b Kaplan–Meier analysis of overall survival among 47 patients. The median overall survival after the initiation of first-line pembrolizumab monotherapy was not reached (95% confidence interval, 10.3 months–not reached)
We also evaluated a number of clinical factors for their prognostic value, in terms of PFS and OS (Table 3). Univariate analyses demonstrated that PS at initiation of pembrolizumab, and pembrolizumab use among patients with non-PD were significantly associated with improved OS. Furthermore, PS at pembrolizumab initiation, smoking status, and pembrolizumab with PR, and non-PD, were significantly associated with longer PFS. After adjusting for multiple clinical variables, multivariate analysis showed that pembrolizumab was independently associated with improved PFS among patients with non-PD (p = 0.0001). Notably, the effect of pembrolizumab monotherapy on median PFS was significantly modified by PD status (PFS among patients with non-PD = 10.6 months vs. 0.6 months in patients with PD; log-rank p < 0.0001). Multivariate analysis also demonstrated that PS (p = 0.01) and efficacy without PD (p = 0.003) were independently associated with improved OS. Patients who began pembrolizumab with a favorable PS (0–1) experienced a longer median OS than those beginning therapy with a poor PS (2–3) (median OS not reached vs. 1.3 months, respectively; log-rank p = 0.0004). Similarly, patients with non-PD had a longer median OS than those with PD (not reached vs. 2.1 months, respectively; log-rank p < 0.0001).
Safety and toxicity profile
Table 4 shows the main adverse events noted during the treatment period. The most common event was skin rash (n = 11, 23.4%; grade ≥ 3 in 4.2%) followed by anorexia (n = 6, 12.7%). Patients with these conditions were managed with standard supportive therapy and cessation of the drug. A total of ten patients (21.3%) discontinued the therapy due to adverse events. Overall, pneumonitis was observed in 3 (6.3%) patients, among whom two recovered after temporarily discontinuing pembrolizumab, or with the use of a corticosteroids, while one patient died of potential treatment-related pneumonitis, as determined by the primary investigator. According to the investigator, pneumonitis may have developed as a secondary complication of bacterial pneumonia. Despite treatment with antibiotics and corticosteroids, the disease progressed rapidly, and the patient ultimately succumbed. A second fatal adverse event that occurred 8 months after treatment initiation was the result of a bacterial infection. Although the deaths were attributed to bacterial infections, the role of pembrolizumab could not be excluded.
Treatment after disease progression
Thirteen patients were still receiving first-line pembrolizumab monotherapy and had not developed PD at the end of the follow-up period. Table 5 lists the secondary therapies, including best supportive care, initiated in 34 patients after failure of first-line pembrolizumab monotherapy. Cytotoxic chemotherapy was administered in the second line in 12 patients; 4 and 8 patients received carboplatin combination therapy and monotherapy with cytotoxic agents (docetaxel, pemetrexed, S-1, or nab-paclitaxel), respectively. One patient underwent immune checkpoint inhibitor re-challenge with nivolumab in the third line. Twenty-two patients opted to receive best supportive care without second-line therapy.
Discussion
This study was the first to investigate the efficacy and tolerability of front-line pembrolizumab monotherapy in elderly patients in a real-world setting. Our findings demonstrated that, among elderly patients with NSCLC expressing high PD-L1, first-line pembrolizumab monotherapy was safe, and provided similar outcomes to that of younger patients.
In a similar cohort (NSCLC treated with first-line pembrolizumab), Reck et al. observed greater improvements in the risk of disease progression or death among patients aged > 65 years than those aged < 65 years (HR 0.45, 95% CI 0.29–0.70 versus HR 0.61, 95% CI 0.40–0.95) (Reck 2016). In a pooled analysis of pembrolizumab monotherapy in elderly patients, one study reported that in advanced NSCLC with PD-L1-positive tumors (PD-L1 TPS ≥ 1%), pembrolizumab improved OS versus chemotherapy, with a favorable safety profile (Nosaki 2019). Outcomes with pembrolizumab in elderly patients were comparable to those of younger patients. However, their analysis included pretreated patients, those with PD-L1 tumor proportion scores (TPS) of 1–49%, and those with good PS. Therefore, the efficacy of front-line pembrolizumab in elderly patients aged ≥ 75 years was not fully evaluated. We found that first-line pembrolizumab monotherapy conferred a good ORR (53.1%), and extended survival in elderly NSCLC patients expressing high PD-L1. The ORR observed in our cohort was higher than that of the KEYNOTE-024 study (44.8%), which included younger patients (Reck 2016). Although we found no significant differences in ORR based on PS, the disease control rate significantly differed between PS 0–1 and 2–3. Since patients with poor PS usually have more aggressive disease, these results were unremarkable. However, the PFS in our study was 7.0 months, which is less than that reported in a previous prospective study (10.3 months) (Reck 2016). This difference may reflect the relatively poor PS of the patients included in our real-world cohort from clinical practice. Our findings may be explained by several factors. First, the elderly participants in our study were more frail, and had higher comorbidities than other cohorts. Second, elderly patients may respond differently to immunotherapy owing to immune-senescence (Solana et al. 2012). However, the PFS of patients in our cohort with good PS (0–1) at pembrolizumab initiation was 8.9 months, which was similar to that reported in a previous study (Reck 2016). Our findings indicate that front-line pembrolizumab monotherapy in elderly patients expressing high PD-L1 confers longer PFS than cytotoxic drugs. In a trial of cytotoxic therapy in elderly patients, the Multicenter Italian Lung Cancer in the Elderly Study (MILES; phase III) compared three chemotherapy regimens in patients aged≥ 70 years, and found a PFS of 18 and 17 weeks for vinorelbine and gemcitabine, respectively (Gridelli 2003). Owing to the relatively short follow-up period, median OS was not reached in our study. A previous large Japanese phase III trial for elderly patients with advanced NSCLC reported an ORR, PFS, and OS of 10–22.7%, 3.1–5.5 months, and 9.9–14.0 months, respectively, for cytotoxic drug monotherapy (vinorelbine or docetaxel) (Kudoh 2006). In a recent Japanese randomized phase III study comparing carboplatin–pemetrexed followed by pemetrexed or docetaxel in elderly patients with advanced non-squamous non-small-cell lung cancer, Okamoto et al. (2019) reported an ORR, PFS, and OS of 36.8%, 6.4 months, and 18.7 months, respectively, in the carboplatin–pemetrexed arm. Despite the relatively small sample size, our findings indicate that pembrolizumab may provide improved survival compared to cytotoxic therapies. Additional clinical studies are needed to further evaluate the nature and magnitude of age-related differences in the efficacy of immune checkpoint inhibitors. The performance of combination therapy with carboplatin, pemetrexed, and pembrolizumab in elderly patients with advanced NSCLC needs to be evaluated.
The patient populations that experience the greatest survival benefit from first-line pembrolizumab monotherapy are yet to be identified. Multivariate analyses performed in the present study demonstrated that tumor response (non-PD or PD) was an independent prognostic factor for PFS, and that PS (0–1 or 2–3) and tumor response (non-PD or PD) were independent prognostic factors for OS. Our findings suggest that patients with good PS may achieve non-PD after front-line treatment with pembrolizumab. Furthermore, patients who achieved non-PD were also likely to achieve prolonged PFS, which is associated with prolonged OS.
In the present analysis, 21.3% patients had a PS of ≥ 2; they typically had a relatively low tolerance to first-line cytotoxic chemotherapy. Since first-line pembrolizumab was initiated at the discretion of the attending physician, our results may have included clinical outcomes of patients who were reluctant to receive any front-line therapy. In this study, poor PS (2–3) at initiation of pembrolizumab was associated with a much shorter median OS than good PS (0–1). In the previous studies from real-world settings, frail patients (e.g., those with a PS of ≥ 2) receiving nivolumab experienced poor outcomes (Dudnik et al. 2018; Fujimoto et al. 2018). Our results suggest that pembrolizumab monotherapy is not suitable for elderly patients with poor PS. Previous studies have reported that first-line pembrolizumab provides better outcomes in NSCLC with a PD-L1 TPS of 75–100% than 50–74% (Alguilar 2018). Immune-related adverse events associated with nivolumab have been observed in patients with NSCLC (Haratani et al. 2018). However, in our cohort, multivariate analysis demonstrated that a PD-L1 TPS of 50–74% or 75–100%, and immune-related adverse events were not independent prognostic factors for either PFS or OS. This may be attributed to the modest sample size, which limited evaluation of the relationship between efficacy, PD-L1 expression, and immune-related adverse events.
In this study, adverse events associated with first-line pembrolizumab monotherapy in elderly patients were typically mild and predictable, and their incidence and severity were either identical to, or less than those observed in previous prospective studies (Garon et al. 2015; Herbst 2016; Reck 2016). Immune-related adverse events were mostly manageable and well controlled, regardless of severity. Treatment-related serious adverse events of grade 3 or greater were observed in less than 5% of patients. However, treatment was discontinued due to adverse events in ten patients (21.3%). Treatment-related deaths occurred in 2 (4.2%) patients; however, both of these events were probably caused by bacterial infections. Although the frequency of grade 5 adverse events in this study may be slightly higher than that of previous reports (Garon et al. 2015; Herbst 2016; Reck 2016), the sample size was too small to evaluate the relationship between treatment-related deaths and pembrolizumab. The discrepancies between our findings and those of previous studies highlight the well-known limitations of clinical trials in assessing drug safety, and emphasize the need for real-world data (Singh and Loke 2012). Nevertheless, the safety profile of pembrolizumab monotherapy observed in this study was generally consistent with that observed in previous studies (Garon et al. 2015; Herbst 2016; Reck 2016), and was favorable in comparison with earlier reports on cytotoxic drugs among elderly patients (Gridelli 2003; Kudoh 2006). In one Japanese phase III study that evaluated patients receiving docetaxel and vinorelbine, grade 3 or 4 neutropenia was observed in 82.9% and 69.3%, respectively (Kudoh 2006). This indicates that the adverse events associated with first-line pembrolizumab monotherapy among elderly patients with NSCLC expressing high PD-L1 are generally mild, suggesting that this treatment is suitable and feasible.
In comparison with conventional cancer therapies, immune checkpoint inhibitors are usually associated with lower rates of adverse events of any grade (Champiat et al. 2016). However, currently available data regarding toxicity in elderly patients treated with immune checkpoint inhibitors are conflicting. Subset analyses in phase II/III trials, including advanced NSCLC, melanoma, and renal cell carcinoma, have confirmed that nivolumab is well tolerated by elderly patients. However, a trend towards increased grades III–V toxicity was apparent in patients older than 70 years (Rizvi et al. 2015). The KEYNOTE trials have investigated the efficacy of pembrolizumab in 3991 patients with melanoma, NSCLC, head and neck cancers, Hodgkin’s lymphoma, and urothelial carcinoma; 46% and 16% were aged 65 and 75 years or older, respectively. No overall differences in safety were observed between older and younger patients (Alkharabsheh et al. 2018). We speculated that older individuals with reduced immunity may respond less favorably to immunotherapy, and develop fewer immune-related adverse events (Daste et al. 2017; Tomihara et al. 2013). However, neither of these differences were observed in our study, and the results appear to confirm that dose adjustment based on age alone, is unnecessary.
No standard second-line treatments have been established for elderly patients with NSCLC. Therefore, the influence of second-line or later therapies on OS in elderly patients with NSCLC expressing high PD-L1 remains unknown. Several options are available for aged patients with advanced NSCLC, including best supportive care, single-agent chemotherapy with a third-generation drug, and non-platinum- or platinum-based combination chemotherapy. In this study, only 12 (35.3%) of the 34 patients who failed first-line pembrolizumab monotherapy received second-line cytotoxic chemotherapy. In clinical practice, the rate of cytotoxic drug monotherapy is relatively high (Table 5), although second-line cytotoxic drug regimens may be offered to elderly patients less frequently. The main reasons for withholding cytotoxic drug chemotherapy in elderly patients include age-related organ dysfunction, comorbid conditions, and potentially lower tolerance to the toxicity of combination chemotherapy compared to younger patients. However, studies on the subsequent treatments in elderly patients with NSCLC expressing high PD-L1 are limited.
Recent reports have suggested promising efficacy for chemotherapy–immunotherapy or dual checkpoint blockade combinations as first-line treatments (Gandhi et al. 2018; Hellmann et al. 2018; Socinski et al. 2018). However, our study included only patients treated with pembrolizumab monotherapy; therefore, the efficacy or toxicity of these therapies in elderly patients with advanced NSCLC could not be evaluated, and requires further evaluation in future studies.
This study had several limitations. First, both, the use of pembrolizumab monotherapy as first-line treatment, and the choice of second-line treatment were at the discretion of the attending physician. Possible selection bias may have affected survival after second-line therapy. Second, this was a retrospective study comprising a selected group of patients. Third, planned pembrolizumab monotherapy may have been delayed or omitted at the treating physician’s discretion. To minimize the impact of these potential sources of bias, all consecutive patients who were treated at our institutions were included during analysis, and their original charts were thoroughly reviewed. Fourth, our results suggested that tumor response was a prognostic factor for PFS and OS in this analysis. However, this study did not examine predictive ability. An additional limitation was the relatively small sample size. Larger prospective trials are needed to confirm and validate our findings in a real-world setting.
In conclusion, our results indicate that first-line pembrolizumab monotherapy among elderly patients with NSCLC expressing high PD-L1 was safe, with similar outcomes to that of younger patients. Front-line pembrolizumab monotherapy should be considered in elderly patients with advanced NSCLC expressing high PD-L1. Future large prospective studies are warranted to confirm and validate our findings.
References
Alguilar EJ et al (2018) MA04. 05 outcomes in NSCLC patients treated with first-line pembrolizumab and a PD-L1 TPS of 50-74% vs 75-100% or 50-89% vs 90-100%. J Thorac Oncol 13:S367–S368
Alkharabsheh O, Kannarkatt P, Kannarkatt J, Karapetyan L, Laird-Fick HS, Al-Janadi A (2018) An overview of the toxicities of checkpoint inhibitors in older patients with cancer. J Geriatr Oncol 9:451–458. https://doi.org/10.1016/j.jgo.2018.02.002
Champiat S et al (2016) Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol 27:559–574. https://doi.org/10.1093/annonc/mdv623
Daste A et al (2017) Immune checkpoint inhibitors and elderly people: a review. Eur J Cancer 82:155–166. https://doi.org/10.1016/j.ejca.2017.05.044
Davidoff AJ, Tang M, Seal B, Edelman MJ (2010) Chemotherapy and survival benefit in elderly patients with advanced non-small-cell lung cancer. J Clin Oncol 28:2191–2197. https://doi.org/10.1200/jco.2009.25.4052
Dudnik E et al (2018) Effectiveness and safety of nivolumab in advanced non-small cell lung cancer: the real-life data. Lung Cancer 126:217–223. https://doi.org/10.1016/j.lungcan.2017.11.015
Eisenhauer EA et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Ferlay J et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359–E386. https://doi.org/10.1002/ijc.29210
Fujimoto D et al (2018) Efficacy and safety of nivolumab in previously treated patients with non-small cell lung cancer: a multicenter retrospective cohort study. Lung Cancer 119:14–20. https://doi.org/10.1016/j.lungcan.2018.02.017
Gandhi L et al (2018) Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378:2078–2092. https://doi.org/10.1056/nejmoa1801005
Garon EB et al (2015) Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 372:2018–2028. https://doi.org/10.1056/nejmoa1501824
Gridelli C et al (2003) Chemotherapy for elderly patients with advanced non-small-cell lung cancer: the Multicenter Italian Lung Cancer in the Elderly Study (MILES) phase III randomized trial. J Natl Cancer Inst 95:362–372
Haratani K et al (2018) Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol 4:374–378. https://doi.org/10.1001/jamaoncol.2017.2925
Hellmann MD et al (2018) Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med 378:2093–2104. https://doi.org/10.1056/nejmoa1801946
Herbst RS et al (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387:1540–1550. https://doi.org/10.1016/s0140-6736(15)01281-7
Kudoh S et al (2006) Phase III study of docetaxel compared with vinorelbine in elderly patients with advanced non-small-cell lung cancer: results of the West Japan Thoracic Oncology Group Trial (WJTOG 9904). J Clin Oncol 24:3657–3663. https://doi.org/10.1200/jco.2006.06.1044
Miller KD et al (2016) Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 66:271–289. https://doi.org/10.3322/caac.21349
Nosaki K et al (2019) Safety and efficacy of pembrolizumab monotherapy in elderly patients with PD-L1-positive advanced non-small-cell lung cancer: pooled analysis from the KEYNOTE-010, KEYNOTE-024, and KEYNOTE-042 studies. Lung Cancer 135:188–195. https://doi.org/10.1016/j.lungcan.2019.07.004
Okamoto I et al (2019) Randomized phase III study comparing carboplatin plus pemetrexed followed by pemetrexed versus docetaxel in elderly patients with advanced non-squamous non-small-cell lung cancer (JCOG1210/WJOG7813L). J Clin Oncol 37 (suppl; abstr 9031)
Owonikoko TK, Ragin CC, Belani CP, Oton AB, Gooding WE, Taioli E, Ramalingam SS (2007) Lung cancer in elderly patients: an analysis of the surveillance, epidemiology, and end results database. J Clin Oncol 25:5570–5577. https://doi.org/10.1200/jco.2007.12.5435
Pang HH et al (2016) Enrollment trends and disparity among patients with lung cancer in National Clinical Trials, 1990 to 2012. J Clin Oncol 34:3992–3999. https://doi.org/10.1200/jco.2016.67.7088
Poropatich K, Fontanarosa J, Samant S, Sosman JA, Zhang B (2017) Cancer immunotherapies: are they as effective in the elderly? Drugs Aging 34:567–581. https://doi.org/10.1007/s40266-017-0479-1
Reck M et al (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375:1823–1833. https://doi.org/10.1056/nejmoa1606774
Rizvi NA et al (2015) Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 16:257–265. https://doi.org/10.1016/s1470-2045(15)70054-9
Roach C et al (2016) Development of a companion diagnostic PD-L1 immunohistochemistry assay for pembrolizumab therapy in non-small-cell lung cancer. Appl Immunohistochem Mol Morphol 24:392–397. https://doi.org/10.1097/pai.0000000000000408
Sacher AG, Le LW, Leighl NB, Coate LE (2013) Elderly patients with advanced NSCLC in phase III clinical trials: are the elderly excluded from practice-changing trials in advanced NSCLC? J Thorac Oncol 8:366–368. https://doi.org/10.1097/jto.0b013e31827e2145
Singh S, Loke YK (2012) Drug safety assessment in clinical trials: methodological challenges and opportunities. Trials 13:138. https://doi.org/10.1186/1745-6215-13-138
Socinski MA et al (2018) Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 378:2288–2301. https://doi.org/10.1056/nejmoa1716948
Solana R, Tarazona R, Gayoso I, Lesur O, Dupuis G, Fulop T (2012) Innate immunosenescence: effect of aging on cells and receptors of the innate immune system in humans. Semin Immunol 24:331–341. https://doi.org/10.1016/j.smim.2012.04.008
Tomihara K, Curiel TJ, Zhang B (2013) Optimization of immunotherapy in elderly cancer patients. Crit Rev Oncog 18:573–583
Townsley CA, Selby R, Siu LL (2005) Systematic review of barriers to the recruitment of older patients with cancer onto clinical trials. J Clin Oncol 23:3112–3124. https://doi.org/10.1200/jco.2005.00.141
Townsley CA, Chan KK, Pond GR, Marquez C, Siu LL, Straus SE (2006) Understanding the attitudes of the elderly towards enrolment into cancer clinical trials. BMC Cancer 6:34. https://doi.org/10.1186/1471-2407-6-34
Acknowledgements
The authors would like to thank Drs. Takako Mouri, Yoichiro Hamamoto, Norimitsu Kasahara, Shinichi Ishihara, and Ichiro Naruse for their assistance in preparing this manuscript. We would like to thank Editage (http://www.editage.jp) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any financial or personal relationships with people or organizations that could inappropriately influence this work.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
The need for informed consent was waived by the institutional review boards of the participating institutions owing to the retrospective nature of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Imai, H., Wasamoto, S., Yamaguchi, O. et al. Efficacy and safety of first-line pembrolizumab monotherapy in elderly patients (aged ≥ 75 years) with non-small cell lung cancer. J Cancer Res Clin Oncol 146, 457–466 (2020). https://doi.org/10.1007/s00432-019-03072-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-019-03072-1