Abstract
Purpose
To evaluate biochemical recurrence (BCR) risk in men with localized prostate cancer (PC) of pathological Gleason score (pGS) 8–10. Although such patients have low BCR-free survival (BCRFS) following radical prostatectomy (RP), they are not recommended for adjuvant radiation therapy (ART) as per current guidelines.
Methods
Among an adjuvant treatment-naïve cohort between 1995 and 2015, 1272 men were identified and categorized into group 1 [pGS7 (3 + 4) and pT3; n = 654], group 2 [pGS7 (4 + 3) and pT3; n = 408], and group 3 (pGS 8–10 and pT2; n = 210). The BCR risk of group 3 was compared with that of groups 1 and 2 who are the candidates for ART.
Results
At a median follow-up of 60 months (interquartile range: 39–86), 432 men experienced BCR. BCRFS was lower in group 3 than in groups 1 and 2 (p < 0.001 and p = 0.021, respectively). In multivariate analysis, this association persisted and surgical margin (SM) was found to be a significant BCR predictor. Although statistically not significant, BCRFS was lower in group 3 with positive SM (PSM) than in group 2 with PSM (p = 0.101). BCRFS was significantly worse in group 3 with negative SM (NSM) than in group 1 with PSM (p = 0.038), while it was better in group 2 with PSM (p = 0.297).
Conclusion
Localized high-grade PC with PSM showed lower BCRFS and that with NSM showed better BCRFS without statistical significance than locally advanced GS 7 PC with PSM that are eligible for ART.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
While radical prostatectomy (RP) is one of the standard treatments for localized and locally advanced prostate cancer (PC), 40% of the patients with localized PC show biochemical recurrence (BCR) following RP, even with excellent surgical cancer control (Chang and Cookson 2006). Despite radiation toxicity, adjuvant radiation therapy (ART) reduces the BCR risk and improves overall survival (Thompson et al. 2004; Van der Kwast et al. 2007). Thus, identification of patients who have a high risk of BCR is important.
During pre-treatment assessment, the clinical staging of PC includes radiological imaging, the prostate-specific antigen (PSA) level and the biopsy Gleason score (GS). GS 8–10, which is considered as high-grade, is one of the most important prognostic factors and indicates a high risk of PC (D’Amico et al. 1998). After RP, the pathological stage of PC is determined by examining the tumor extent in the prostate, seminal vesicles, and pelvic lymph nodes (if lymphadenectomy is performed). Pathological staging is more useful than clinical staging in predicting outcomes. The pathological features that are associated with BCR include a high GS (8–10), positive surgical margin (PSM), extraprostatic extension (EPE), seminal vesicle invasion (SVI), and lymph node invasion (LNI) (American Joint Committee on C and Amin 2017; Menon et al. 2010; Pound et al. 1997). As expected, a pathologically localized tumor (pT2) shows significantly better outcomes than a locally advanced one (pT3) (Van Poppel et al. 2000).
The current guidelines recommend an adjuvant androgen deprivation for patients who have LNI and ART for patients who either have adverse pathological features (PSM, EPE, or SVI) or detectable PSA levels after RP without consideration of RP GS (Cornford et al. 2017; Mohler et al. 2016; Thompson et al. 2013). However, the 5-year disease-free survival rate following RP is low in patients with RP GS 8–10 and localized PC (Han et al. 2003; Lau et al. 2002; Mian et al. 2002). Whether an adjuvant therapy is necessary in case of high RP GS remains controversial (de la Taille et al. 2002; Kamat et al. 2003; Van der Kwast et al. 2007). Several studies have analyzed the predictors of BCR after ART to identify patients who might benefit from radiotherapy; nonetheless, the predictive impact of high RP GS was not proved consistently.
In cases of pathologically localized high-grade PC, where GS and pT stage do not match with regard to the risk classification, the oncological outcomes, and application of ART are questionable. We hypothesized that the oncological outcomes in men with localized high-grade PC may be worse than or similar to those in men with locally advanced PC with RP GS 7 who are currently the candidates for ART. We evaluated BCR risk in a single-center cohort of patients who did not receive adjuvant treatment.
Patients and methods
This retrospective study received approval from the institutional review board (IRB) of Yonsei University Severance Hospital (IRB number: 4-2018-0206) for the collection of data of the 4969 patients who underwent RP for PC at our institution between 1995 and 2015.
Among 1787 patients with RP GS 7 and pT3 and RP GS 8–10 and pT2, we excluded those patients who: had received a neoadjuvant treatment (n = 138), had metastatic disease at initial diagnosis (n = 19) or post-RP LNI (n = 33), had an incomplete clinicopathological or follow-up data (n = 12), had received an adjuvant treatment (n = 17), and had persistent PSA after RP (n = 296). In total 1272 men were identified. G7 (3 + 4) and G7 (4 + 3) show different prognosis (Epstein et al. 2016); thus, based on the RP GS and the pT stage, the cohort was divided into the following three groups: group 1, consisting of 654 men with G7 (3 + 4) and T3; group 2, consisting of 408 men with GS 7 (4 + 3) and T3; and group 3, consisting of 210 men with GS 8–10 and T2 (Fig. 1).
The pathological stages were assigned in accordance with the American Joint Committee on Cancer staging system (American Joint Committee on C and Amin 2017). Pathological analysis of the RP specimens was performed at our institute by an experienced uropathologist (NH Cho). Briefly, the entire surface of the resected prostate specimens was coated with India ink, fixed in neutral-buffered formalin, and embedded in paraffin blocks. Whole-mount step sections were then transversely cut at regular intervals, from the apex of the prostate to the tips of the seminal vesicles. Each section was examined for EPE, SVI, and PSM (Jang et al. 2016).
Postoperative PSA follow-up was undertaken at 3-month intervals for the first 2 years and at 6-month intervals for the subsequent 3 years; an annual PSA follow-up was recommended thereafter. BCR was defined as two consecutive increases over 0.2 ng/mL in the PSA levels following an undetectable PSA after RP, or any secondary treatment for PSA elevation (Cookson et al. 2007; Cooperberg et al. 2011). Biochemical recurrence-free survival (BCRFS) was defined as the time from RP to BCR.
Baseline characteristics and pathological outcomes were compared using the Chi-squared test for categorical data and the Kruskal–Wallis test for continuous data. BCRFS was estimated using the Kaplan–Meier method and log-rank test was used to compare these estimates among groups. Multivariate Cox regression analyses were performed to identify the predictive factors for BCR. The level of significance was set at 0.05 in all analyses. All statistical analyses were performed using SPSS version 23.0 (IBM Corp., Armonk, NY, USA).
Results
The baseline characteristics of the patients are summarized in Table 1. Significant intergroup differences in PSA levels, year of surgery, and PSM were observed (all p < 0.05), while there was no difference in age among the three groups (p = 0.058). Preoperative PSA levels of groups 2 and 3 differed significantly (p = 0.176), but they were higher than those of group 1 (group 2: p = 0.005, group 3: p = 0.001). While the PSM rates of groups 1 and 2 were not significantly different (p = 0.555), group 3 showed a significantly lower PSM rate than the other two (all p < 0.001). Group 1 showed a greater EPE than group 2 (92% vs. 87%).
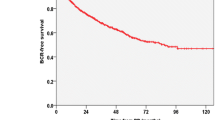
Among the 1272 men studied, 432 (34.0%) experienced BCR at a median follow-up of 60 months (interquartile range: 39–86). The 5-year BCRFS rates were 72.1%, 60.3%, and 49.2% in groups 1, 2, and 3, respectively. The 10-year BCRFS rates were 59.7%, 49.4%, and 36.6% in groups 1, 2, and 3, respectively. The BCRFS was significantly lower in group 3 than in the other two groups (group 1: p < 0.001, group 2: p = 0.021, overall: p < 0.001; Fig. 2).
In multivariate models (Table 2), the BCR was higher in group 2 (adjusted hazard ratio [AHR]: 1.626, 95% confidence interval [CI] 1.311–2.018, p < 0.001) and in group 3 (AHR: 2.185, 95% CI 1.673–2.852, p < 0.001), than in group 1. The PSA level (AHR: 1.008, 95% CI 1.006–1.010, p < 0.001) and year of surgery (AHR: 1.073, 95% CI 1.036–1.111, p < 0.001) were found to be associated with the BCR. PSM was also a significant predictor of BCR (AHR: 1.955, 95% CI 1.598–2.393, p < 0.001).
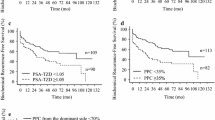
Additionally, we analyzed the relationship between BCR and the surgical margin status. The BCR rate was higher when the RP GS was higher, irrespective of whether the surgical margin status was positive or not. When the GS was identical, the BCR rate in case of PSM was higher than in case of negative surgical margin (NSM) (Table 3). In case of PSM, BCRFS in group 3 was significantly lower than in group 1 (p < 0.001), whereas though not statistically significant, it was lower than in group 2 (p = 0.101). Regarding NSM, BCRFS in group 3 was lower than in groups 1 and 2 (p < 0.001 and p = 0.001, respectively). Furthermore, group 3 with NSM showed significantly lower BCRFS than group 1 with PSM (p = 0.038); however, it showed better BCRFS, though not statistically significant, compared to group 2 with PSM (p = 0.297) (Fig. 3).
Discussion
Management after RP depends on the preoperative PSA level and the pathological features such as RP GS, surgical margin status, EPE, SVI, and LNI. About 30% of the patients who underwent an RP showed PSM, EPE, and SVI. Such patients had a 60% recurrence rate at 5 years after RP (Bolla et al. 2012; Swanson et al. 2007). According to the results of the European Organisation for Research and Treatment of Cancer (EORTC) trial 22911, ART in patients with adverse risk factors (PSM, EPE, or SVI) diminished the risk of BCR and improved the local control of the disease despite the associated radiation toxicity (Thompson et al. 2004; Van der Kwast et al. 2007). Thus, the current guidelines recommend ART for patients with adverse pathological features or detectable PSA levels after RP. However, these guidelines do not include RP GS 8–10 as a criterion for selecting patients eligible for ART (Cornford et al. 2017; Mohler et al. 2016; Thompson et al. 2013). Previous studies have indicated that high RP GS is related to BCR after RP. Furthermore, Menon et al. also reported that one of the strongest predictors of BCR was RP GS 8–10 (HR: 5.37, 95% CI 2.99–9.65, p < 0.001) (Menon et al. 2010). Eisenberg et al. evaluated the outcomes in patients with pT3aN0 PC and observed that RP GS was significantly associated with BCR (HR: 1.84, 95% CI 1.6–2.1, p < 0.001) (Eisenberg et al. 2013). Furthermore, Suardi et al. reported that the 3-year, 5-year, and 7-year BCRFS rates for RP GS 8–10 were lower than those for RP GS ≤ 7 and that RP GS 8–10 was an independent predictor of BCR (HR 5.14, p = 0.004) (Suardi et al. 2012).
Indeed, there has been a controversy over whether ART is necessary in patients with a high-grade PC. De la Taille et al. evaluated ART failure in patients with BCR after RP and showed that GS (p = 0.0395) was an independent predictive factor (de la Taille et al. 2002). Conversely, Van der Kwast et al. analyzed pathological data on specimens from participants of the randomized controlled EORTC trial 22911 and identified no statistically significant predictive impact of RP GS (p > 0.1) (Van der Kwast et al. 2007). Kamat et al. investigated BCRFS in patients who received ART for PSM and reported that GS ≥ 7 (4 + 3) was predictive of BCR in univariate analysis, but not in multivariate analysis (Kamat et al. 2003). Thus, it is crucial to identify patients who are at a high risk for the development of BCR.
While patients with RP GS 8–10 PC are at a high risk of BCR, combinations of favorable pathological features could modify the BCR risk. Although the definition of recurrence differed, previous studies have evaluated the relationship between localized high-grade PC and recurrence-free survival. Lau et al. reported that 104 patients had a 5-year progression-free survival rate of 53%. They defined progression as local recurrence, systemic progression, or a PSA value of 0.4 ng/mL or greater. However, 41% of the localized high-grade PC patients had PSM and the survival rate was not estimated according to the surgical margin status (Lau et al. 2002). Mian et al. reported that the 5-year disease-free survival rates were 82% for 58 patients with NSM and 80% for 14 patients with PSM, while disease recurrence was defined as a local or distant disease, or a PSA value of 0.1 ng/mL or greater (Mian et al. 2002). Fischer et al. also examined the association between localized high-grade PC and BCR. They defined BCR as a single PSA value greater than 0.2 ng/mL, two values of 0.2 ng/mL, or a secondary treatment for an elevated postoperative PSA level. The 5-year BCRFS rates were 38% for 140 patients with NSM and 16% for 89 patients with PSM (Fischer et al. 2016).
In the present study, the 5-year BCRFS of patients with localized high-grade PC was significantly lower than that of patients with locally advanced RP GS 7 PC. We also analyzed BCR according to the surgical margin status after consideration of the verified relationship between PSM and BCR on multivariable Cox regression analysis. The 5-year BCRFS rates of patients with localized high-grade PC were 53.9% for men with NSM and 38% for men with PSM. Moreover, while the BCRFS in group 3 with both PSM and NSM was significantly lower than that in group 1 with PSM, it was not significantly different from that in group 2 with PSM. Localized high-grade PC with NSM, which is currently ineligible for an ART, showed a lower or similar BCRFS compared to locally advanced RP GS 7 PC with PSM. This finding was notable, as PSM is one of the adverse pathological features taken into consideration for ART. Overall, even without the presence of such features, high RP GS indicates a high BCR risk. Hence, patients with localized high-grade PC are excellent candidates for ART. Irrespective of the guidelines, physicians differ in their opinions with respect to the adoption of ART, regarding the possible urinary, bowel, and sexual side-effects of radiotherapy (Kim et al. 2013; Showalter et al. 2012). With additional concern regarding overtreatment, localized high-grade PC with NSM could be considered for salvage radiotherapy instead of ART (Pisansky et al. 2019). To confirm our results, further studies evaluating the benefit of adjuvant treatment in large number of patients with localized high-grade and locally advanced low-grade PC are required.
The present study has some limitations. First, our results may not be generalizable, because all data were collected from a single institution and retrospectively reviewed. Second, the present cohort was not reclassified according to the updated Gleason grading system in 2014, because our institution adopted the system in 2016. As major update was the restricted definition of the grade pattern 3 (Epstein et al. 2016), some cases of RP GS 7 may have been reclassified as RP GS 8 and the reclassified RP GS 8 disease may have a better prognosis than our findings. Therefore, the current results should be reassessed in accordance with the updated Gleason grading system. Finally, the comorbidity and tertiary Gleason pattern in RP GS were not included in the analysis.
Conclusion
Pathologically localized high-grade PC with PSM showed lower BCRFS and that with NSM showed better BCRFS without statistical significance compared to locally advanced RP GS 7 PC with PSM that are eligible for ART. These findings suggest that high RP GS, though localized stage, indicates a high BCR risk.
References
American Joint Committee on C, Amin MB (2017) AJCC cancer staging manual. Springer, New York
Bolla M et al (2012) Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911). Lancet 380:2018–2027. https://doi.org/10.1016/s0140-6736(12)61253-7
Chang SS, Cookson MS (2006) Impact of positive surgical margins after radical prostatectomy. Urology 68:249–252. https://doi.org/10.1016/j.urology.2006.03.053
Cookson MS et al (2007) Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: the American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel report and recommendations for a standard in the reporting of surgical outcomes. J Urol 177:540–545. https://doi.org/10.1016/j.juro.2006.10.097
Cooperberg MR, Hilton JF, Carroll PR (2011) The CAPRA-S score: a straightforward tool for improved prediction of outcomes after radical prostatectomy. Cancer 117:5039–5046
Cornford P et al (2017) EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol 71:630–642. https://doi.org/10.1016/j.eururo.2016.08.002
D’Amico AV et al (1998) Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 280:969–974
de la Taille A, Flam TA, Thiounn N, Pontvert D, Saighi D, Zerbib M, Debre B (2002) Predictive factors of radiation therapy for patients with prostate specific antigen recurrence after radical prostatectomy. BJU Int 90:887–892
Eisenberg MS, Karnes RJ, Kaushik D, Rangel L, Bergstralh EJ, Boorjian SA (2013) Risk stratification of patients with extraprostatic extension and negative lymph nodes at radical prostatectomy: identifying optimal candidates for adjuvant therapy. J Urol 190:1735–1741. https://doi.org/10.1016/j.juro.2013.05.053
Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA, Grading C (2016) The 2014 International Society of Urological Pathology (ISUP) consensus conference on gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol 40:244–252
Fischer S et al (2016) Do all men with pathological Gleason score 8-10 prostate cancer have poor outcomes? Results from the SEARCH database. BJU Int 118:250–257. https://doi.org/10.1111/bju.13319
Han M, Partin AW, Zahurak M, Piantadosi S, Epstein JI, Walsh PC (2003) Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J Urol 169:517–523. https://doi.org/10.1097/01.ju.0000045749.90353.c7
Jang WS, Kim LH, Yoon CY, Rha KH, Choi YD, Hong SJ, Ham WS (2016) Effect of preoperative risk group stratification on oncologic outcomes of patients with adverse pathologic findings at radical prostatectomy. PLoS One 11:e0164497. https://doi.org/10.1371/journal.pone.0164497
Kamat AM, Babaian K, Cheung MR, Naya Y, Huang SH, Kuban D, Babaian RJ (2003) Identification of factors predicting response to adjuvant radiation therapy in patients with positive margins after radical prostatectomy. J Urol 170:1860–1863. https://doi.org/10.1097/01.ju.0000092503.45951.c2
Kim SP et al (2013) Variation in treatment recommendations of adjuvant radiation therapy for high-risk prostate cancer by physician specialty. Urology 82:807–812. https://doi.org/10.1016/j.urology.2013.04.060
Lau WK, Bergstralh EJ, Blute ML, Slezak JM, Zincke H (2002) Radical prostatectomy for pathological Gleason 8 or greater prostate cancer: influence of concomitant pathological variables. J Urol 167:117–122
Menon M et al (2010) Biochemical recurrence following robot-assisted radical prostatectomy: analysis of 1384 patients with a median 5-year follow-up. Eur Urol 58:838–846. https://doi.org/10.1016/j.eururo.2010.09.010
Mian BM, Troncoso P, Okihara K, Bhadkamkar V, Johnston D, Reyes AO, Babaian RJ (2002) Outcome of patients with Gleason score 8 or higher prostate cancer following radical prostatectomy alone. J Urol. 167:1675–1680
Mohler JL et al (2016) Prostate Cancer, Version 1.2016. J Natl Compr Canc Netw 14:19–30
Pisansky TM, Thompson IM, Valicenti RK, D’Amico AV, Selvarajah S (2019) Adjuvant and Salvage Radiotherapy after Prostatectomy: ASTRO/AUA Guideline Amendment 2018–2019. J Urol 202:533–538. https://doi.org/10.1097/ju.0000000000000295
Pound CR, Partin AW, Epstein JI, Walsh PC (1997) Prostate-specific antigen after anatomic radical retropubic prostatectomy. Patterns of recurrence and cancer control. Urol Clin North Am 24:395–406
Showalter TN et al (2012) Physician beliefs and practices for adjuvant and salvage radiation therapy after prostatectomy. Int J Radiat Oncol Biol Phys 82:e233–238. https://doi.org/10.1016/j.ijrobp.2011.04.003
Suardi N et al (2012) Long-term biochemical recurrence rates after robot-assisted radical prostatectomy: analysis of a single-center series of patients with a minimum follow-up of 5 years. Urology 79:133–138. https://doi.org/10.1016/j.urology.2011.08.045
Swanson GP, Riggs M, Hermans M (2007) Pathologic findings at radical prostatectomy: risk factors for failure and death. Urol Oncol 25:110–114. https://doi.org/10.1016/j.urolonc.2006.06.003
Thompson IM et al (2004) Prevalence of prostate cancer among men with a prostate-specific antigen level < or = 4.0 ng per milliliter. N Engl J Med 350:2239–2246. https://doi.org/10.1056/nejmoa031918
Thompson IM et al (2013) Adjuvant and salvage radiotherapy after prostatectomy: AUA/ASTRO Guideline. J Urol 190:441–449. https://doi.org/10.1016/j.juro.2013.05.032
Van der Kwast TH et al (2007) Identification of patients with prostate cancer who benefit from immediate postoperative radiotherapy: EORTC 22911. J Clin Oncol 25:4178–4186. https://doi.org/10.1200/jco.2006.10.4067
Van Poppel H, Goethuys H, Callewaert P, Vanuytsel L, Van de Voorde W, Baert L (2000) Radical prostatectomy can provide a cure for well-selected clinical stage T3 prostate cancer. Eur Urol 38:372–379. https://doi.org/10.1159/000020311
Funding
This study was supported by a faculty research grant from the Yonsei University College of Medicine (6-2016-0067).
Author information
Authors and Affiliations
Contributions
Conceptualization: JEH, WSH. Methodology: JEH, JSP, JSL, WSH. Formal analysis and investigation: JEH, JK, WSJ, NHC. Writing-original draft preparation: JEH. Writing-review and editing: WSH. Funding acquisition: WSH. Resources: KHR, YDC, SJH. Supervision: WSH.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Institutional review board approval was obtained by the participating center and institutional data sharing agreements were adhered to in accordance with the ethical standards of the institutional and/or national research committee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Heo, J.E., Park, J.S., Lee, J.S. et al. Postoperative biochemical recurrence of pathologically localized high-grade prostate cancer in adjuvant treatment-naïve patients. J Cancer Res Clin Oncol 146, 221–227 (2020). https://doi.org/10.1007/s00432-019-03049-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-019-03049-0