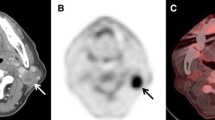
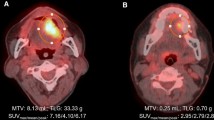
Abstract
Purpose
Subclinical lymph node (LN) metastasis is associated with poor survival outcome in oral cavity squamous cell carcinoma (OCC), which alleges elective neck LN dissection. Preoperative detection of metastatic LNs may improve prognosis and proper management of OCC. We examined the clinical usefulness of fluorine 18-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET)/computed tomography (CT) for the detection of occult neck LN metastasis in OCC patients in comparison with conventional CT/magnetic resonance (MR) imaging.
Methods
A total of 178 OCC patients with negative neck palpation findings were assessed prospectively with 18F-FDG PET/CT and CT/MR imaging. Histopathological analyses of neck dissection samples served as reference. Diagnostic values of 18F-FDG PET/CT versus CT/MR imaging were compared with the McNemar test and logistic regression with generalized estimating equations.
Results
Forty-two patients (23.6%) had metastasis in 44 sides and 58 levels of the neck. The sensitivity for detection of occult metastasis was higher for 18F-FDG PET/CT than that for CT/MR imaging on a per-patient (69.1% vs 35.7%), per-side (70.5% vs 36.4%), and per-level (62.1% vs 29.3%) basis (all P ≤ 0.001). However, the specificity for metastatic detection was higher for CT/MR imaging than that for 18F-FDG PET/CT (all P < 0.005). 18F-FDG PET/CT improved detection of occult metastasis up to 33.4% in these patients compared to CT/MR imaging.
Conclusions
18F-FDG PET/CT can better detect occult neck metastasis than CT/MR imaging, which may potentially impact the clinical management of OCC patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Oral cavity squamous cell carcinoma (OCC) can spread to regional neck lymph node (LN) through the lymphatic drainage (Farmer et al. 2015). An early study reported that the rate of subclinical neck metastasis was 34% in patients with clinically LN-negative (cN0) OCC (Shah et al. 1990). Metastatic LNs are predominantly found in cervical levels I–III (Shah et al. 1990). Metastasis to LNs of cervical level IV or V is less common, particularly in patients with clinically LN-positive (cN+) OCC (Shah et al. 1990). Regional metastasis of OCC is an ominous factor associated with increased recurrence and disease-specific and overall death (D’Cruz et al. 2015; Ho et al. 2017). Neck metastasis confers up to 50% decrease of overall survival along with increased metastatic LN burden (Ho et al. 2017). Occult neck metastasis, only pathologically defined upon histological examination of elective neck dissection samples, is also a poor prognostic factor in OCC cN0 patients (Mucke et al. 2014). Therefore, an elective neck dissection has been advocated, rather than a watchful waiting approach, until therapeutic neck dissection, even in early stage OCC cN0 patients (D’Cruz et al. 2015; Dik et al. 2016; Joo et al. 2019; Joo and Koo 2019). However, additional neck dissection may cause surgical morbidities; it is thus being avoided in up to 70% of patients (Shah et al. 1990), and sentinel LN biopsy has been applied for staging and surgical management of OCC cN0 patients (Schilling et al. 2015, 2017).
Neck metastasis of OCC may be preoperatively detected with contrast-enhanced computed tomography (CT), magnetic resonance (MR) imaging, fluorine 18-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET)/CT, and single photon emission CT. (Arya et al. 2014). 18F-FDG PET/CT is being actively performed to assess initial staging and treatment responses and for post-treatment selection and prognosis (Evangelista et al. 2014; Manca et al. 2016; Chong et al. 2017). The combined use of 18F-FDG PET with CT has enhanced the anatomic delineation of metabolic lesions (Pelosi et al. 2004) and facilitated the evaluation of both the metabolic and anatomic characteristics of metastatic diseases (Ceylan et al. 2018). Previous studies have shown the superior sensitivity of 18F-FDG PET or PET/CT to CT/MR imaging for the detection of neck LN metastasis in OCC patients, including both cN0 and cN+ neck diseases (Ng et al. 2005; Roh et al. 2007). However, the limited sensitivity for detection of small metastatic deposits and a relatively high number of false-positive findings has been reported in cN0 patients with OCC or other head and neck cancer (Schöder et al. 2006; Kim et al. 2019). Therefore, the diagnostic value of 18F-FDG PET/CT should be further examined in prospective settings against other conventional imaging modalities. Herein, we examined the clinical usefulness of 18F-FDG PET/CT for the detection of occult neck metastasis in OCC patients in comparison with conventional CT/MR imaging.
Materials and methods
Study patients
This prospective study included OCC patients who underwent surgery between September 2010 and August 2017. Patients received prospective imaging evaluation of whole body 18F-FDG PET/CT and CT/MR imaging within 3 weeks before primary surgery. OCC patients with negative palpation findings were considered for detection of occult LN metastasis with 18F-FDG PET/CT and CT/MR imaging. Exclusion criteria were patients with pathologies other than squamous cell carcinoma, a previous history of head and neck cancer, neck surgery or irradiation, and refusal of preoperative imaging workups. Tumor was staged using the tumor-node-metastasis staging system proposed by the American Joint Committee on Cancer (7th ed.). This study was reviewed and approved by the institutional review board and informed consent from each patient was obtained.
Patients underwent complete extirpation of tumor and elective neck dissection. Primary tumor was removed with tumor-free margins more than 1–1.5 cm and all resection margins and beds were confirmed as tumor-free with intraoperative frozen section examinations. Deficits of the oral cavity following tumor resection were primarily closed or reconstructed with regional or free flaps. All patients underwent elective dissection of LNs levels I to III or IV on the affected neck side or on both neck sides, if tumors were located on median or bilateral sides. Cervical level V was dissected in patients with advanced tumor classification. Neck dissection samples were divided along the neck sides and levels and sent for histological examination. All tumor and neck dissection samples were stained with hematoxylin–eosin and examined by a board-certified pathologist with clinical experience over 30 years. LN tissues were serially sectioned every 50 μm and occasionally stained with cytokeratin immunohistochemistry to identify small occult metastasis.
Imaging studies and interpretation
All patients underwent 18F-FDG PET/CT and CT/MR imaging at initial staging within 3 weeks before surgery. The three different imaging studies were acquired within 2 days. Patients underwent 18F-FDG PET/CT scanning with different PET/CT equipment using a Biograph Sensation 16 (BIO16)/TruePoint 40 (BIO40) system (Siemens Medical Systems, Knoxville, TN, USA) or Discovery STE 8 (DSTE)/Discovery 690 (D690) system/Discovery 710 (D710) (GE Healthcare, Milwaukee, WI, USA). Median blood glucose levels were 96 mg/dL (range 63–135 mg/dL) prior to intravenous injection of median 440 MBq (range 290–620 MBq) followed by PET scanning. PET attenuation correction and image fusion were obtained using non-contrast-enhanced CT scans (100 mAs, 120 kV, 5 mm section width, and 0.75 mm collimation). Caudocranial PET emission scans were obtained with 2 min (for BIO40, D690, and D710) or 2.5 min (for BIO16 and DSTE) acquisition times per bed position with five to eight bed positions for the whole body and 5 min per bed position with two bed positions for the head and neck. PET data were reconstructed using CT-based attenuation correction, an iterative reconstruction algorithm (two iterations, 16 subsets for BIO16; three iterations, 21 subsets for BIO40; two iterations, 20 subsets for DSTE8; and four iterations, 18 subsets for D690 and D710), and a post-reconstruction smoothing Gaussian filter (full width at half-maximum = 4 mm). Images were reconstructed with a 168 × 168 matrix (pixel size = 5.3 mm). FDG PET/CT images were reviewed with a viewing platform (Syngo MMWP VE40A and Syngo VE32E; Siemens Medical Systems, Erlangen, Germany).
All patients underwent both contrast-enhanced CT and MR imaging. CT scanning was performed on several commercially available CT systems with multi-detector capabilities and 64–128 channels (Siemens Medical Solutions, Erlangen, Germany). Contrast-enhanced CT images were obtained 70 s after intravenous injection with a 140-mL bolus of non-ionic iodinated contrast material (iopamidol, Isovue-370; Bracco Diagnostics, Princeton, NJ). Imaging was performed with the following parameters: section thickness, 3 mm; field of view, 22 cm; 120 kV; 200 mA; matrix, 256 × 256. MR imaging was performed with a 3-T unit MR machine (Achieva, Philips Medical Systems, Best, the Netherlands) with a slice thickness of 3 mm in the axial, coronal, and sagittal projections from the skull base to the upper chest. Subsequently, T1-weighted post-gadolinum with fat-suppressed images were sequentially obtained with the following parameters: T1-weighted turbo-spin echo sequence (TR/TE, 600/8.8 ms; FOV, 230 × 190 mm; matrix, 512 × 512) and T2-weighted turbo-spin echo sequence (TR/TE, 4100/100 ms; FOV, 230 × 190 mm; matrix size, 512 × 512). Images were acquired in the axial, sagittal, and coronal planes with 3 mm section thickness. Post-contrast images were acquired 3–8 min after IV contrast administration using gadoterate meglumine (0.1 mmol/kg; Dotarem; Guerbet, Paris, France).
18F-FDG PET/CT images were interpreted by two board-certified nuclear medicine physicians with clinical experience over 10 years and disagreements were resolved by consensus. Abnormal 18F-FDG uptakes in the neck were carefully interpreted relative to the background and to blood-pool activity. Foci with increased 18F-FDG uptake were graded from 1 to 4, with grades 3 and 4 indicating LN metastasis. Foci were separately described in neck sides and levels, based on visual and semiquantitative analyses of abnormal 18F-FDG uptakes without strict cutoffs of standardized uptake values. CT and MR images were interpreted in combination by two radiologists with clinical experience over 10 years and disagreements were resolved by consensus. Neck LNs were graded using the same four-point scale, separately at neck sides and levels. The 18F-FDG PET/CT and CT/MR images were separately interpreted by different physicians from different departments without information on other imaging results.
Statistical analysis
Histological nodal findings after neck dissection were considered as a reference, to compare the results on occult neck metastasis between 18F-FDG PET/CT and CT/MR imaging. Sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV) of each imaging method were calculated, for identification of neck LN metastasis. 18F-FDG PET/CT and CT/MR imaging were compared in terms of sensitivity and specificity using the McNemar test. The comparison of the diagnostic values between 18F-FDG PET/CT and CT/MRI at a per-side and a per-level basis was performed with logistic regression using generalized estimating equations that accounted for the clustering of observations within patients. To compare between imaging procedures in terms of their diagnostic performances for total detection of occult metastatic LNs, receiver operating characteristic (ROC) curves were generated using a four-point scale and the area under the ROC curve (AUC) was then calculated. Comparison of the AUC was performed using block bootstrap resampling in R package version R 2.14.0 (The R Project for Statistical Computing, http://www.r-project.org) and analyzed using package “pcvsuite” in R. All tests were two-sided and P < 0.05 was considered statistically significant. All statistical analyses were performed using the IBM SPSS software version 24.0 (IBM, Armonk, NY).
Results
A total of 178 patients were included in this study. The characteristics of the patients are summarized in Table 1. Tumor site included the oral tongue (n = 141, 83.1%), followed by the mouth floor (n = 14, 7.9%); buccal region, hard palate, and lip (n = 4 each, 2.2%); gingiva (n = 3, 1.7%); and retromolar trigone (n = 1, 0.6%). Median size and invasion depth of tumors were 1.8 cm (range 0.2–6.8 cm) and 0.6 cm (0.1–3.1 cm), respectively. Among 178 patients, 149 (83.7%) were in early T1 or T2 classification. Postoperative radiotherapy or chemoradiotherapy was performed in 59 (33.2%) patients.
LNs in neck levels I to III were dissected in all 178 patients. Additionally, LNs in neck levels IV and V were dissected in 50 (28.1%) and 31 (17.4%) patients, respectively. Bilateral neck levels I to III were dissected in 21 (11.8%) patients. Therefore, 199 LNs from neck sides and 678 from all levels were dissected from all patients. A total of 6498 neck LNs was harvested, 76 (1.2%) of which were pathologically positive. The median number of harvested LNs was 33 (range 14–108). A single positive LN (N1) was found in 26 (14.6%) patients and two or three positive LNs (N2) were found in 16 (9.0%). Two patients had bilateral neck metastases (N2c). Finally, pathological LN metastasis was confirmed in 42 (23.6%) patients at 44 sides and 58 levels of the neck. The median size of metastatic LNs in the longest diameter was 0.6 cm (range 0.1–1.8 cm). Microscopic extranodal extension was found in six (3.3%) patients.
Table 2 shows the comparison of 18F-FDG PET/CT versus CT/MR imaging for the detection of occult neck metastasis. 18F-FDG PET/CT showed higher sensitivity than CT/MR imaging on a per-patient (69.1% vs 35.7%, P = 0.001), per-side (70.5% vs 36.4%, P < 0.001), and per-level (62.1% vs 29.3%, P < 0.001) basis. However, the specificity for metastatic detection was higher for CT/MR imaging than 18F-FDG PET/CT on a per-patient (89.0% vs 77.9%, P = 0.001), per-side (89.7% vs 78.7%, P < 0.001), and per-level (96.8% vs 89.2%, P < 0.001) basis. The area under the ROC curve showed that the diagnostic performance for total detection of occult neck metastatic LNs was significantly better for 18F-FDG PET/CT than CT/MRI: 0.780 (95% CI 0.695–0.864) and 0.649 (0.543–0.754) on a per-patient basis (P = 0.023); 0.776 (0.694–0.858) vs 0.652 (0.550–0.754) on a per-side basis (P = 0.019); and 0.813 (0.746–0.881) vs 0.629 (0.543–0.715) on a per-level basis (P < 0.001). Overall, high NPV and low PPV were found in both 18F-FDG PET/CT and CT/MR imaging. However, the NPVs for 18F-FDG PET/CT were > 89% on a per-patient (89.1%), per-side (90.4%), and per-level (96.2%) basis, which was 2.6–7.8% higher than for CT/MR imaging.
Table 3 shows the comparison of 18F-FDG PET/CT versus CT/MR imaging for the detection of occult metastasis at each dissected level. 18F-FDG PET/CT showed superior sensitivity to CT/MR imaging for neck levels I and II and inferior specificity to CT/MR imaging for neck levels I to III.
Overall, 18F-FDG PET/CT improved the detection of occult metastasis up to 33.4% in patients with negative neck palpation findings, up to 34.1% in negative neck sides, and up to 32.8% in negative neck levels, which were unidentified on CT/MR imaging. Table 4 shows the comparisons between CT/MR imaging and 18F-FDG PET/CT false results according to tumor classification. False-positive results appeared to be more frequent in 18F-FDG PET/CT, particularly in the cases of T4 classification. False-negative results appeared to be more frequent in the CT/MR imaging, regardless of T classifications. The mean size of occult metastatic LNs was significantly lower with the PET/CT-negative findings than with the PET/CT-positive findings (0.43 cm vs 0.84 cm; t test, P = 0.004). The same finding was observed between CT/MR-negative and -positive LNs (0.50 cm vs 1.07 cm; t test, P < 0.001).
Discussion
The present study showed the superior sensitivity of 18F-FDG PET/CT to conventional CT/MR imaging. 18F-FDG PET/CT improved detection of occult metastasis up to 33.4% in patients with palpably negative LN findings compared to CT/MR imaging. An early study prospectively compared 18F-FDG PET vs CT/MR imaging for the detection of neck LN metastasis in 124 OCC patients regardless of cN+ (Ng et al. 2005). The study showed higher sensitivity for 18F-FDG PET than CT/MR imaging at a level-based analysis.
The role of 18F-FDG PET or PET/CT for detection of LN metastasis has been unclear. A previous study prospectively examined the role of 18F-FDG PET/CT for the detection of occult neck metastasis in 31 OCC patients with cN0 neck by clinical examination or CT/MR imaging (Schöder et al. 2006). Pathological LN metastasis was found in 25% of the patients and the sensitivity of 18F-FDG PET/CT was 67% at a per-level basis. The false-negative results were found in all three sides and levels, particularly in metastatic LNs smaller than 3 mm. A recent systematic review showed that the pooled sensitivity and specificity of 18F-FDG PET and PET/CT for the detection of occult neck LN metastasis were 0.58 (95% CI 0.42–0.72) and 0.87 (0.79–0.92) on a per-patient basis, respectively, in 1044 head and neck cancer patients from 18 studies (Kim et al. 2019). The pooled sensitivity and specificity were 0.53 (0.40–0.65) and 0.97 (0.95–0.98) on a per-level basis, respectively. The meta-analysis concluded low sensitivity and moderate specificity of 18F-FDG PET or PET/CT for the detection of occult LN metastasis in cN0 head and neck cancer patients. Sensitivity appeared to be slightly higher in our study than that in the meta-analysis. The increased sensitivity may be due to our prospective evaluation of the imaging studies in a single organ site (OCC) that is generally treated with primary surgery combined with elective or therapeutic neck dissection. The recent meta-analysis did not include the comparison between 18F-FDG PET or PET/CT and the conventional imaging with CT/MR imaging for the detection of occult neck metastasis. Our study suggested the superior sensitivity of 18F-FDG PET/CT to CT/MR imaging, leading to better detection of occult neck metastasis and potentially improving clinical neck management of OCC cN0 patients.
The present study showed a high incidence of false-positive PET/CT results for the detection of occult LN metastasis. This resulted in relatively lower specificity of 18F-FDG PET/CT compared with that of CT/MR imaging, consistent with a recent systematic review (Kim et al. 2019). Abnormal 18F-FDG uptakes in the head and neck region were commonly interpreted by comparing to background and blood-pool activity and by referring to normal 18F-FDG distribution patterns previously reported (Nakamoto et al. 2005). Inflammation, infection, or other benign conditions may cause false-positive PET/CT results, because 18F-FDG uptake is not tumor-specific, but occurs at all sites of increased glucose metabolism (Nakamoto et al. 2005; Rosenbaum et al. 2006). 18F-FDG avidity in reactive lymph nodes may result from increased glucose uptake in lymphoid follicles due to the overexpression of glucose transporter type 1 (Chung et al. 2004; Nakagawa et al. 2008). False-positive results contributed to low PPV for 18F-FDG PET/CT in our cohort, thus potentially leading to increased chance of overtreatment indicating unnecessary elective neck dissection in OCC cN0 patients. Much higher rates of false-positive PET/CT results as compared to the other modalities might be a serious disadvantage. This might be reduced by a combination with high-resolution neck ultrasonography that shows important architecture of the LNs and direct fine needle aspiration accordingly and offered similar diagnostic accuracy comparing to other different imaging modalities of CT, MRI, and PET (Liao et al. 2012).
Our study showed relatively high NPVs for 18F-FDG PET/CT compared to CT/MR imaging for the detection of occult neck LN metastasis. NPVs for 18F-FDG PET/CT were as high as 96.2% on a per-level basis, similar to previous studies (94–98%) (Ng et al. 2005; Schöder et al. 2006; Roh et al. 2007). NPVs for 18F-FDG PET/CT may be lower on a per-patient basis than a per-side or a per-level basis. Nonetheless, high NPVs over 90% may imply the potential clinical use of 18F-FDG PET/CT by excluding elective neck dissection in OCC cN0 patients with LN-negative PET/CT findings. However, among 42 OCC patients with occult LN metastasis, 13 (31%) showed false-negative LN findings in 18F-FDG PET/CT, which is significantly lower than CT/MR imaging (n = 27, 64%). This may result in increased chance of undertreatment omitting necessary neck dissection in OCC cN0 patients with occult LN metastasis. The false-negative PET/CT results are found in cervical micrometastatic LNs with small metastatic deposits, also commonly were undetected by CT/MR imaging, possibly due to technical limitations of 18F-FDG PET/CT to detect small-volume diseases (Fukui et al. 2005). This may be improved by future development of imaging techniques or modalities, in combination with sentinel LN biopsy (Arya et al. 2014; Schilling et al. 2017).
In conclusion, the present study suggests that 18F-FDG PET/CT has superior sensitivity to CT/MR imaging in a prospective cohort of 178 OCC patients with negative neck palpation findings. 18F-FDG PET/CT improved detection of occult LN metastasis up to 33.4% in these patients with positive cervical LNs that was unidentified with CT/MR imaging. 18F-FDG PET/CT may provide better detection of occult lymph node metastasis than CT/MR imaging, which potentially improves prognosis and proper management of OCC patients. However, false-positive PET/CT findings resulting in relatively lower specificity compared with that of CT/MR imaging might be a serious disadvantage. In addition, the presence of false-negative PET/CT findings cannot entirely exclude the necessity of sentinel LN biopsy or elective neck dissection in OCC cN0 patients.
References
Arya S, Rane P, Deshmukh A (2014) Oral cavity squamous cell carcinoma: role of pretreatment imaging and its influence on management. Clin Radiol 69(9):916–930
Ceylan Y, Omur O, Hatipoglu F (2018) Contribution of (18)F-FDG PET/CT to staging of head and neck malignancies. Mol Imaging Radionucl Ther 27(1):19–24
Chong A, Ha JM, Han YH, Kong E, Choi Y, Hong KH, Park JH, Kim SH, Park JM (2017) Preoperative lymph node staging by FDG PET/CT with contrast enhancement for thyroid cancer: a multicenter study and comparison with neck CT. Clin Exp Otorhinolaryngol 10(1):121–128
Chung JH, Cho KJ, Lee SS, Baek HJ, Park JH, Cheon GJ, Choi CW, Lim SM (2004) Overexpression of Glut1 in lymphoid follicles correlates with false-positive (18)F-FDG PET results in lung cancer staging. J Nucl Med 45(6):999–1003
D’Cruz AK, Vaish R, Kapre N, Dandekar M, Gupta S, Hawaldar R, Agarwal JP, Pantvaidya G, Chaukar D, Deshmukh A, Kane S, Arya S, Ghosh-Laskar S, Chaturvedi P, Pai P, Nair S, Nair D, Badwe R (2015) Elective versus therapeutic neck dissection in node-negative oral cancer. N Engl J Med 373(6):521–529
Dik EA, Willems SM, Ipenburg NA, Rosenberg AJ, Van Cann EM, van Es RJ (2016) Watchful waiting of the neck in early stage oral cancer is unfavourable for patients with occult nodal disease. Int J Oral Maxillofac Surg 45(8):945–950
Evangelista L, Cervino AR, Chondrogiannis S, Marzola MC, Maffione AM, Colletti PM, Muzzio PC, Rubello D (2014) Comparison between anatomical cross-sectional imaging and 18F-FDG PET/CT in the staging, restaging, treatment response, and long-term surveillance of squamous cell head and neck cancer: a systematic literature overview. Nucl Med Commun 35(2):123–134
Farmer RW, McCall L, Civantos FJ, Myers JN, Yarbrough WG, Murphy B, O’Leary M, Zitsch R, Siegel BA (2015) Lymphatic drainage patterns in oral squamous cell carcinoma: findings of the ACOSOG Z0360 (Alliance) study. Otolaryngol Head Neck Surg 152(4):673–677
Fukui MB, Blodgett TM, Snyderman CH, Johnson JJ, Myers EN, Townsend DW, Meltzer CC (2005) Combined PET-CT in the head and neck: part 2. Diagnostic uses and pitfalls of oncologic imaging. Radiographics 25(4):913–930
Ho AS, Kim S, Tighiouart M, Gudino C, Mita A, Scher KS, Laury A, Prasad R, Shiao SL, Van Eyk JE, Zumsteg ZS (2017) Metastatic lymph node burden and survival in oral cavity cancer. J Clin Oncol 35(31):3601–3609
Joo YH, Koo BS (2019) Evolving strategy for surgical management of oral cancer: present and future. Clin Exp Otorhinolaryngol 12(2):101–102
Joo YH, Cho JK, Koo BS, Kwon M, Kwon SK, Kwon SY, Kim MS, Kim JK, Kim H, Nam I, Roh JL, Park YM, Park IS, Park JJ, Shin SC, Ahn SH, Won S, Ryu CH, Yoon TM, Lee G, Lee DY, Lee MC, Lee JK, Lee JC, Lim JY, Chang JW, Jang JY, Chung MK, Jung YS, Cho JG, Choi YS, Choi JS, Lee GH, Chung PS (2019) Guidelines for the surgical management of oral cancer: Korean Society of thyroid-head and neck surgery. Clin Exp Otorhinolaryngol 12(2):107–144
Kim SJ, Pak K, Kim K (2019) Diagnostic accuracy of F-18 FDG PET or PET/CT for detection of lymph node metastasis in clinically node negative head and neck cancer patients; A systematic review and meta-analysis. Am J Otolaryngol 40(2):297–305
Liao LJ, Lo WC, Hsu WL, Wang CT, Lai MS (2012) Detection of cervical lymph node metastasis in head and neck cancer patients with clinically N0 neck-a meta-analysis comparing different imaging modalities. BMC Cancer 12:236
Manca G, Vanzi E, Rubello D, Giammarile F, Grassetto G, Wong KK, Perkins AC, Colletti PM, Volterrani D (2016) (18)F-FDG PET/CT quantification in head and neck squamous cell cancer: principles, technical issues and clinical applications. Eur J Nucl Med Mol Imaging 43(7):1360–1375
Mucke T, Mitchell DA, Wagenpfeil S, Ritschl LM, Wolff KD, Kanatas A (2014) Incidence and outcome for patients with occult lymph node involvement in T1 and T2 oral squamous cell carcinoma: a prospective study. BMC Cancer 14:346
Nakagawa T, Yamada M, Suzuki Y (2008) 18F-FDG uptake in reactive neck lymph nodes of oral cancer: relationship to lymphoid follicles. J Nucl Med 49(7):1053–1059
Nakamoto Y, Tatsumi M, Hammoud D, Cohade C, Osman MM, Wahl RL (2005) Normal FDG distribution patterns in the head and neck: PET/CT evaluation. Radiology 234(3):879–885
Ng SH, Yen TC, Liao CT, Chang JT, Chan SC, Ko SF, Wang HM, Wong HF (2005) 18F-FDG PET and CT/MRI in oral cavity squamous cell carcinoma: a prospective study of 124 patients with histologic correlation. J Nucl Med 46(7):1136–1143
Pelosi E, Messa C, Sironi S, Picchio M, Landoni C, Bettinardi V, Gianolli L, Del Maschio A, Gilardi MC, Fazio F (2004) Value of integrated PET/CT for lesion localisation in cancer patients: a comparative study. Eur J Nucl Med Mol Imaging 31(7):932–939
Roh JL, Yeo NK, Kim JS, Lee JH, Cho KJ, Choi SH, Nam SY, Kim SY (2007) Utility of 2-[18F] fluoro-2-deoxy-d-glucose positron emission tomography and positron emission tomography/computed tomography imaging in the preoperative staging of head and neck squamous cell carcinoma. Oral Oncol 43(9):887–893
Rosenbaum SJ, Lind T, Antoch G, Bockisch A (2006) False-positive FDG PET uptake–the role of PET/CT. Eur Radiol 16(5):1054–1065
Schilling C, Stoeckli SJ, Haerle SK, Broglie MA, Huber GF, Sorensen JA, Bakholdt V, Krogdahl A, von Buchwald C, Bilde A, Sebbesen LR, Odell E, Gurney B, O’Doherty M, de Bree R, Bloemena E, Flach GB, Villarreal PM, Fresno Forcelledo MF, Junquera Gutierrez LM, Amezaga JA, Barbier L, Santamaria-Zuazua J, Moreira A, Jacome M, Vigili MG, Rahimi S, Tartaglione G, Lawson G, Nollevaux MC, Grandi C, Donner D, Bragantini E, Dequanter D, Lothaire P, Poli T, Silini EM, Sesenna E, Dolivet G, Mastronicola R, Leroux A, Sassoon I, Sloan P, McGurk M (2015) Sentinel European Node Trial (SENT): 3-year results of sentinel node biopsy in oral cancer. Eur J Cancer 51(18):2777–2784
Schilling C, Shaw R, Schache A, McMahon J, Chegini S, Kerawala C, McGurk M (2017) Sentinel lymph node biopsy for oral squamous cell carcinoma. Where are we now? Br J Oral Maxillofac Surg 55(8):757–762
Schöder H, Carlson DL, Kraus DH, Stambuk HE, Gonen M, Erdi YE, Yeung HW, Huvos AG, Shah JP, Larson SM, Wong RJ (2006) 18F-FDG PET/CT for detecting nodal metastases in patients with oral cancer staged N0 by clinical examination and CT/MRI. J Nucl Med 47(5):755–762
Shah JP, Candela FC, Poddar AK (1990) The patterns of cervical lymph node metastases from squamous carcinoma of the oral cavity. Cancer 66(1):109–113
Funding
This study was supported by the National Research Foundation of Korea (NRF) grant, funded by the Ministry of Science and ICT (MSIT), the Government of Korea (No. 2019R1A2C2002259) (J.-L.R.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to disclose.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research board and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors. Informed consent from all individual participants was obtained.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bae, M.R., Roh, JL., Kim, J.S. et al. 18F-FDG PET/CT versus CT/MR imaging for detection of neck lymph node metastasis in palpably node-negative oral cavity cancer. J Cancer Res Clin Oncol 146, 237–244 (2020). https://doi.org/10.1007/s00432-019-03054-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-019-03054-3