Abstract
Purpose
Growing solid tumors mostly outstrip blood supply and become hypoxic (low oxygen supply). To survive under this pathological milieu, tumors overexpress a potent oncogenic factor, hypoxia-inducible factor-1α (HIF-1α). HIF-1α up-regulate HIF-1 signaling pathways and subsequently activate genes that promote cancer growth even under hypoxia. Also, HIF-1 pathway activation leads to aggressive tumor growth, metastasis, therapy resistance and ultimately poor patient prognosis as evidential by several clinical studies. Hence, targeting HIF-1 pathway is regarded as a promising strategy to treat cancer. To date, several synthetic HIF-1 pathway inhibitors have been developed to treat hypoxic tumors; however, they are clinically ineffective due to off-target effects, low potency and high toxicity. Hence, there is an urgent need to explore safe and promising drugs to combat hypoxic tumors.
Results
This article extensively reviews the therapeutic potential of various herbal nutraceuticals against wide varieties of hypoxic tumors. The inhibitory effects of each herbal nutraceutical on the pathological consequences of HIF-1 signaling pathway and also their ability to improve the response of hypoxic cancer cells to conventional cancer therapies are discussed. Furthermore, we have provided new directions to overcome challenges behind conducting in vivo and preclinical hypoxia research and developing herbal nutraceuticals into pharmaceuticals to treat cancer.
Conclusions
The present review strongly suggests that herbal nutraceuticals are highly effective in combating the oncogenic effects of the HIF-1 pathway in wide varieties of tumors. However, more in vivo studies using zebrafish as a model system and extensive clinical studies in cancer patients with elevated tumor HIF-1α levels are highly warranted to ascertain the effective utilization of herbal nutraceuticals as adjunct/ alternative medicine in clinical practice to treat cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer, the second leading cause of death globally has accounted for nearly 9.6 million mortalities in 2018 (WHO Cancer Fact Sheet 2018). According to World Health Organization (WHO) reports, one in six deaths globally is due to cancer. The major risk factors of cancer are low fruit and vegetable consumption, sedentary lifestyle, obesity, increased exposure to cancer-causing agents including tobacco and alcohol, etc. (Sauer et al. 2017). During development, tumor cells relish an uninterrupted supply of nutrients and oxygen up to a certain size of about 2–3 mm2. However, when they progress beyond this size, tumors outgrow blood supply which in turn results in lack of oxygen supplementation to cancer cells. This pathological condition is defined as “Hypoxia” (Hockel and Vaupel 2001). To overcome this pathological milieu and grow under hypoxic conditions, cancer cells overexpress a potent oncogenic protein known as HIF-1α. HIF-1α promotes tumor progression even under hostile tumor microenvironment by inducing significant adaptive cellular changes in them through the activation of HIF-1 signaling pathways. This in turn results in aggressive tumor growth, survival, metastasis, therapy resistance and poor patient survival (Masoud and Li 2015). Hence, down-regulating HIF-1 pathway is currently regarded as a promising approach to treat hypoxic tumors.
To date, several drugs including romidepsin, perifosine, 2-methoxyestradiol, echinomycin, geldanamycin, tanespimycin, chetomin and also other small molecule inhibitors have been developed to inhibit HIF-1 pathways in tumor cells. However, the beneficial and effective applications of these drugs are limited by low potency and high toxicity (Burroughs et al. 2013). The need for the discovery of promising therapeutics with low toxicity to combat tumor hypoxia led to the advent of numerous studies that explored the inhibitory action of various herbal nutraceuticals on the oncogenic potential of HIF-1α and its downstream signaling events. This review will comprehensively discuss the clinical, in vitro and in vivo studies that demonstrate the therapeutic efficacy of various plant-based nutraceuticals against hypoxic tumors.
Pathological effects of HIF-1α in tumor cells
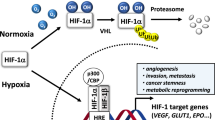
HIF-1α is a potent transcription factor produced by most solid tumors when they are deprived of oxygen supply (Koh et al. 2010; Masoud and Li 2015). It orchestrates key events that enable the cancer cells to adapt and grow efficiently even under stressful hypoxic tumor microenvironments. Structurally, HIF-1α is a component of the heterodimeric transcription factor, HIF-1. HIF-1 is made of two subunits—HIF-1α subunit and HIF-1β subunit [also known as aryl hydrocarbon receptor nuclear translocator (ARNT)]. Furthermore, HIF-1α is also a member of the basic helix loop helix (bHLH) and PER-ARNT-SIM (PAS) superfamily of proteins (Wang et al. 1995) (13). Basically, cells express both HIF-1α and HIF-1β in equal proportions. However, during normal oxygen supply, the proline residues (P402 and P564) present in the oxygen-dependent degradation (ODD) domain of HIF-1α undergo hydroxylation by prolyl hydroxylase domain (PHD) proteins. This, in turn, enables HIF-1α to bind with a tumor suppressor called Von Hippel Lindau protein (pVHL) (Min et al. 2002). pVHL then adds polyubiquitin tail to HIF-1α and makes it a target for degradation by proteasomes. Hence, HIF-1α produced under normoxic conditions undergoes ubiquitin-mediated proteasomal degradation. However, under hypoxic conditions, the proline residues of HIF-1α do not undergo hydroxylation due to lack of oxygen supply. Hence, pVHL cannot bind to HIF-1α and target it for proteasomal degradation (Liu et al. 2015). The stable HIF-1α present in the cytoplasm then enters the nucleus and binds to HIF-1β, to form a potent transcription factor, HIF-1. This in turn leads to tremendous modulation in the expression pattern of the genes by the cells.
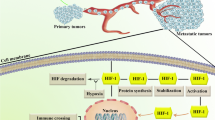
Mounting clinical studies show significantly elevated levels of HIF-1α in the tumor tissues of a wide variety of cancer patients including breast, prostate, brain, cervix, colon, pancreas, skin, stomach, oropharynx, etc. (Semenza 2010). Furthermore, these clinical studies also show that overexpression of HIF-1α in the tumor tissues of cancer patients contribute independently to their poor prognosis, poor clinical outcome and increased mortality (Semenza 2010). The oncogenic potential of HIF-1α majorly resides in its ability to up-regulate HIF-1 signaling pathway and subsequently augments crucial genes and key events that promote tumor cell survival, progression and metastasis (Koh et al. 2010; Masoud and Li 2015; Eales et al. 2016; Petrova et al. 2018). The key events augmented by HIF-1 pathway in tumor cells are given in (Fig. 1).
One of the major events triggered by HIF-1 pathway in the hypoxic tumor microenvironment is angiogenesis. To ensure sufficient supply of nutrients and oxygen, HIF-1 pathway ‘turns on’ the angiogenic switch in the hypoxic tumor cells by up-regulating their expression of vascular endothelial growth factor (VEGF) (Koh et al. 2010; Petrova et al. 2018; Krock et al. 2011). However, the new blood vessels formed are abnormal, immature and leaky with no basement membrane and increased vascular permeability. This in turn provides an easy way for the tumor cells to intravasate the blood vessels and metastasize to distant organs. Furthermore, HIF-1 pathway promotes epithelial-mesenchymal transition (EMT) in tumor cells and enables them to acquire plastic and mobile phenotype to cross the extracellular matrix and invade the surrounding tissues. To conserve energy, the HIF-1 pathway induces replicative quiescence in tumor cells and arrests them at G1/S phase of the cell cycle (Muz et al. 2015). Since most drugs used for treating cancer are targeted against the rapidly dividing cells, this cell cycle arrest by HIF-1 pathway acts as a major cause for the development of chemoresistance in cancer patients (Muz et al. 2015; Warfel and El-Deiry 2014; Rohwer and Cramer 2011). Moreover, HIF-1 pathway also reprograms glucose and energy metabolism in the hypoxic tumor cells to generate energy even in the absence of oxygen (Eales et al. 2016). This occurs by a metabolic shift from oxidative phosphorylation in mitochondria towards glycolysis which ultimately leads to elevated glucose uptake, glycolysis, and lactate synthesis accompanied by a reciprocal reduction in mitochondrial respiration. These effects are collectively referred to as the “Warburg effect”. More importantly, the HIF-1 pathway has been reported to increase the aggressive clones from heterogeneous tumor cells and promote a lethal phenotype (Petrova et al. 2018). Thus, taken together, the HIF-1 pathway has been found to promote tumor growth and metastasis by augmenting neovascularization, EMT, abnormal metabolism, cell cycle arrest and generation of aggressive clones of tumor cells. Furthermore, these pathological events triggered by the HIF-1 pathway have been shown to induce drug resistance and therapy failure in cancer patients via following mechanisms: (i) hindered drug diffusion due to abnormal vasculature, (ii) replicative quiescence due to cell cycle halt at G1/S phase and (iii) EMT-induced stemness of cancer cells (Muz et al. 2015). Hence, targeting HIF-1 pathway is currently regarded as a promising and novel approach to treat cancer.
Herbal nutraceuticals and hypoxic tumors
Herbal nutraceuticals are bioactive phytochemicals derived from plants as secondary or tertiary metabolites (Dillard and German 2000). These non-toxic food-extract supplements possess a fascinating spectrum of medicinal properties which in turn enable them to exert a tremendous impact on healthcare system and offer great protection against many dreadful diseases including cancer (Das et al. 2012). Based on their structure and function, herbal nutraceuticals are classified into different groups as polyphenols, cannabinoids, flavonoids, saponins, sitosterols, anthocyanins, carotenoids, isothiocyanates, terpenes, isoflavones, etc (Fig. 2). Mounting studies show that herbal nutraceuticals could act as safe and potent agents to (i) detoxify environmental and ingested carcinogens by activating antioxidant enzymes, tumor suppressor proteins and DNA repair pathways and (ii) also fight cancer progression by promoting apoptosis and inhibiting EMT, angiogenesis and metastasis (Wargovich et al. 2010; Ranzato et al. 2014). Furthermore, accumulating studies show that herbal nutraceuticals could also act against hypoxic cancer cells by effectively attenuating their survival, growth, and progression through the inhibition of HIF-1 signaling pathways. Herbal nutraceuticals act both by inhibiting the pathways that promote the synthesis of HIF-1α and also by degrading HIF-1α and attenuating downstream signaling events. On top of exerting these anti-cancer effects, herbal nutraceuticals have also been reported to (i) reverse hypoxia-induced drug resistance, (ii) improve the therapeutic index of cancer therapies against hypoxic tumors and (iii) reduce the harmful side effects of these cytotoxic therapies. The mechanism of action of herbal nutraceuticals and their chemo/radiosensitizing potential on hypoxic tumor cells have been summarized in (Fig. 3) and (Table 1), respectively.
Combating tumor hypoxia with herbal nutraceuticals
Resveratrol
Resveratrol (3,4,5-trihydroxystilbene) is a natural polyphenolic stilbenoid found in diverse plant species including grapes, berries, peanuts, and other plant sources. Over the past decades, resveratrol has received more attention from medical, nutraceutical and scientific world due to its immense estrogenic, antioxidant and anti-inflammatory properties. Numerous studies show that resveratrol could act as a promising chemopreventive and chemoprotective drug against wide varieties of cancers. It has been reported to induce apoptosis and abrogate cell proliferation, EMT, neovascularization, and metastasis in tumor cells through the modulation of diverse molecular and cell signaling pathways (Rauf et al. 2018a, b; Carter et al. 2014). More interestingly, emerging studies show that resveratrol could exhibit its cytotoxic, anti-angiogenic and anti-metastatic activities against varieties of hypoxic tumors as well via diverse mechanisms.
Li et al. intensively evaluated the therapeutic potential of resveratrol against hypoxic BxPC-3 and Panc-1 pancreatic cancer cells (Li et al. 2016). Interestingly, they have found that resveratrol could decrease hypoxia-driven (i) production of reactive oxygen species (ROS), (ii) ROS-induced invasion and migration, (iii) HIF-1α protein synthesis and (iv) expression of metastatic-related factors including uPA and MMP-2 in these cells through the activation of a hedgehog signaling pathway. In a study done by Zhang et al., resveratrol inhibited tumor neovascularization in hypoxic SCC-9 human tongue squamous cell carcinoma and HepG2 hepatoma cells by impeding hypoxia-mediated HIF-1α accrual and VEGF expression via p42/44 MAPK and PI3K/Akt mechanisms (Zhang et al. 2005). In U87 and U251 glioma cells, resveratrol repressed hypoxia-induced migration and invasion via p-STAT3/miR-34a axis in a time and dose-dependent manner (Wang et al. 2016). Furthermore, in colon cancer cells, resveratrol inhibited metastasis under both normoxic and hypoxic conditions by blocking cell migration, invasion, adhesion, MMP-2, and MMP-9 secretion through the suppression of HIF-1α protein synthesis and stabilization (Wu et al. 2008). Similarly, in Saos-2 osteosarcoma cells, resveratrol abrogated hypoxia-induced cell growth, invasion, and EMT by decreasing the levels of HIF-1α protein (Sun et al. 2015).
Castration-induced HIF-1α promotes the synthesis of androgen receptor even at low androgen concentration thereby imitating the castration-resistant stage and hence, HIF-1α acts as a promising target to suppress the growth of castration-resistant prostate cancer (Mitani et al. 2014a, b). Fascinatingly, studies done by Mitani et al. using hypoxic LNCaP human prostate cancer cells (in vitro study) and castrated male BALB/cSlc-nu/nu mice (in vivo study) bearing LNCaP xenografts show that resveratrol could retard the progression of castration-resistant prostate tumor by suppressing β-catenin-mediated androgen receptor function through the inhibition of HIF-1α expression.
Human Papillomavirus (HPV)-16 oncoproteins, E6 and E7 have been found to worsen the prognosis of cervical cancer patients by augmenting neovascularization through the up-regulation of HIF-1α-mediated VEGF synthesis (Guo et al. 2014). Fortunately, in a study done by Tang et al., resveratrol effectively impeded capillary formation in C-33A and HeLa human cervical cancer cells by inhibiting HPV 16 E6- and E7-induced expression of HIF-1α (Tang et al. 2007).
Besides resveratrol, HS-1793, a novel resveratrol analog with enhanced stability has also been found to act efficiently against hypoxic tumors. Kim et al. conducted two independent studies with PC-3 human prostate (Kim et al. 2013) and MDA-MB-231 and MCF-7 breast cancer cells (Kim et al. 2017a, b) under both normoxic and hypoxic conditions to evaluate the anti-tumor potential of HS-1793. Interestingly, their study results show that HS-1793 could impede hypoxia-mediated HIF-1α and VEGF synthesis in all these cells. Furthermore, HS-1793 also inhibited the growth of MDA-MB-231 breast cancer xenografts in athymic mice without any apparent toxicity by decreasing the levels of Ki-67 (proliferation index marker) and CD31 (a biomarker of microvessel density).
Apart from exerting these chemotherapeutic effects, resveratrol and its analogs have also been reported to improve the sensitivity of hypoxic tumor cells to chemotherapy and radiotherapy by repressing the expression of crucial genes involved in drug resistance. Carbonyl reductase 1 (CBR 1), a HIF-1α-driven enzyme is majorly responsible for doxorubicin resistance in hypoxic cancer cells. It acts by catalyzing the formation of therapeutically less effective doxorubicinol from doxorubicin. Astonishingly, studies done by Mitani et al. show that resveratrol could reverse the hypoxia-induced resistance to doxorubicin in MCF-7 cells and sensitize these hypoxic cells to doxorubicin therapy by decreasing the activity of HIF-1α and thus CBR 1 (Mitani et al. 2014a, b). Furthermore, in another study done by Quan et al., resveratrol enhanced the sensitivity of CNE2 nasopharyngeal carcinoma cells to paclitaxel therapy under hypoxia by suppressing the effects of HIF-1α, multidrug resistance gene and multidrug resistance protein (Quan et al. 2009). Choi et al. have shown that HS-1793 could promote IR-induced programmed cell death of hypoxic FM3A mouse breast cancer cells through the modulation of hypoxic conditions and also inhibit the metastasis of these cells by abrogating the expression of HIF-1α and VEGF (Choi et al. 2016).
All these findings strongly suggest that resveratrol and its analog HS-1793 could act as effective inhibitors against the growth of various hypoxic tumors and also improve their sensitivity to chemo and radiotherapies by down-regulating HIF-1α-induced signaling pathways.
Curcumin
Curcumin, the active component of turmeric is a dietary polyphenol obtained from the dried rhizome of the Curcuma longa plant (Kocaadam and Sanlier 2017). It is a potent and non-toxic nutraceutical with significantly high potential to combat cancer, inflammation, infection, etc. Owing to its safe and multiple health benefits, curcumin has emerged as a high interest phytochemical in the nutraceutical industry. It employs multiple biochemical and molecular mechanisms to both prevent the transformation of normal cells to cancer cells and also kill the cancer cells in the event of their development (Bar-Sela et al. 2010). Besides possessing these chemotherapeutic properties, curcumin has also been reported to effectively reverse intra-tumoral hypoxia and the adaptations of tumor cells to hypoxic microenvironment by down-regulating HIF-1α and its downstream signaling events.
Choi et al. extensively studied the pharmacological effects of curcumin against wide varieties of hypoxic tumors including HT29 colon carcinoma, Hep3B hepatoma, H596 non-small-cell lung carcinoma, MCF7 mammary carcinoma, PC3 prostate carcinoma, Caki-1 renal carcinoma, SiHa cervical carcinoma and MKN28 gastric carcinoma (Choi et al. 2006). Interestingly, in most of these cell lines, curcumin has been found to retard tumor growth by inactivating HIF-1α activity through the degradation of ARNT (Aryl hydrocarbon receptor nuclear translocator). To substantiate these results, Choi et al. conducted in vivo studies too using xenografted mice-bearing Hep3B tumors. Fascinatingly, curcumin also arrested tumor progression and repressed ARNT, erythropoietin and VEGF activity in these mice, thereby suggesting that it could act as a potent anti-HIF-1α agent in hypoxic tumors.
In HepG2 cells, curcumin retarded tumor growth and metastasis by suppressing HIF-1α-driven proliferation, migration, invasion and EMT in a hypoxic microenvironment (Duan et al. 2014). Also, curcumin attenuated new blood vessel formation in a variety of hypoxic cells including HepG2 human hepatoma cells (Bae et al. 2006), vascular endothelial cells (Bae et al. 2006), by inhibiting the expression of HIF-1α and its major downstream target, VEGF. In K1 papillary thyroid cancer cells, curcumin has strongly repressed HIF-1α expression, hypoxia-induced ROS generation, and the DNA-binding potential of HIF-1α to hypoxia response element under hypoxic conditions (Tan et al. 2015). Also, curcumin has attenuated the migration of these cells by up-regulating E-cadherin expression and inhibiting MMP-9 enzyme activity. In hypoxic HT29 colon cancer cells, curcumin has triggered apoptosis by repressing AMP-activated protein kinase-α1 (AMPK α1)/HIF-1α pathway. Moreover, curcumin has also decreased glucose uptake and lactate synthesis (Warburg effect) in a variety of cancer cell lines including H1299, HEK293, PC3, HeLa and MCF-7 by suppressing pyruvate kinase M2 expression via inhibition of mTOR-HIF-1α axis (Siddiqui et al. 2018).
Cancer-associated fibroblasts (CAFs) are one of the key components of tumor microenvironment which promotes tumorigenesis, EMT, metastasis, and acquisition of stem cell traits in cancer cells via multiple mechanisms (Liu et al. 2019). It has been found to augment EMT, invasion, ROS generation and expression of CXC4 and IL-6 receptor in prostate cancer cells by activating monoamine oxidase A/mTOR/HIF-1α signaling. However, in a study by Du et al., curcumin effectively impeded CAF is driven (i) invasion and EMT, (ii) ROS generation and (iii) expression of CXC4 and IL-6 receptor expression in prostate cancer cells through the down-regulation of monoamine oxidase A/mTOR/HIF-1α signaling (Du et al. 2015).
Apart from curcumin, EF24 and tertahydrocurcumin (novel analog and active in vivo metabolite of curcumin, respectively) have also been shown to inhibit the activity of HIF-1α during cancer. EF24 reduced HIF-1α protein levels in MDA-MB231 breast and PC3 prostate cancer cells in a VHL-dependent but proteasome-independent manner (Thomas et al. 2008). Tetrahydrocurcumin abrogated tumor neovascularization in CaSki (cervical cancer cell line) implanted female nude mice by downregulating HIF-1α/VEGF/VEGF R2 pathway (Yoysungnoen et al. 2015). These observations suggest that curcumin acts in multifaceted manner to inhibit all the pathological pathways triggered by HIF-1α in tumor cells.
Quercetin
Quercetin is a plant flavonoid found in many leaves, fruits, and vegetables including green tea, apples, berries, onions, red wine, etc. (Anand-David et al. 2016). It is a well-known anti-oxidant with potent anti-cancer properties and is a safe phytochemical which shows no or minimal side effects even at higher doses. Quercetin can effectively inhibit the growth and progression of human cancers by directly exhibiting its pro-apoptotic effect on tumor cells (Rauf et al. 2018a, b). In this section, we will discuss the anti-cancer effects of quercetin against hypoxic tumors.
In majority of the hypoxic tumors including LNCaP human prostate cancer cells, CX-1 colon cancer cells, SkBr3 breast cancer cells (Lee and Lee 2008), NCI-H157 lung cancer cells (Ansó et al. 2010) and HOS and MG 63 osteosarcoma cells (Wang et al. 2011), quercetin has been found to exert its chemotherapeutic effects by attenuating the synthesis of HIF-1α and its downstream target, VEGF.
Kim et al. intensively evaluated the chemotherapeutic efficacy of quercetin on HCT 116 colon cancer cells under both normoxic and hypoxic conditions (Kim et al. 2012). Fascinatingly, quercetin has been found to act more effectively under hypoxic conditions than under normoxic conditions in these cells. It has been found to promote apoptosis of HCT 116 cells in vitro and suppress tumor growth in HCT 116 cells xenografted in vivo model by attenuating the activity of hypoxia-induced AMPK (an enzyme that plays a protective role against hypoxia) and decreasing the activity of HIF. More importantly, quercetin also improved the therapeutic response of hypoxic HCT116 cells to cisplatin and etoposide.
Du et al. analyzed the chemosensitizing potential of quercetin in BALB/c mice bearing 4T1 breast cancer to doxorubicin therapy (Du et al. 2010). They have found that quercetin could retard the tumor growth and prolong the survival of these mice by enhancing the apoptotic potential and systemic toxicity of doxorubicin and simultaneously reducing doxorubicin-induced toxic side effects by suppressing intratumoral HIF-1α in a hypoxia-dependent way in tumor cells.
While most studies suggest that quercetin exhibits its anti-tumor potential by abolishing the activity of HIF-1α, a study done by Bach et al. shows that quercetin inhibits the proliferation of human HepG2 hepatoma cells through HIF-1-dependent induction of cell cycle inhibitor p21WAF (Bach et al. 2010). They have found that quercetin inhibits the growth of human HepG2 hepatoma by prolonging the half-life of HIF-1α protein. These findings, in turn, suggest that quercetin exhibits its chemotherapeutic potential by targeting HIF-1α through different mechanisms in different types of cancer.
Genistein
Genistein, a natural isoflavonoid found abundantly in soy products, is a potent phytochemical with amazing anti-cancer properties (Spagnuolo et al. 2015). Numerous epidemiological studies show that consumption of soy products rich in genistein is responsible for the lower incidence of cancer in Asian populations (Messina 2016). Genistein acts via multiple mechanisms to fight wide varieties of cancer under both normoxic and hypoxic conditions. This section will discuss the pro-apoptotic, anti-angiogenic and radiosensitizing potential of genistein on hypoxic tumor cells.
Li et al. intensively evaluated the inhibitory effects of genistein on HIF-1α activity and aerobic glycolysis using five human hepatocellular carcinoma (HCC) cell lines (HCC-LM3, SMMC-7721, Hep3B, Bel-7402, and Huh-7) and a normal hepatic cell line (LO2) (Li et al. 2017). Interestingly, they have found that genistein could abrogate aerobic glycolysis and trigger apoptosis of HCC cell lines by down-regulating HIF-1α and thereby inactivating glucose transporter 1 (GLUT1) and hexokinase-2 (HK2) activity. Furthermore, in this study, Li et al. have also investigated the potential of genistein to overcome sorafenib resistance in HCC cells in vivo using subcutaneous xenograft mouse models. Surprisingly, genistein has augmented the ability of sorafenib to reduce tumor size and increased the apoptotic area in hepatoma-bearing mice by down-regulating HIF-1α/GLUT1/HK2 activity. These shreds of evidence show that genistein could act effectively against advanced stage HCC by targeting HIF-1α pathway. Moreover, in A549 lung cancer cells, genistein has inhibited proliferation and promoted apoptosis by attenuating the expression of HIF-1α through the down-regulation of PI3K/Akt signaling pathways (Zhang et al. 2017).
Bilir et al. conducted a randomized, placebo-controlled, double-blinded clinical trial on prostate cancer patients by supplementing them with 30 mg genistein or placebo capsules daily for 3–6 weeks before prostatectomy and analyzed the genes modulated by genistein prior to prostatectomy using microarray technology. Intriguingly, HIF-1α is one of the genes significantly down-regulated by genistein in these patients (Bilir et al. 2017).
Buchler et al. investigated the anti-angiogenic efficacy of genistein on Capan-1, Capan-2, AsPc-1, PANC-1, and Mia PaCa-2 human pancreatic cancer cells under both normoxic and hypoxic conditions. Surprisingly, genistein has been found to inhibit tumor neovascularization particularly under low oxygen levels in these cells by decreasing the expression of HIF-1α and VEGF (Büchler et al. 2004). Likewise, genistein has also retarded hypoxia-mediated retinal neovascularization in retinal pigment epithelial cells by blocking the expression of HIF-1α protein (Wang et al. 2005).
APE1/Ref-1, a protein involved in the activation of HIF-1α and NF-κB is associated with increased resistance of cancer patients to chemoradiotherapy. In two independent studies done by Gupta et al., genistein along with other soy isoflavones including daidzein and glycetin have been shown to effectively enhance the response of prostate cancer cells (Singh-Gupta et al. 2009) and A549 lung cancer cells (Singh-Gupta et al. 2011) to radiotherapy by inhibiting the expression of HIF-1α, NF-κB and APE1/Ref-1. Moreover, in hypoxic MCF-7 cancer cells, pre-treatment with genistein has been found to promote their death when subject to radiotherapy via down-regulation of 4 Gy radiation-induced NF-κB signaling (Aravindan et al. 2013). Fascinatingly, in this study, the same chemosensitizing effects exhibited by genistein were found to be exhibited by other herbal nutraceuticals including resveratrol and curcumin. Collectively, all these evidence suggest that genistein could act as a potent anti-cancer agent on hypoxic tumors.
Berberine
Berberine, an isoquinoline alkaloid found in roots, rhizomes, stems, and bark of Berberis plants is a potent anti-cancer agent with anti-proliferative and pro-apoptotic effects (Zou et al. 2017). Current evidence shows that berberine majorly targets HIF-1 pathway to regulate the activity of various enzymes, genes, and proteins involved in cancer pathogenesis.
Lin et al. investigated the anti-angiogenic potential of berberine by co-culturing gastric adenocarcinoma cell lines, SC-M1 with HUVEC under hypoxic conditions and analyzing the cross-talk between these cells (Lin et al. 2004). Interestingly, berberine has been found to inhibit the stimulatory potential of hypoxic SC-M1 cells on HUVEC migration by repressing the expression of HIF-1α and VEGF in SC-M1 cells and promoting HIF-1α degradation via a proteasomal proteolytic pathway and lysine acetylation. Moreover, in studies done by Mao et al. using colon cancer cells, berberine impeded hyperactive glucose uptake and glycolysis in these cells by suppressing mTOR-mediated HIF-1α synthesis (Mao et al. 2018). In non-small cell lung cancer cells, this Chinese herbal extract retarded tumor growth by simultaneously targeting HIF-1α/VEGF, AP-2/hTERT, NF-κB/COX-2, PI3K/Akt, Raf/MEK/ERK and Cytochrome C/Caspase signaling pathways (Fu et al. 2013).
Mounting evidence shows that berberine could act as a potent chemo/radiosensitizer and enhance the response of wide varieties of hypoxic cancer cells to chemo and radiotherapy. In a study done by Zhang et al., berberine improved the sensitivity of hypoxic LNCaP and DU-145 prostate cancer cell lines to ionizing radiation by repressing the expression of HIF-1α and VEGF (Zhang et al. 2014a, b). Fascinatingly, similar results have been obtained by the same research team in another study wherein berberine sensitized hypoxic CNE-1 and CNE-2 nasopharyngeal carcinoma cells and male nude mice inoculated subcutaneously with CNE-2 cells to radiotherapy and promoted their apoptosis by inhibiting hypoxia/radiation-induced synthesis of HIF-1α and VEGF (Zhang et al. 2014a, b). Even more interestingly, results similar to that of Zhang et al. have also been obtained by Yang et al. in esophageal squamous cell carcinoma cells. In their in vitro and in vivo studies, Yang et al. have found that berberine could radiosensitize hypoxic ECA109 esophageal squamous cell carcinoma cells and ECA109 xenografted nude mice by impeding HIF-1α and VEGF expression (Yang et al. 2013). Pan et al. investigated the potential of berberine to reverse doxorubicin resistance in breast cancer cells by conducting in vitro and in vivo studies using doxorubicin-resistant MCF-7 breast cancer cells and xenografted mice model bearing doxorubicin resistant MCF-7 tumor, respectively (Pan et al. 2017). Doxorubicin resistance was induced in MCF-7 cells by constantly exposing them to hypoxia for 1 week. These drug-resistant cells were then treated with different doses of berberine. At low doses, berberine has been found to augment the chemosensitivity of MCF-7 cells to doxorubicin by inactivating AMPK/HIF-1α/P-glycoprotein pathway. However, at high doses, berberine directly triggered cell apoptosis by activating p53 through the down-regulation of AMPK/HIF-1α pathway. All these studies show that berberine could act in a pleiotropic manner to fight the oncogenic effects of HIF-1α pathway in tumor cells.
Saponins
Saponins are naturally occurring amphipathic glycosides with diverse pharmacological properties. They are found abundantly throughout the plant kingdom and are characterized by the formation of soap-like foam in aqueous solutions. Structurally, they possess one or more hydrophilic glycoside moieties combined with a lipophilic triterpene derivative. There are 11 main classes of saponins including dammarane, oleananes, ursane, lanostanes, cucurbitane, tirucallane, hopane, cycloartane, lupane, taraxasterane and steroids (Vincken et al. 2007). All these classes of saponins are well known for their potent anti-tumor and antioxidant properties (Xu et al. 2016). Interestingly, emerging studies show that the majority of these saponins could also act effectively against the survival, growth, and progression of hypoxic tumors by targeting HIF-1 signaling pathways. This section will discuss the molecular mechanisms underlying the therapeutic efficacy of various saponins on hypoxic tumors.
20 ®-Ginsenoside (Rg3) is a dammarane saponin extracted from ginseng (Xu et al. 2016). Liu et al. conducted two independent studies both in vitro and in vivo to investigate the pharmacological effects of Rg3 in ovarian cancer cells. Fascinatingly, Rg3 inhibited tumor growth (Liu et al. 2017) and also blocked EMT (Liu et al. 2014) in these cells by promoting the ubiquitin–proteasome-mediated degradation of HIF-1α. In Eca-109 and 786-o human oesophageal carcinoma cell lines, Rg3 retarded tumor growth and angiogenesis under both normoxic and hypoxic conditions by attenuating the expression of VEGF via down-regulation of HIF-1α, COX-2, NF-κB, hypoxia-induced phosphorylation of STAT3, ERK 1/2 and JNK (Chen et al. 2010). Zeng et al. investigated the anti-angiogenic potential of Rg3 in the bone marrow stromal cells derived from patients with acute leukemia (Zeng et al. 2014). Interestingly, Rg3 inhibited the HIF-1α and VEGF expression at both transcriptional and translational level in the bone marrow stromal cells by down-regulating PI3K/Akt and ERK1/2 pathways. Moreover, Rg3 also significantly reduced the serum levels of HIF-1α and VEGF in acute leukemia patients. This evidence shows that Rg3 could effectively abrogate the metastatic events including EMT and angiogenesis in hypoxic cancer cells. Furthermore, in a study done by Ge et al., pre-treatment of human oesophageal carcinoma cell lines (EC109, Te1 and KYSE 170) with Rg3 under hypoxic conditions led to an increased response of these cells to radiotherapy via inhibition of HIF-1α and VEGF (Ge et al. 2014). Likewise, Rg3 also sensitized the hypoxic lung cancer cells to cisplatin therapy by blocking hypoxia and abrogating hypoxia-induced EMT and stemness through the modulation of the NF-κB pathway (Wang et al. 2018). These studies, in turn, suggest that Rg3 could act as a potent chemo/radiosensitizer of hypoxic tumor cells.
DT-13 is a saponin monomer of Dwarf lilyturf tuber which is a traditional Chinese medicinal plant with potent medicinal values. It has been shown to inhibit hypoxia-induced adhesion and migration of MDA-MB-435 breast cancer cells by suppressing the expression of αvβ3 integrin, tissue factor, early growth response gene-1 (Egr-1) and MMP-9 (Sun et al. 2010). Furthermore, DT-13 also suppressed tube formation and migration in human umbilical vein endothelial cells under both normoxia and hypoxia by down-regulating the expression of HIF-1α, p-ERK 1/2, p-Akt, VEGF and p-VEGFR2 (Zhao et al. 2013). Like DT-13 from Dwarf lilyturf tuber, DT-13 extracted from a plant named Ophiopogon japonicas is also found to be highly effective against breast cancer cells. In a study done by Pin et al., DT-13 extracted from Ophiopogon japonicas retarded MDA-MB-435 cell proliferation, adhesion, and migration in vitro and lung metastasis in vivo by abrogating the expression of HIF-1α, VEGF and C–C chemokine receptor type 5 (CCR5) (Ren-Ping et al. 2014).
Astragalus saponins are the total saponins isolated from the medicinal herb Radix Astragali. In hypoxic HCT 116 colon cancer cells and the tumor tissues of HCT 116 cells xenografted athymic nude mice, they have been found to dramatically attenuate the production of HIF-1α and VEGF, p-Akt, p-mTOR, VEGFR1 and VEGFR2 (Law et al. 2012).
Investigations done by Jia et al. on the effect of tea saponins (triterpenoid saponins composed of sapogenins, glycosides and organic acids) against OVCAR-3 and A2780/CP70 ovarian cancer cell lines show that tea saponins exert their pro-apoptotic and anti-angiogenic activities by augmenting the extrinsic pathway and lowering VEGF protein levels, respectively, through the modulation of HIF-1α signaling pathway (Jia et al. 2017).
Oleanolic acid, a naturally occurring pentacyclic triterpenoid saponin found in garlic, olive oil, etc., has been reported to retard the proliferation of rectal cancer cells by targeting NADPH oxidase 2/ROS/HIF-1α signaling (Guo et al. 2017).
α-solanine is a bitter-tasting steroidal alkaloid saponin found in potato, tomato, and eggplant. Under hypoxic conditions, α-solanine has been shown to retard the growth of tumor cells by suppressing the expression of VEGF, E-cadherin and down-regulating ERK1/2/HIF-1α and STAT3 signaling pathways (Wen et al. 2016). In HCC SMMC-7721 hepatocellular carcinoma cells, Saikosaponin-d (a saponin derivative extracted from several species of Bupleurum), has been found to exhibit its anticancer activity by inhibiting the expression of COX-2 via p-STAT3/HIF-1α pathway (He et al. 2014). Son et al. investigated the therapeutic efficacy of SB365 Pulsatilla saponin D on five types of human pancreatic cancer cell lines (MIAPaCa-2, BXPC-3, PANC-1, AsPC-1 and HPAC) and have found that SB365 could down-regulate neovascularization in these cells by blocking the expression of HIF-1α and VEGF (Son et al. 2013). Diosgenin, a steroid saponin constituent of yam and fenugreek has been shown to promote apoptosis and suppress invasion in hypoxic gastric cancer BGC-823 cells (Mao et al. 2012). More interestingly, the anticancer effects of diosgenin in these cells have been found to be ameliorated in the presence of HIF-1α specific short hairpin RNA. Polyphyllin D, a steroidal saponin extracted from the Chinese medicinal herb Paris polyphylla has been demonstrated to inhibit proliferation and enhance apoptosis of Lewis lung cancer cells and mouse tracheal epithelial cells under both normoxic and hypoxic conditions. Also, polyphyllin D abrogated the expression of HIF-1α and VEGF in Lewis cells (Ma et al. 2009). All these shreds of evidence show that different classes of saponins derived from different plant species act by down-regulating HIF-1α to suppress the growth and progression of hypoxic tumors.
Carotenoids
Carotenoids are a ubiquitous group of isoprenoid pigments belonging to a parent class of molecules called tetraterpenoids. These organic pigments produced majorly by plants, algae, bacteria, and fungi are responsible for the classic colors of carrots, corn, daffodils, corn, bananas, canaries, rutabagas, and buttercups. There are different types of carotenoids including zeaxanthin, α-carotene, β-carotene, crocetinate, lutein, lycopene, etc. They are potent anti-oxidants with fascinating chemopreventive and chemoprotective properties (Tanaka et al. 2012). In this section, we will discuss the therapeutic potential of various carotenoids against hypoxic tumors.
Trans sodium crocetinate (TSC) is an antineoplastic carotenoid which is known for its ability to enhance oxygen diffusion to the hypoxic tissues (Lapchak 2010). Sheehan and his research team have conducted many in vivo and preclinical studies to investigate the therapeutic benefits of TSC on intratumoral hypoxia in C6 glioma cells using oxygen-specific positron emission tomography imaging or magnetic resonance imaging. In vivo studies were conducted in rats whose right frontal region of brains was stereotactically implanted with C6 glioma cells. Pre-clinical studies were conducted in glioblastoma multiforme (GBM) patients. In one in vivo study, Sheehan et al. evaluated the pharmacological effects of TSC on glioma cells implanted rats. As expected, TSC significantly reduced intratumoral hypoxia and increased the oxygen diffusion to tumor cells in these rats (Sheehan et al. 2011). In another in vivo study, Sheehan et al. elucidated the chemo and radiosensitizing potential of TSC by treating the glioma cells implanted rats with TSC, temozolomide and radiation therapy (Sheehan et al. 2010). Interestingly, TSC improved the response of glioma cells to radiation therapy and temozolomide treatment. The results obtained in the in vivo studies were further validated by Gainer et al. in phase I/II clinical trial wherein the chemo radiosensitizing potential of TSC was tested on 59 GBM patients receiving temozolomide and radiotherapy (Gainer et al. 2017). For this study, GBM patients were subject to 6 weeks of radiotherapy consisting of 2 Gy/day for 5 days/week. Temozolomide (TMZ), 75 mg/m2, was given before each radiotherapy session. TSC, 0.25 mg/kg, was intravenously administered around 45 min before a radiotherapy session 3 days/week. Fascinatingly, GBM patients treated with TSC showed an amazingly enhanced response to chemo and radiotherapy. Taken together, these study results suggest that TSC is highly effective in augmenting the response of glioma cells to chemotherapy and radiotherapy. Furthermore, Sheehan et al. also conducted in vivo studies to elucidate the molecular mechanism underlying the radiosensitizing potential of TSC. They have found that the efficacy of TSC to induce transient tissue oxygenation in the hypoxic gliomas is majorly responsible for its ability to augment the response of glioma cells to radiotherapy (Sheehan et al. 2009).
Lutein is a xanthophylls carotenoid produced only by plants and is found abundantly in green leafy vegetables. Li et al. intensively studied the anti-proliferative and pro-apoptotic potential of lutein on hypoxic MDA-MB-157 and MCF-7 human breast cancer cells and have found that lutein acts by abrogating hypoxia-induced (i) activation of HIF-1α, NOTCH signaling and HES1 expression, (ii) expression of EMT-associated factors, (iii) invasion and migration of breast cancer cells and (iv) synthesis of ROS in these cells (Li et al. 2018). This evidence shows that dietary supplementation with lutein could counteract the pathogenic events triggered in hypoxic breast cancer cells.
β-carotene, the most common form of carotene in plants, has been found to inhibit the invasion and metastasis of neuroblastoma cells by targeting HIF-1α (Kim et al. 2014). Furthermore, lycopene, a bright red carotenoid pigment found in tomatoes, other red fruits and vegetables, has been shown to counteract the N-nitrosodiethylamine-induced HCC in female Balb/c mice by decreasing the protein levels of prominent biomarkers of hypoxia, angiogenesis, and metastasis including HIF-1α, VEGF, CD31, AFP, MMP-2, and MMP-9 (Bhatia et al. 2015). Zeaxanthin, the most common carotenoid alcohol found in nature has also been shown to impede hypoxia-mediated neovascularization in retinal pigment epithelial cells by decreasing the protein levels of HIF-1α (Rosen et al. 2015). Taken together, these study results show that carotenoids act very effectively against hypoxic tumors by down-regulating the expression, synthesis, and activity of HIF-1α.
Isothiocyanates
Isothiocyanates are sulfur-containing nutraceuticals found abundantly in vegetables including wasabi, horseradish, mustard, radish, Brussels sprouts, watercress, papaya seeds, nasturtiums, and capers. The most common isothiocyanates are phenethyl isothiocyanate (PEITC), benzyl isothiocyanate (BITC), and sulforaphane. Isothiocyanates are highly effective in inactivating and detoxifying the ingested carcinogens. Their anti-tumorigenic effects are proven by various epidemiological studies which show an inverse correlation between the increased dietary intake of isothiocyanates and reduced incidence of cancer (Gupta et al. 2014; Anubhuti et al. 2016). This section will discuss in detail about the anti-cancer effects of these isothiocyanates on hypoxic tumors.
PEITC is a potent isothiocyanate derivative found in various cruciferous vegetables. It has been found to impede hypoxia-driven HIF-1α expression and accrual in a variety of cancer cell lines including human glioma U87, human prostate cancer DU145, colon cancer HCT116, liver cancer HepG2, and breast cancer SkBr3 cells through the down-regulation of PI3K and MAPK signaling pathways (Gupta et al. 2013). In MCF-7 breast cancer cells, PEITC decreased HIF-1α translation by impeding mTORC1 activity and dephosphorylating 4E-BP1 and p70 S6K, well-characterized downstream substrates of the mTOR-containing mTORC1 complex (Cavell et al. 2012) (153). Wang et al. investigated the therapeutic efficacy of PEITC on hypoxic tumor cells and have found that PEITC could retard tumor growth and neovascularization in these cells by abrogating the activity of HIF-α and its downstream endogenous targets genes including CAIX, GLUT1, BNIP3 AND VEGF-A (Wang et al. 2009). Also, in a study done by Sarkar et al., PEITC suppressed the metastatic events including migration, adhesion, aggregation and angiogenesis in both normoxic and hypoxic cancer cells by down-regulating the expression of HSP90 and thus HIF-1α through the (i) induction of nuclear accumulation of Nrf2, (ii) activation of several antioxidant enzymes and (iii) reduction of ROS encumbrance (Sarkar et al. 2015).
Sulforaphane is another natural dietary isothiocyanate obtained from broccoli and other vegetables including Brussels sprouts and cabbage. Like PEITC, sulforaphane has also been shown to act diversely in different tumor cells to counteract the pathological effects of HIF-1α. In human tongue squamous cell carcinoma and prostate cancer cells, sulforaphane blocked the tumor proliferation by inhibiting hypoxia-induced HIF-1α synthesis through the up-regulation JNK and ERK signaling pathways (Yao et al. 2008). In A549 lung cancer cells, sulforaphane inhibited tumor progression by impeding the transcription of microsomal prostaglandin E synthase-1 and thus the production of the prostaglandin E2, a potent tumor growth promoter by targeting HIF-1α (Zhou et al. 2012). In HCT 116 colon cancer cells, sulforaphane retarded tumor progression and angiogenesis by abrogating the expression of HIF-1α and VEGF (Kim et al. 2015). In hepatic cancer cells, sulforaphane attenuated glycolysis by down-regulating the expression of a key molecule of glycolysis namely, 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase4 (PFKFB4) protein in a HIF-1α dependent pathway (Jeon et al. 2011). On top of exerting these therapeutic effects, sulforaphane has also augmented the response of hypoxic Adriamycin-resistant A2780/ADR and cisplatin-resistant A2780/CP ovarian cancer cells to adriamycin and cisplatin, respectively, by effectively suppressing the expression of HIF-1α and its major downstream target, CA IX (which safeguards the tumor cells from hypoxia-mediated pH imbalance and enable their migration/invasion) and stimulating the expression of tumor suppressor proteins including p53, ARE, IRF-1, Pax-6, and XRE (Pastorek et al. 2015).
Terpenoids
Terpenoids, the largest class of naturally occurring terpene-derived organic chemicals have been reported to inhibit cell proliferation, angiogenesis, and metastasis of a wide variety of cancers by inhibiting or degrading HIF-1α (Tholl 2015). In the previous sections, we have already discussed the therapeutic potential of various terpenoids including α-solanine, diosgenin, saikosaponin, etc. against hypoxic tumors. In this section, we will discuss the efficacy of other terpenoids to counteract the pathogenic effects of HIF-1α in cancer.
Tanshinone IIA (T2A), a phenanthrenequinone constituent of Chinese medicinal herb Danshen has been shown to effectively inhibit neovascularization in a variety of hypoxic tumor cells via diverse mechanisms. In colorectal cancer, it inhibited β-catenin/VEGF-mediated angiogenesis by targeting TGF-β1 in normoxic and HIF-1α in hypoxic microenvironments (Sui et al. 2017) (166). In hypoxic MDA-MB-231 and MCF-7 breast cancer cells, T2A suppressed angiogenesis and thus their growth by repressing VEGF expression through the inhibition of HIF-1α activity via mTOR/P70S6K/4E-BP1 signaling pathways (Li et al. 2015). Likewise, T2A also impeded angiogenesis and growth of human breast xenograft in nude mice through the suppression of HIF-1α and VEGF (Wang et al. 2015). Apart from inhibiting angiogenesis, T2A is also found to be effective in ameliorating hypoxia-mediated doxorubicin resistance and EMT in MCF-7 and HCC1973 breast cancer cell lines by decreasing the expression of HIF-1α (Fu et al. 2014).
Celastrol is a pentacyclic triterpenoid isolated from the root extracts of Tripterygium wilfordii. Emerging reports show that celastrol is highly effective in impeding the oncogenic effects of HIF-α in hypoxic tumor cells. It blocked hypoxia-induced neovascularization and metastasis of HepG2 hepatoma and A549 human lung adenocarcinoma epithelial cells by regulating HIF-1α activity at multiple levels (Huang et al. 2011). Furthermore, celastrol also retarded proliferation, migration and tube formation in EA/hy926 human umbilical vein endothelial cells under hypoxic conditions by targeting HIF-1α (Huang et al. 2011). In a study done by Ma et al., celastrol impeded hypoxia-mediated VEGF and erythropoietin production in human hepatoma cells by blocking HIF-1α protein synthesis through the downregulation of mTOR/P70S6K/eIF4E and ERK1/2 pathways and decreased the growth of Hep3B cells in xenograft tumor models by inhibiting the synthesis of HIF-1α proteins (Ma et al. 2014a, b). Similarly, andrographolide, a labdane diterpenoid isolated from the medicinal herb Andrographis paniculata Nees, has also been shown to inhibit the growth and neovascularization in Hep3B and HepG2 hepatoma cells by promoting ubiquitin-mediated HIF-1α protein degradation via JNK and MTA1/HDAC1 pathways (Shi et al. 2017).
Terpenoids including brusatol, parthenolide, genipin, oleuropein have been found to act effectively against colon cancer by targeting HIF-1α and its gene targets. Brusatol has been shown to kill a variety of hypoxic colorectal cancer cell lines including RKO, HCT116, SW480, CoLo205, DLD-1, HT29 and HCT115 by inhibiting HIF-1α expression in a dose-dependent manner through the down-regulation of c-MYC and ROS production and up-regulation of PHD activity (Oh et al. 2017). Moreover, in studies done by Lu et al. brusatol, it has also been found to (i) degrade HIF-1α, (ii) suppress the expression of HIF-1 target genes including VEGF, GLUT1, HK-2 and LDHA in HCT 116 colon cancer cells, (iii) reduce excess glucose consumption and (iv) decrease intracellular and mitochondrial ROS activity in hypoxic HCT116 colon cancer cells (Lu et al. 2016). Parthenolide, a sesquiterpene lactone has been demonstrated to suppress growth, angiogenesis, and invasion of colon cancer cells by impeding HIF-1α signaling and hypoxia-mediated EMT through the inactivation of NF-κB (Kim et al. 2017a, b). Furthermore, genipin, a monoterpene derived from gardenia fruit extract, has been found to inhibit migration and invasion of colon cancer cells by preventing HIF-1α accumulation and VEGF synthesis (Lee et al. 2018). Similarly, oleuropein, a secoiridoid obtained from an olive tree, has also been demonstrated to block the proliferation of colorectal cancer cells by downregulating HIF-1α (Cárdeno et al. 2013).
Studies done by Karna et al. show that betulinic acid, a naturally occurring pentacyclic triterpenoid, could retard blood vessel formation in human endometrial adenocarcinoma cells by preventing the synthesis of collagen, HIF-1α and VEGF (Karna et al. 2010). Triptolide, another terpenoid isolated from Tripterygium wilfordii, has been reported to block the growth of PANC1, ASPC2 and SW1990 pancreatic cancer cells by impeding HIF-1α expression via c-MYC-dependent mechanisms (Chen et al. 2013). Cucurbitacin B, a triterpenoid compound obtained from Trichosanthes kirilowii Maximowicz, inhibited the growth of various hypoxic cancer cells at in vitro and in vivo level by inactivating HIF-1α (Ma et al. 2014a, b). Furthermore, pristimerin, a bioactive terpenoid retarded HIF-1α accrual in the dose- and time-dependent manner in hypoxic PC-3 prostate cancer cells through the suppression of SPHK-1 pathway (Lee et al. 2016).
In addition to executing their anti-tumor potential, terpenoids are also found to be effective in sensitizing tumor cells to chemo and radiotherapy by inhibiting HIF-1α activity. Oleuropein enhanced the sensitivity of nasopharyngeal carcinoma cells HNE1 and HONE 1 to radiotherapy at in vitro and in vivo level by decreasing the activity of HIF-1α/miR519d/PDRG pathway (Xu and Xiao 2017). Triptolide triggered the apoptotic potential of doxorubicin and imatinib in HL60/A and K562/G myeloid leukemia cells by down-regulating the expression of HIF-1α and Nrf2 at the transcriptional and translational level (Chen et al. 2013). Furthermore, Pachymic acid, a natural triterpenoid found in Poria cocos has been found to aggravate the sensitivity of gastric cancer cells to radiation therapy at in vitro and in vivo level by inducing the expression of pro-apoptotic Bax through the inhibition of hypoxia-mediated HIF-1α (Lu et al. 2018). Collectively, these studies show that terpenoids could effectively suppress cancer-promoting mechanisms including proliferation, progression, metastasis and drug resistance in hypoxic tumors by targeting HIF-1α.
Conclusions and future perspectives
The pathogenic role played by hypoxia-induced HIF-1α and its downstream HIF-1 signaling pathways in the development of aggressive disease, drug resistance, and metastasis acts as a major hurdle for the efficient treatment of cancer. Despite the fact that cancer can be effectively treated by down-regulating HIF-1 pathways, currently existing therapies do not deal with the same. Also, most of the synthetic inhibitors developed against HIF-1 pathways do not meet all expectations concerning efficacy and safety. Hence, cancer remains a dreadful disease till date. This review has put forth numerous evidences that obviously show that herbal nutraceuticals possess a great potential to combat the oncogenic effects of HIF-1α. As described in the previous sections, every herbal nutraceutical acts as a multitarget drug and regulates the key components of HIF-1α-mediated signal transduction pathways. They interfere with the progression of angiogenesis, epithelial-mesenchymal transition, cell proliferation, invasion and metastasis in hypoxic tumor cells (Fig. 3). Furthermore, emerging studies also show that herbal nutraceuticals could act as potent chemo/radiosensitizers and improve the response of hypoxic cancer cells to conventional therapies by targeting HIF-1 pathway (Table 1). Taken together, the present review strongly suggests that herbal nutraceuticals are highly effective in combating the oncogenic effects of the HIF-1 pathway in wide varieties of tumors.
Nevertheless, most of these studies validating the potential of herbal nutraceuticals to inhibit the survival, growth, and progression of hypoxic tumors have been carried out in vitro using different cancer cell lines. Very few in vivo studies have been done to date using xenografted athymic mice. This is due to the limitations and difficulties in establishing a hypoxic microenvironment in a mouse model system. Moreover, athymic mice are very expensive to procure and maintain and it requires at least 2–6 months to deliver a verdict. However, these problems could be easily overcome by employing zebrafish as an alternative model system (Lee et al. 2009). Zebrafish are cheap and easy to maintain and produce results within 2 weeks. Most importantly, hypoxia-induced pathological events in cancer cells including survival, growth, angiogenesis, metastasis and drug resistance can be easily and effectively studied in vivo using zebrafish embryos, because these embryos could be easily placed in hypoxic water (7.5% air saturation). Also, the transparent and immunoprivileged nature of zebrafish embryos allows the researchers to easily inject the fluorescently labeled cancer cells into their perivitelline cavity and monitor the pathological events in tumors easily under a confocal microscope. These advantages of zebrafish embryos could be effectively utilized to investigate the therapeutic efficacy of various herbal nutraceuticals against the metastatic events (including tumor cell dissemination, invasion, and angiogenesis) of cancer under both normoxic and hypoxic conditions. The chemosensitizing effects of herbal nutraceuticals against hypoxic tumors could also be easily studied by treating the tumor-bearing zebrafish embryos with both the conventional drugs and the herbal nutraceuticals of interest under hypoxic conditions. To investigate the therapeutic effects of herbal nutraceuticals against hypoxic tumors pre-clinically, the tumor cells derived from the cancer patients during biopsy could be injected into the zebrafish embryos and then treated with herbal nutraceuticals under hypoxic conditions.
Many of the synthetic HIF-1 pathway inhibitors fail clinical trials in cancer patients, because these patients are randomly chosen without determining their circulating and tumor HIF-1α levels. Hence, in the future clinical trials, the effect of herbal nutraceuticals and other inhibitors against hypoxic tumors could be effectively assessed if the cancer patients with elevated serum and tumor HIF-1α levels are chosen for the study (Baba et al. 2010; He et al. 2016).
To conclude, the robust and multifaceted approach of herbal nutraceuticals to ameliorate the pathological events augmented by HIF-1α in hypoxic tumors is well documented. Hence, it is plausible that herbal nutraceuticals have enormous potential for consideration as important, safe and pharmacologically active phytotherapeutics to fight tumor hypoxia. However, more investigations at in vivo level using zebrafish as a model system and at the clinical level in cancer patients with elevated tumor HIF-1α is highly warranted to ascertain their effective utilization as adjunct/alternative medicine in clinical practice to treat cancer.
References
Anand-David AV, Arulmoli R, Parasuraman S (2016) Overviews of biological importance of quercetin: a bioactive flavonoid. Pharmacogn Rev 10(20):84–89
Ansó E, Zuazo A, Irigoyen M, Urdaci MC, Rouzaut A, Martínez-Irujo JJ (2010) Flavonoids inhibit hypoxia-induced vascular endothelial growth factor expression by a HIF-1 independent mechanism. Biochem Pharmacol 79(11):1600–1609
Anubhuti Sh, Ashok Sh, Prashant Y, Dhiraj S (2016) Isothiocyanates in brassica: potential anti cancer agents. Asian Pac J Cancer Prev 17(9):4507–4510
Aravindan S, Natarajan M, Herman TS, Awasthi V, Aravindan N (2013) Molecular basis of ‘hypoxic’ breast cancer cell radio-sensitization: phytochemicals converge on radiation induced Rel signaling. Radiat Oncol 8(46):1–12
Baba Y, Nosho K, Shima K, Irahara N, Chan AT, Meyerhardt JA, Chung DC, Giovannucci EL, Fuchs CS, Ogino S (2010) HIF1A overexpression is associated with poor prognosis in a cohort of 731 colorectal cancers. Am J Pathol 176:2292–2301
Bach A, Bender-Sigel J, Schrenk D, Flügel D, Kietzmann T (2010) The antioxidant quercetin inhibits cellular proliferation via HIF-1-dependent induction of p21WAF. Antioxid Redox Signal 13:437–448
Bae MK, Kim SH, Jeong JW, Lee YM, Kim HS, Kim SR, Yun I, Bae SK, Kim KW (2006) Curcumin inhibits hypoxia-induced angiogenesis via down-regulation of HIF-1. Oncol Rep 15(6):1557–15562
Bar-Sela G, Epelbaum R, Schaffer M (2010) Curcumin as an anti-cancer agent: review of the gap between basic and clinical applications. Curr Med Chem 17(3):190–197
Bhatia N, Gupta P, Singh B, Koul A (2015) Lycopene enriched tomato extract inhibits hypoxia, angiogenesis, and metastatic markers in early stage N-nitrosodiethylamine induced hepatocellular carcinoma. Nutr Cancer 67(8):1268–12675
Bilir B, Sharma NV, Lee J, Hammarstrom B, Svindland A, Kucuk O, Moreno CS (2017) Effects of genistein supplementation on genome-wide DNA methylation and gene expression in patients with localized prostate cancer. Int J Oncol 51:223–234
Büchler P, Reber HA, Büchler MW, Friess H, Lavey RS, Hines OJ (2004) Anti-angiogenic activity of genistein in pancreatic carcinoma cells is mediated by the inhibition of hypoxia-inducible factor-1 and the down-regulation of VEGF gene expression. Cancer 100(1):201–210
Burroughs SK, Kaluz S, Wang D, Wang K, Van Meir EG, Wang B (2013) Hypoxia inducible factor pathway inhibitors as anticancer therapeutics. Future Med Chem 5:553–572
Cárdeno A, Sánchez-Hidalgo M, Rosillo MA, de la Lastra CA (2013) oleuropein, a secoiridoid derived from olive tree, inhibits the proliferation of human colorectal cancer cell through downregulation of HIF-1α. Nutr Cancer 65(1):147–156
Carter LG, D’Orazio JA, Pearson KJ (2014) Resveratrol and cancer: focus on in vivo evidence. Endocr Relat Cancer. 21:R209–R225
Cavell BE, Syed Alwi SS, Donlevy AM, Proud CG, Packham G (2012) Natural product- derived antitumor compound phenethyl isothiocyanate inhibits mTORC1 activity via TSC2. J Nat Prod 75(6):1051–1057
Chen F, Liu Y, Wang S, Guo X, Shi P, Wang W, Xu B (2013) Triptolide, a Chinese herbal extract, enhances drug sensitivity of resistant myeloid leukemia cell lines through downregulation of HIF-1α and Nrf2. Pharmacogenomics 14(11):1305–1317
Chen QJ, Zhang MZ, Wang LX (2010) Gensenoside Rg3 inhibits hypoxia- induced VEGF expression in human cancer cells. Cell Physiol Biochem 26(6):849–858
Choi H, Chun YS, Kim SW, Kim MS, Park JW (2006) Curcumin inhibits hypoxia-inducible factor-1 by degrading aryl hydrocarbon receptor nuclear translocator: a mechanism of tumor growth inhibition. Mol Pharmacol 70(5):1664–1671
Choi YJ, Heo K, Park HS, Yang KM, Jeong MH (2016) The resveratrol analog HS-1793 enhances radiosensitivity of mouse-derived breast cancer cells under hypoxic conditions. Int J Oncol 49(4):1479–1488
Das L, Bhaumik E, Raychaudhuri U, Chakraborty R (2012) Role of nutraceuticals in human health. J Food Sci Technol 49:173–183
Dillard CJ, German JB (2000) Phytochemicals: nutraceuticals and human health. J Sci Food Agric 80:1744–1756
Du G, Lin H, Wang M, Zhang S, Wu X, Lu L, Ji L, Yu L (2010) Quercetin greatly improved therapeutic index of doxorubicin against 4T1 breast cancer by its opposing effects on HIF-1α in tumor and normal cells. Cancer Chemother Pharmacol 65:277–287
Du Y, Long Q, Zhang L, Shi Y, Liu X, Li X, Guan B, Tian Y, Wang X, Li L, He D (2015) Curcumin inhibits cancer- associated fibroblast- driven prostate cancer invasion through MAOA/mTOR/HIF-1α signaling. Int J Oncol 47:2015–2064
Duan W, Chang Y, Li R, Xu Q, Lei J, Yin C, Li T, Wu Y, Ma Q, Li X (2014) Curcumin inhibits hypoxia-inducible factor- 1α- induced epithelial-mesenchymal transition in HepG2 hepatocellular carcinoma cells. Mol Med Rep 10(5):2505–2510
Eales KL, Hollinshead KER, Tennant DA (2016) Hypoxia and metabolic adaptation of cancer cells. Oncogenesis 5:1–8
Fu L, Chen W, Guo W, Wang J, Tian Y, Shi D, Zhang X, Qiu H, Xiao X, Kang T, Huang W, Wang S, Deng W (2013) Berberine Targets AP-2/hTERT, NF-κB/COX-2, HIF- 1α/VEGF and cytochrome-c/caspase signaling to suppress human cancer cell growth. PLoS One 8:1–13
Fu P, Du F, Chen W, Yao M, Lv K, Liu Y (2014) Tanshinone IIA blocks epithelial-mesenchymal transition through HIF-1α downregulation, reversing hypoxia-induced chemotherapy resistance in breast cancer cell lines. Oncol Rep 31(6):2561–2568
Gainer JL, Sheehan JP, Larner JM, Jones DR (2017) Trans sodium crocetinate with temozolomide and radiation therapy for glioblastoma multiforme. J Neurosurg 126(2):460–466
Ge X, Zhen F, Yang B, Yang X, Cai J, Zhang C, Zhang S, Cao Y, Ma J, Cheng H, Sun X (2014) Ginsenoside Rg3 enhances radiosensitization of hypoxic oesophageal cancer cell lines through vascular endothelial growth factor and hypoxia-inducible factor 1α. J Int Med Res 42:628–640
Guo Y, Han B, Luo K, Ren Z, Cai L, Sun L (2017) NOX2-ROS-HIF-1α signaling is critical for the inhibitory effect of oleanolic acid on rectal cancer cell proliferation. Biomed Pharmacother 85:733–739
Guo Y, Meng X, Ma J, Zheng Y, Wang Q, Wang Y, Shang H (2014) Human papillomavirus 16 E6 contributes HIF-1α induced warburg effect by attenuating the VHL-HIF-1α interaction. Int J Mol Sci 15:7974–7986
Gupta B, Chiang L, Chae K, Lee DH (2013) Phenethyl isothiocyanate inhibits hypoxia-induced accumulation of HIF-1α and VEGF expression in human glioma cells. Food Chem 141(3):1841–1846
Gupta P, Wright SE, Kim SH, Srivastava SK (2014) Phenethyl isothiocyanate: a comprehensive review of anti-cancer mechanisms. Biochim Biophys Acta 1846(2):405–424
He J, Hu Y, Hu M, Zhang S, Li B (2016) The relationship between the pro-operative plasma level of HIF-1α and clinic pathological features, prognosis in non-small cell lung cancer. Sci Rep 6:1–12
He S, Lu G, Hou H, Zhao Z, Zhu Z, Lu X, Wang Z (2014) Saikosaponin-d suppresses the expression of cyclooxygenase-2 through the phospho-signal transducer and activator of transcription 3/ hypoxia-inducible factor-1α pathway in hepatocellular carcinoma cells. Mol Med Rep 10(5):2556–2562
Hockel M, Vaupel P (2001) Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst 93:266–276
Huang L, Zhang Z, Zhang S, Ren J, Zhang R, Zeng H, Li Q, Wu G (2011) Inhibitory action of celastrol on hypoxia-mediated angiogenesis and metastasis via the HIF-1α pathway. Int J Mol. 27(3):407–415
Jeon YK, Yoo DR, Jang YH, Jang SY, Nam MJ (2011) Sulforaphane induces apoptosis in human hepatic cancer cells through inhibition of 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase4, mediated by hypoxia inducible factor-1-dependent pathway. Biochim Biophys Acta 1814(10):1340–1348
Jia LY, Wu XJ, Gao Y, Rankin GO, Pigliacampi A, Bucur H, Chen YC (2017) Inhibitory effects of total triterpenoid saponins isolated from the seeds of the tea plant (Camellia sinensis) on human ovarian cancer cells. Molecules 22(1649):1–16
Karna E, Szoka L, Palka JA (2010) Betulinic acid inhibits the expression of hypoxia-inducible factor 1α and vascular endothelial growth factor in human endometrial adenocarcinoma cells. Mol Cell Biochem 340(1–2):15–20
Kim DH, Sung B, Kang YJ, Hwang SY, Kim MJ, Yoon JH, Kim ND (2015) Sulforaphane inhibits hypoxia-induced HIF-1α and VEGF expression and migration of human colon cancer cells. Int J Oncol 47(6):2226–2232
Kim DH, Hossain MA, Kim MY, Kim JA, Yoon JH, Suh HS, Kim GY, Choi YH, Chung HY, Kim ND (2013) A novel resveratrol analogue, HS-1793, inhibits hypoxia-induced HIF-1α and VEGF expression, and migration in human prostate cancer cells. Int J Oncol 43:1915–1924
Kim DH, Sung B, Kim JA, Kang YJ, Hwang SY, Hwang NL, Suh H, Choi YH, Im E, Chung HY, Kim ND (2017a) HS-1793, a resveratrol analogue, downregulates the expression of hypoxia-induced HIF-1 and VEGF and inhibits tumor growth of human breast cancer cells in a nude mouse xenograft model. Int J Oncol 51:715–723
Kim HS, Wannatung T, Lee S, Yang WK, Chung SH, Lim JS, Choe W, Kang I, Kim SS, Ha J (2012) Quercetin enhances hypoxia-mediated apoptosis via direct inhibition of AMPK activity in HCT116 colon cancer. Apoptosis. 17(9):938–949
Kim SL, Park YR, Lee ST, Kim SW (2017b) Parthenolide suppresses hypoxia-inducible factor-1α signaling and hypoxia induced epithelial-mesenchymal transition in colorectal cancer. Int J Oncol 51(6):1809–1820
Kim YS, Lee HA, Lim JY, Kim Y, Jung CH, Yoo SH, Kim Y (2014) β-Carotene inhibits neuroblastoma cell invasion and metastasis in vitro and in vivo by decreasing level of hypoxia-inducible factor-1α. J Nutr Biochem 25(6):655–664
Kocaadam B, Şanlier N (2017) Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit Rev Food Sci Nutr 57:2889–2895
Koh MY, Spivak-Kroizman TR, Powis G (2010) HIF-1alpha and cancer therapy. Recent Results Cancer Res 180:15–34
Krock BL, Skuli N, Simon MC (2011) Hypoxia-induced angiogenesis. Good and evil. Genes Cancer 2(12):1117–1133
Lapchak PA (2010) Efficacy and safety profile of the carotenoid trans sodium crocetinate administered to rabbits following multiple infarct ischemic strokes: a combination therapy study with tissue plasminogen activator. Brain Res 1309:136–145
Law PC, Auyeung KK, Chan LY, Ko JK (2012) Astragalus saponins downregulate vascular endothelial growth factor under cobalt chloride-stimulated hypoxia in colon cancer cells. BMC Complement Altern Med 12(160):1–12
Lee DH, Lee YJ (2008) Quercetin suppresses hypoxia-induced accumulation of hypoxia- inducible factor-1alpha (HIF-1alpha) through inhibiting protein synthesis. J Cell Biochem 105:546–553
Lee S, Kim HJ, Oh SC, Lee DH (2018) Genipin inhibits the invasion and migration of colon cancer cells by the suppression of HIF-1α accumulation and VEGF expression. Food Chem Toxicol 116:70–76
Lee SL, Rouhi P, Dahl Jensen L, Zhang D, Ji H, Hauptmann G, Ingham P, Cao Y (2009) Hypoxia-induced pathological angiogenesis mediates tumor cell dissemination, invasion, and metastasis in a zebrafish tumor model. Proc Natl Acad Sci U.S.A. 106:19485–19490
Lee SO, Kim JS, Lee MS, Lee HJ (2016) Anti-cancer effect of pristimerin by inhibition of HIF-1α involves the SPHK-1 pathway in hypoxic prostate cancer cells. BMC Cancer 16(701):1–10
Li G, Shan C, Liu L, Zhou T, Zhou J, Hu X, Chen Y, Cui H, Gao N (2015) Tanshinone IIA inhibits HIF-1α and VEGF expression in breast cancer cells via mTOR/p70S6K/RPS6/4E-BP1 signaling pathway. PLoS One 10(2):1–14
Li S, Li J, Dai W, Zhang Q, Feng J, Wu L, Liu T, Yu Q, Xu S, Wang W, Lu X, Chen K, Xia Y, Lu J, Zhou Y, Fan X, Mo W, Xu L, Guo C (2017) Genistein suppresses aerobic glycolysis and induces hepatocellular carcinoma cell death. Br J Cancer 117:1518–1528
Li W, Cao L, Chen X, Lei J, Ma Q (2016) Resveratrol inhibits hypoxia-driven ROS-induced invasive and migratory ability of pancreatic cancer cells via suppression of the Hedgehog signaling pathway. Oncol Rep. 35:1718–1726
Li Y, Zhang Y, Liu X, Wang M, Wang P, Yang J, Zhang S (2018) Lutein inhibits proliferation, invasion and migration of hypoxic breast cancer cells via downregulation of HES1. Int J Oncol 52(6):2119–2129
Lin S, Tsai SC, Lee CC, Wang BW, Liou JY, Shyu KG (2004) Berberine inhibits HIF-1alpha expression via enhanced proteolysis. Mol Pharmacol 66:612–619
Liu T, Zhao L, Hou H, Ding L, Chen W, Li X (2017) Ginsenoside 20(S)-Rg3 suppresses ovarian cancer migration via hypoxia-inducible factor 1 alpha and nuclear factor-kappa B signals. Tumour Biol 39:1–10
Liu T, Zhao L, Zhang Y, Chen W, Liu D, Hou H, Ding L, Li X (2014) Ginsenoside 20(S)-Rg3 targets HIF-1α to block hypoxia-induced epithelial-mesenchymal transition in ovarian cancer cells. PLoS One. 9:1–12
Liu T, Han C, Wang S, Fang P, Ma Z, Xu L, Yin R (2019) Cancer-associated fibroblasts: an emerging target of anti-cancer immunotherapy. J Hematol Oncol 12(1):1–15
Liu ZJ, Semenza GL, Zhang HF (2015) Hypoxia-inducible factor 1 and breast cancer metastasis. J Zhejiang Univ Sci B. 16:32–43
Lu C, Cai D, Ma J (2018) Pachymic acid sensitizes gastric cancer cells to radiation therapy by upregulating bax through hypoxia. Am J Chin Med 46(04):875–890
Lu Y, Wang B, Shi Q, Wang X, Wang D, Zhu L (2016) Brusatol inhibits HIF-1 signaling pathway and suppresses glucose uptake under hypoxic conditions in HCT116 cells. Sci Rep 6(1):1–12
Ma DD, Lu HX, Xu LS, Xiao W (2009) Polyphyllin D exerts potent anti-tumour effects on lewis cancer cells under hypoxic conditions. J Int Med Res 37(3):631–640
Ma J, Han LZ, Liang H, Mi C, Shi H, Lee JJ, Jin X (2014a) Celastrol inhibits the HIF-1α pathway by inhibition of mTOR/p70S6K/eIF4E and ERK1/2 phosphorylation in human hepatoma cells. Oncol Rep 32(1):235–242
Ma J, Zi Jiang Y, Shi H, Mi C, Li J, Xing Nan J, Jin X (2014b) Cucurbitacin B inhibits the translational of hypoxia-inducible factor-1α. Eur J Pharmacol 723:46–54
Mao L, Chen Q, Gong K, Xu X, Xie Y, Zhang W, Cao H, Hu T, Hong X, Zhan YY (2018) Berberine decelerates glucose metabolism via suppression of mTOR- dependent HIF- 1α protein synthesis in colon cancer cells. Oncol Rep 39(5):2436–2442
Mao ZJ, Tang QJ, Zhang CA, Qin ZF, Pang B, Wei P, Chou YN (2012) Anti-proliferation and anti-invasion effects of diosgenin on gastric cancer BGC-823 cells with HIF-1α shRNAs. Int J Mol Sci 13(5):6521–6533
Masoud GN, Li W (2015) HIF-1α pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B 5:378–389
Messina M (2016) Soy and health update: evaluation of the clinical and epidemiologic literature. Nutrients. 8(12):1–42
Min JH, Yang H, Ivan M, Gertler F, Kaelin WG, Pavletich NP (2002) Structure of an HIF-1alpha -pVHL complex: hydroxyproline recognition in signaling. Science 296:1886–1889
Mitani T, Harada N, Tanimori S, Nakano Y, Inui H, Yamaji R (2014a) Resveratrol inhibits hypoxia-inducible factor-1α-mediated androgen receptor signaling and represses tumor progression in castration-resistant prostate cancer. J Nutr Sci Vitaminol (Tokyo) 60:276–282
Mitani T, Ito Y, Harada N, Nakano Y, Inui H, Ashida H, Yamaji R (2014b) Resveratrol reduces the hypoxia-induced resistance to doxorubicin in breast cancer cells. J Nutr Sci Vitaminol 60:122–128
Muz B, de la Puente P, Azab F, Azab AK (2015) The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia (Auckl) 3:83–92
Oh ET, Kim CW, Kim HG, Lee JS, Park HJ (2017) Brusatol-mediated inhibition of c-Myc increases HIF-1α degradation and causes cell death in colorectal cancer under hypoxia. Theranostics 7(14):3415–3431
Pan Y, Shao D, Zhao Y, Zhang F, Zheng X, Tan Y, He K, Li J, Chen L (2017) Berberine reverses hypoxia-induced chemoresistance in breast cancer through the inhibition of AMPK-HIF-1α. Int J Biol Sci 13:794–803
Pastorek M, Simko V, Takacova M, Barathova M, Bartosova M, Hunakova L, Sedlak J (2015) Sulforaphane reduces molecular response to hypoxia in ovarian tumor cells independently of their resistance to chemotherapy. Int J Oncol 47(1):51–60
Petrova V, Petruzzelli MA, Melino G, Amelio I (2018) The hypoxic tumour microenvironment. Oncogenesis 7:1–13
Quan F, Zhang SQ, Bai YX, Yao XB, Li HH, Yu L, Pan CE (2009) Resveratrol increases sensitivity of CNE2 cells to chemotherapeutic drugs under hypoxia. Zhong Xi Yi Jie He Xue Bao 7:952–957
Ranzato E, Martinotti S, Calabrese CM, Giorgio C (2014) Role of nutraceuticals in cancer therapy. J Food Res 3:1–8
Rauf A, Imran M, Butt MS, Nadeem M, Peters DG, Mubarak MS (2018a) Resveratrol as an anti- cancer agent: a review. Crit Rev Food Sci Nutr 58:1428–1447
Rauf A, Imran M, Khan IA, Ur-Rehman M, Gilani SA, Mehmood Z, Mubarak MS (2018b) Anticancer potential of quercetin: a comprehensive review. Phytother Res 32:2109–2130
Ren-Ping Z, Sen-Sen L, Yuan ST, Yu BY, Bai XS, Sun L, Zhang LY (2014) DT-13, a saponin of dwarf lilyturf tuber, exhibits anti-cancer activity by down-regulating C–C chemokine receptor type 5 and vascular endothelial growth factor in MDA-MB-435 cells. Chin J Nat Med 12(1):24–29
Rohwer N, Cramer T (2011) Hypoxia-mediated drug resistance: novel insights on the functional interaction of HIFs and cell death pathways. Drug Resist Updat 14:191–201
Rosen R, Vagaggini T, Chen Y, Hu DN (2015) Zeaxanthin inhibits hypoxia-induced VEGF secretion by RPE cells through decreased protein levels of hypoxia-inducible factors-1α. Biomed Res Int 2015:1–11
Sarkar R, Mukherjee S, Biswas J, Roy M (2015) Phenethyl isothiocyanate, by virtue of its antioxidant activity, inhibits invasiveness and metastatic potential of breast cancer cells: HIF-1α as a putative target. Free Radic Res 50(1):84–100
Sauer AG, Siegel RL, Jemal A, Fedewa SA (2017) Updated review of prevalence of major risk factors and use of screening tests for cancer in the United States. Cancer Epidemiol Biomarkers Prev 26:1192–1208
Semenza GL (2010) Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene 29:625–634
Sheehan J, Cifarelli CP, Dassoulas K, Olson C, Rainey J, Han S (2010) Trans-sodium crocetinate enhancing survival and glioma response on magnetic resonance imaging to radiation and temozolomide. J Neurosurg 113(2):234–239
Sheehan J, Sherman J, Cifarelli C, Jagannathan J, Dassoulas K, Olson C, Rainey J, Han S (2009) Effect of trans sodium crocetinate on brain tumor oxygenation. Laboratory investigation. J Neurosurg 111(2):226–229
Sheehan JP, Popp B, Monteith S, Toulmin S, Tomlinson J, Martin J, Cifarelli CP, Lee DH, Park DM (2011) Trans sodium crocetinate: functional neuroimaging studies in a hypoxic brain tumor. J Neurosurg 115(4):749–753
Shi L, Zhang G, Zheng Z, Lu B, Ji L (2017) Andrographolide reduced VEGFA expression in hepatoma cancer cells by inactivating HIF-1α: The involvement of JNK and MTA1/HDCA. Chem Biol Interact 273:228–236
Siddiqui FA, Prakasam G, Chattopadhyay S, Rehman AU, Padder RA, Ansari MA, Irshad R, Mangalhara K, Bamezai RNK, Husain M, Ali SM, Iqbal MA (2018) Curcumin decreases Warburg effect in cancer cells by down-regulating pyruvate kinase M2 via mTOR-HIF1α inhibition. Sci Rep 8:1–9
Singh-Gupta V, Zhang H, Banerjee S, Kong D, Raffoul JJ, Sarkar FH, Hillman GG (2009) Radiation-induced HIF-1alpha cell survival pathway is inhibited by soy isoflavones in prostate cancer cells. Int J Cancer 124(7):1675–1684
Singh-Gupta V, Joiner MC, Runyan L, Yunker CK, Sarkar FH, Miller S, Gadgeel SM, Konski AA, Hillman GG (2011) Soy isoflavones augment radiation effect by inhibiting APE1/Ref-1 DNA repair activity in non-small cell lung cancer. J Thorac Oncol 6:688–698
Son MK, Jung KH, Lee HS, Lee H, Kim SJ, Yan HH, Hong SS (2013) SB365, Pulsatilla saponin D suppresses proliferation and induces apoptosis of pancreatic cancer cells. Oncol Rep 30(2):801–808
Spagnuolo C, Russo GL, Orhan IE, Habtemariam S, Daglia M, Sureda A, Nabavi SF, Devi KP, Loizzo MR, Tundis R, Nabavi SM (2015) Genistein and cancer: current status, challenges, and future directions. Adv Nutr 6:408–419
Sui H, Zhao J, Zhou L, Wen H, Deng W, Li C, Li Q (2017) Tanshinone IIA inhibits β- catenin/VEGF-mediated angiogenesis by targeting TGF-β1 in normoxic and HIF-1α in hypoxic microenvironments in human colorectal cancer. Cancer Lett 403:86–97
Sun L, Lin S, Zhao R, Yu B, Yuan S, Zhang L (2010) The saponin monomer of dwarf lilyturf tuber, DT-13, reduces human breast cancer cell adhesion and migration during hypoxia via regulation of tissue factor. Biol Pharm Bull 33(7):1192–1198
Sun Y, Wang H, Liu M, Lin F, Hua J (2015) Resveratrol abrogates the effects of hypoxia on cell proliferation, invasion and EMT in osteosarcoma cells through downregulation of the HIF-1α protein. Mol Med Rep 11:1975–1981
Tan C, Zhang L, Cheng X, Lin XF, Lu RR, Bao JD, Yu HX (2015) Curcumin inhibits hypoxia- induced migration in K1 papillary thyroid cancer cells. Exp Biol Med (Maywood) 240:925–935
Tanaka T, Shnimizu M, Moriwaki H (2012) Cancer Chemoprevention by Carotenoids. Molecules 17:3202–3242
Tang X, Zhang Q, Nishitani J, Brown J, Shi S, Le AD (2007) Overexpression of human papillomavirus type 16 oncoproteins enhances hypoxia-inducible factor 1 alpha protein accumulation and vascular endothelial growth factor expression in human cervical carcinoma cells. Clin Cancer Res 13:2568–2576
Tholl D (2015) Biosynthesis and biological functions of terpenoids in plants. Adv Biochem Eng Biotechnol 148:63–106
Thomas SL, Zhong D, Zhou W, Malik S, Liotta D, Snyder JP, Hamel E, Giannakakou P (2008) EF24, a novel curcumin analog, disrupts the microtubule cytoskeleton and inhibits HIF-1. Cell Cycle 7(15):2409–2417
Vincken JP, Heng L, de Groot A, Gruppen H (2007) Saponins, classification and occurrence in the plant kingdom. Phytochemistry 68:275–297
Wang B, Li H, Yan H, Xiao JG (2005) Genistein inhibited hypoxia-inducible factor-1alpha expression induced by hypoxia and cobalt chloride in human retinal pigment epithelium cells. Methods Find Exp Clin Pharmacol 27(3):179–184
Wang GL, Jiang BH, Rue EA, Semenza GL (1995) Hypoxia-inducible factor 1 is a basic-helix- loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA 92:5510–5514
Wang H, Feng H, Zhang Y (2016) Resveratrol inhibits hypoxia-induced glioma cell migration and invasion by the p-STAT3/miR-34a axis. Neoplasma 63:532–539
Wang J, Tian L, Khan MN, Zhang L, Chen Q, Zhao Y, Yan Q, Fu L, Liu J (2018) Ginsenoside Rg3 sensitizes hypoxic lung cancer cells to cisplatin via blocking of NF-κB mediated epithelial-mesenchymal transition and stemness. Cancer Lett 415:73–85
Wang K, Liu R, Li J, Mao J, Lei Y, Wu J, Zeng J, Zhang T, Wu H, Chen L, Huang C, Wei Y (2011) Quercetin induces protective autophagy in gastric cancer cells: involvement of Akt-mTOR- and hypoxia-induced factor 1α-mediated signaling. Autophagy 7(9):966–978
Wang XH, Cavell BE, Syed Alwi SS, Packham G (2009) Inhibition of hypoxia inducible factor by phenethyl isothiocyanate. Biochem Pharmacol 78(3):261–272
Wang Y, Li JX, Wang YQ, Miao ZH (2015) Tanshinone I inhibits tumor angiogenesis by reducing STAT3 phosphorylation at TYR705 and hypoxia-induced HIF-1α accumulation in both endothelial and tumor cells. Oncotarget 6(18):16031–16042
Warfel NA, El-Deiry WS (2014) HIF-1 signaling in drug resistance to chemotherapy. Curr Med Chem 21:3021–3028
Wargovich MJ, Morris J, Brown V, Ellis J, Logothetis B, Weber R (2010) Nutraceutical use in late-stage cancer. Cancer Metastasis Rev 29:503–510
Wen Z, Huang C, Xu Y, Xiao Y, Tang L, Dai J, Sun H, Chen B, Zhou M (2016) α-Solanine inhibits vascular endothelial growth factor expression by down-regulating the ERK1/2-HIF-1α and STAT3 signaling pathways. Eur J Pharmacol 15(771):93–98
WHO (2018) https://www.who.int/news-room/fact-sheets/detail/cancer
Wu H, Liang X, Fang Y, Qin X, Zhang Y, Liu J (2008) Resveratrol inhibits hypoxia-induced metastasis potential enhancement by restricting hypoxia-induced factor-1α expression in colon carcinoma cells. Biomed Pharmacother 62:613–621
Xu T, Xiao D (2017) Oleuropein enhances radiation sensitivity of nasopharyngeal carcinoma by downregulating PDRG1 through HIF1α-repressed microRNA-519d. J Exp Clin Cancer Res 36(1):1–10
Xu XH, Li T, Fong CM, Chen X, Chen XJ, Wang YT, Huang MQ, Lu JJ (2016) Saponins from Chinese medicines as anticancer agents. Molecules 21:1–27
Yang X, Yang B, Cai J, Zhang C, Zhang Q, Xu L, Qin Q, Zhu H, Ma J, Tao G, Cheng H, Sun X (2013) Berberine enhances radiosensitivity of esophageal squamous cancer by targeting HIF-1α in vitro and in vivo. Cancer Biol Ther 14:1068–1073
Yao H, Wan H, Zhang Z, Jiang B, Luo J, Shi X (2008) Sulforaphane inhibited expression of hypoxia inducible factor1α in human tongue squamous cancer cells and prostate cancer cells. Int J Cancer 123(6):1255–1261
Yoysungnoen B, Bhattarakosol P, Patumraj S, Changtam C (2015) Effects of tetra hydrocurcumin on hypoxia-inducible factor-1α and vascular endothelial growth factor expression in cervical cancer cell-induced angiogenesis in nude mice. Biomed Res Int 2015:1–12
Zeng D, Wang J, Kong P, Chang C, Li J, Li J (2014) Ginsenoside Rg3 inhibits HIF-1α and VEGF expression in patient with acute leukemia via inhibiting the activation of PI3K/ Akt and ERK1/2 pathways. Int J Clin Exp Pathol 7(5):2172–2178
Zhang C, Yang X, Zhang Q, Yang B, Xu L, Qin Q, Sun X (2014a) Berberine radiosensitizes human nasopharyngeal carcinoma by suppressing hypoxia- inducible factor-1α expression. Acta Otolaryngol 134:185–192
Zhang J, Su H, Li Q, Li J, Zhao Q (2017) Genistein decreases A549 cell viability via inhibition of the PI3K/AKT/HIF- 1α/VEGF and NF- κB/COX- 2 signaling pathways. Mol Med Rep 15:2296–2302
Zhang Q, Tang X, Lu QY, Zhang ZF, Brown J, Le AD (2005) Resveratrol inhibits hypoxia-induced accumulation of hypoxia-inducible factor-1 and VEGF expression in human tongue squamous cell carcinoma and hepatoma cells. Mol Cancer Ther 4:1465–1474
Zhang Q, Zhang C, Yang X, Yang B, Wang J, Kang Y, Wang Z, Li D, Huang G, Ma Z, Sun X, Cai J, Tao G, Dai S, Mao W, Ma J (2014b) Berberine inhibits the expression of hypoxia induction factor-1alpha and increases the radiosensitivity of prostate cancer. Diagn Pathol 9:1–7
Zhao R, Sun L, Lin S, Bai X, Yu B, Yuan S, Zhang L (2013) The saponin monomer of dwarf lilyturf tuber, DT-13, inhibits angiogenesis under hypoxia and normoxia via multi-targeting activity. Oncol Rep 29(4):1379–1386
Zhou J, Joplin DG, Cross JV, Templeton DJ (2012) Sulforaphane inhibits prostaglandin E2 synthesis by suppressing microsomal prostaglandin E synthase 1. PLoS ONE. 7(11):1–12
Zou K, Li Z, Zhang Y, Zhang HY, Li B, Zhu WL, Shi JY, Jia Q, Li YM (2017) Advances in the study of berberine and its derivatives: a focus on anti-inflammatory and anti-tumor effects in the digestive system. Acta Pharmacol Sin 38(2):157–167
Acknowledgements
The author’s thanks to Dr. V. Purshoshothaman and Dr. K. Revathi for the critical review of the manuscript. The authors sincerely apologize to any authors whose work was omitted.
Funding
Dr. DN was supported by the Start-up-grant from Meenakshi Academy of Higher Education and Research. Dr. GSK acknowledges CSIR-IICB for seed grant. Dr.JS was supported by ECRA, DST-SERB.
Author information
Authors and Affiliations
Contributions
DN and GSK have given an equal contribution in framing review topics, collecting study materials, preparing a manuscript, proofreading, etc. JS has written the terpenoid and isothiocyanate section of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nalini, D., Selvaraj, J. & Kumar, G.S. Herbal nutraceuticals: safe and potent therapeutics to battle tumor hypoxia. J Cancer Res Clin Oncol 146, 1–18 (2020). https://doi.org/10.1007/s00432-019-03068-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-019-03068-x