Abstract
Purpose
Anti-programmed cell death-1 (PD-1)/programmed cell death-ligand 1 (PD-L1) therapy has shown promise in tumor immunotherapy. Our objectives were to measure pre-treatment serum-soluble PD-L1 (sPD-L1) levels and to assess the relationships between sPD-L1 levels and clinical characteristics, prognosis, and tumor tissue PD-L1 expression in patients with hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC).
Methods
Pre-treatment serum sPD-L1 levels were measured with an enzyme-linked immunosorbent assay (ELISA) in 81 patients with HBV-related HCC and compared to those in 49 healthy controls. The association between serum sPD-L1 levels and prognosis was assessed using survival analysis. The correlation between paired serum sPD-L1 levels and tumor PD-L1 expression (in resected tissue homogenates) was assessed in a separate group of 20 patients with HBV-related HCC.
Results
Median sPD-L1 concentration in patients with HBV-related HCC was 5.129 (range 0.140–12.391) ng/mL and in healthy controls was 0.836 (range 0.105–2.168) ng/mL (p < 0.001). On multivariate analysis, sPD-L1 levels were significant independent predictors of disease-free survival (hazard ratio [HR] 3.503; 95% confidence interval [CI], 1.559–7.871; p = 0.002) and overall survival (HR 3.399; 95% CI 1.308–8.831; p = 0.012). Positive correlation (r = 0.527, p = 0.017) between serum sPD-L1 and tumor PD-L1 expression was observed. Tumor expression of PD-L1 was significantly higher in those with serum sPD-L1 concentrations above vs. below the median level of 5.471 ng/ml (p = 0.012).
Conclusions
In patients with HBV-related HCC, serum sPD-L1 concentrations were elevated, and positively correlated with tumor PD-L1 expression. Lower pre-treatment serum sPD-L1 levels were predictors of more favorable disease-free and overall survival. Serum sPD-L1 testing has a potential role in HBV-related HCC disease assessment, systemic therapy choices and survival prediction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the most prevalent primary liver cancer, and it is one of the most common causes of cancer-related mortality worldwide. Most cases (80%) of hepatocellular carcinoma occur in sub-Saharan Africa and eastern Asia, where the main risk factor is chronic hepatitis B (Ferlay et al. 2015). During chronic viral infection, sustained innate and adaptive immune responses contribute to immune tolerance and tumor generation. The programmed cell death-1 (PD-1)/programmed cell death-ligand 1 (PD-L1) pathway plays a critical role in these compromised chronic infections, in tumor immune escape, and in the formation of the tumor microenvironment (Kudo 2017).
The expression of PD-L1 in HCC lesions has been observed in several studies (Gao et al. 2009; Calderaro et al. 2016; Xie et al. 2016). In some studies, elevated PD-L1 expression in HCC tumors has been identified as a poor prognostic factor for HCC (Gao et al. 2009; Calderaro et al. 2016). Conversely, others have reported that patients with higher PD-L1 expression in tumors have better disease-free and overall survival (Xie et al. 2016). Despite these contradictory results, the anti-PD-1 antibody nivolumab has shown promising efficacy in treating patients with HCC, and it was recently approved for use by the US Food and Drug Administration (FDA). However, responses to this agent have also occurred in PD-L1-negative HCC patients, and response rates to it have not correlated with the expression of PD-L1, specifically when measured with immunohistochemical (IHC) testing (El-Khoueiry et al. 2017). Taken together, these findings indicate that IHC-measured PD-L1 expression in local tumor tissue may not be a good predictive biomarker for prognosis or sensitivity to immunotherapy in patients with HCC.
As with some other members of the B7 family of immune-regulatory ligands, a soluble form of PD-L1, which maintains the ability to bind to PD-1 receptors, can be detected in the serum of patients (Chen et al. 2011). In vitro studies have revealed that this soluble programmed cell death-ligand 1 (sPD-L1) is released by PD-L1-positive cell lines and that its levels in the supernatants of these cell lines correlate with the number of cells expressing PD-L1 (Simon et al. 2006). Also, elevated serum concentrations of sPD-L1 have been observed in patients with hepatitis C and several types of cancer, including lymphoma, gastric cancer, and multiple myeloma (Rossille et al. 2014; Wang et al. 2015; Takahashi et al. 2016; Yamagiwa et al. 2017).
Although the prognostic value of sPD-L1 has been explored in some cancers, little attention has been paid to measuring the levels or assessing the prognostic role of sPD-L1 in patients with HCC, and particularly in those with hepatitis B virus (HBV)-related HCC. In this study of patients with HBV-related HCC, our objectives were to measure the pre-treatment serum levels of sPD-L1 and to assess the degree of association between sPD-L1 levels and clinical characteristics, prognosis, and PD-L1 expression in tumor tissue.
Patients and methods
The study was approved by the Institutional Review Committee of Sun Yat-sen University Cancer Centre in Guangzhou, China (YB2015-018-01). Written informed consent for procedures and publishing of data was obtained from patients and their families. All procedures were performed after obtaining informed consent, in accordance with the approved protocol.
Patients and samples
In this study, two independent groups of patients with HBV-related HCC from Sun Yat-sen University Cancer Center were enrolled (Fig. 1). The main study group (Group 1) consisted of 81 patients who were selected retrospectively from a larger group of consecutive patients who were initially diagnosed from 2010 to 2012. The smaller study group (Group 2) consisted of 20 patients who were selected retrospectively from a larger group of consecutive patients who were initially diagnosed from March 1, 2016 to June 30, 2016, who had surgical resections, and who had frozen tumor tissue samples available from those resections. For both groups, to be considered for the study, patients needed to meet the following inclusion criteria: (1) newly diagnosed HCC based on National Comprehensive Cancer Network (NCCN) guidelines; (2) serum positive for hepatitis B antigen and negative for both anti-hepatitis A and C antibodies; (3) Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) 0–2; (4) Class A Child–Pugh Classification for Severity of Liver Disease; (5) received curative-intent therapy (surgical excision or thermal ablation) according to Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China (2017 Edition) (Zhou et al. 2018); (6) no other malignancies or immune-related disease; (7) serum from time of diagnosis available; (8) complete follow-up information available.
Study enrollment and flow of two groups of patients with hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC), diagnosed at Sun Yat-sen University Cancer Center, and one group of healthy blood donors. sPD-L1, soluble programmed cell death-ligand 1; ELISA, enzyme-linked immunosorbent assay; PD-L1, programmed cell death-ligand 1
For the main study group of 81 patients, follow-up records through June 2018 were evaluated. Contrast-enhanced liver computed tomography (CT) or magnetic resonance imaging (MRI) scans were obtained every 2 months during the first year after curative-intent therapy and at least every 4 months thereafter. All follow-up examinations were performed by independent physicians who had no knowledge of the study. Therapeutic responses were evaluated by CT or MRI according to modified Response Evaluation Criteria in Solid Tumors (mRECIST) criteria. The endpoints determined were disease-free survival (DFS) and overall survival (OS), or the time to death from any cause. These were measured from the date of treatment to the date of last follow-up or death.
The control group (Group 3) for the study consisted of 49 age- and sex-matched healthy blood donors, whose serum was obtained from the blood bank of the Red Cross Blood Donation Service (Fig. 1).
Measurements of sPD-L1 in serum
Serum samples were collected from all Group 1 and Group 2 patients at the time of diagnosis and stored at − 80 °C until further processing. Serum concentrations of sPD-L1 were measured using a specific enzyme-linked immunosorbent assay (ELISA) kit from USCN Life Science (Wuhan, China), according to the manufacturer’s protocol, as follows: (1) all reagents, standard dilutions, and serum samples were prepared according to the manufacturer’s instructions; (2) 100 µL of the standard and the serum sample was added to each well; (3) plates were covered with a plate sealer and incubated at 37 °C for 2 h; (4) each well was aspirated, 100 µL of detection reagent A (1:100) was added to each well, and the plates were re-sealed and incubated at 37 °C for 2 h; (5) each well was aspirated and washed four times in wash buffer; (6) 100 µL of detection reagent B (1:100) was added to each well, and the plates were re-sealed and incubated at 37 °C for 30 min; (7) each well was aspirated and washed five times, 90 µL of substrate solution was added, and the plates were newly sealed and incubated in a darkroom at 37 °C for 20 min; (8) 50µL of stop solution was added to each well, and the contents was then immediately measured at 450 nm using a spectrophotometer (Tecan, Mannedorf, Switzerland). Each serum sample was tested in duplicates. The detection limit used for the ELISA was 57 pg/mL.
Measurement of PD-L1 expression in tumor tissue
For the patients in Group 2, liver tumor tissue (T) was taken from the non-necrotic peripheral zone of tumors. Peritumoral liver tissue (P) and normal liver tissue (N) were taken from regions close (< 1 cm) to the tumor and distant (> 1 cm) from the tumor, respectively (Nara et al. 2012). Tissues obtained from surgical resections were stored at − 80 °C until homogenization was performed for ELISA. Tissues were rinsed in ice-cold phosphate-buffered saline (PBS) (0.02 mol/L, pH 7.0–7.2) to thoroughly remove excess blood and were weighed before homogenization. Tissues were minced into small pieces and homogenized in 1 mL of PBS with a high-speed tissue grinder on ice (OSE-Y30, Tiangen Biotech, Beijing, China). The resulting suspension was sonicated with an ultrasonic cell disrupter (Diagenode Bioruptor Pico, Belgium) to further break down the cell membranes. After that, the homogenates were centrifuged at 5000×g for 10 min. The supernatant was collected and assayed immediately. Before the measurement of PD-L1 expression, tissue total protein content was quantified, using a bicinchoninic acid (BCA) Protein Assay Kit (Beyotime Biotechnology, China). PD-L1 levels were measured using a commercially-available target-specific ELISA kit (SEA788Hu, USCN Life Science, Wuhan, China), according to the manufacturer’s protocol. The resulting PD-L1 levels were then adjusted by the total protein content for each tissue sample, with subsequent results reported as relative PD-L1 expression. Each tissue sample was tested in duplicates.
Statistical methods
Serum sPD-L1 concentrations were reported as means and standard deviations (SD). Cut-off values for defining high and low values of each variable were determined using receiver operating characteristics (ROC) analysis. Differences in sPD-L1 concentrations between categorical variables were analyzed using χ2 tests and t-tests. Differences in levels of expression of PD-L1 between tumor (T), peritumor (P), and normal (N) tissues were analyzed using one-way ANOVA. Spearman rank correlation analysis was performed, and results were reported using Spearman’s correlation coefficient (r).
Survival curves were analyzed using the Kaplan–Meier method and compared using log-rank tests with the following covariates: Age (< 50 years, ≥ 50 years); sex (female, male); maximum tumor diameter (< 50 mm, ≥ 50 mm); tumor number (single, multiple); portal vein invasion (yes, no); lymph node (LN) metastasis (yes, no); HBV genomic DNA copy number (< 1 IU/mL, ≥ 1 IU/mL); alpha-fetoprotein (AFP) concentration (< 400 ng/mL, ≥ 400 ng/mL); carcinoembryonic antigen (CEA) concentration (< 5 ng/mL, ≥ 5 ng/mL); Barcelona Clinic Liver Cancer (BCLC) stage (0 or A, B or C); Tumor, Node, Metastasis (TNM) stage (1 or 2, 3 or 4), curative-intent therapy (surgery, thermal ablation); and sPD-L1 concentration (< 2.825 ng/mL, ≥ 2.825 ng/mL). The cut point of 400 ng/mL for AFP was chosen because, according to the updated European Association for the Study of the Liver [EASL] guidelines, concentrations of AFP above 400 ng/mL are considered diagnostic for HCC in patients with cirrhosis (Galle et al. 2018).
Covariates with significant sPD-L1 concentration differences on univariate analysis were subsequently entered into multivariate time-to-event analysis using Cox proportional hazards regression, with results presented as hazards ratios (HR) and 95% confidence intervals (CI). Alpha was set at 0.05, and all tests were two sided. Data were analyzed with GraphPad Prism 7.0 software (GraphPad Software, Inc., La Jolla, CA, USA) and the SPSS statistics package (version 19, SPSS, Inc., Chicago, IL, USA).
Results
For the main study group (Group 1), there were 561 consecutive patients with HBV-related HCC diagnosed at Sun Yat-Sen University Cancer Center from 2010 to 2012, of which 169 had undergone curative-intent therapy (Fig. 1). Of these, 81 met the inclusion criteria and had follow-up that was adequate for analysis. For the smaller study group (Group 2), there were 84 patients who were diagnosed with HBV-related HCC in 2016, met the inclusion criteria, and had frozen tissue samples available. Out of this group, 20 patients were randomly selected.
Patient characteristics and serum sPD-L1 levels in HBV-related HCC patients
The median follow-up in the main study group was 39.5 (range 2.1–103.0) months. No patients had distant metastases. Curative-intent therapy consisted of surgical resection in 69 (85%) patients and thermal (radiofrequency or microwave) ablation in 12 (15%) patients (Table 1). Among the 81 patients, the average age was 50 years, 75 (93%) were male, 61 (75%) had a low BCLC stage HCC, and 61 (75%) had TNM stage 1 or 2 disease. Although all patients were HBV positive, 55 (68%) had an HBV DNA copy number of 100 IU/mL or higher.
The median serum concentration of sPD-L1 for all 81 HBV-related HCC patients was 5.129 (range 0.140–12.390) ng/mL, significantly higher than that of the 49 healthy controls, which was 0.836 (range 0.105–2.168) ng/mL (p < 0.001) (Fig. 2). The mean sPD-L1 concentration was significantly higher for the patients with maximum tumor diameters ≥ 50 mm compared to those with maximum diameters < 50 mm (p = 0.010). The mean sPD-L1 concentration did not differ significantly between any of the other covariates.
Serum-soluble programmed death-ligand 1 (sPD-L1) levels in 81 patients with hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC) diagnosed in 2010–2012 at Sun Yat-sen University Cancer Center, and 49 healthy control subjects. The median concentration of serum sPD-L1 in HBV-related HCC patients was 5.129 (range 0.14–12.39) ng/mL, significantly higher than that in healthy controls, which was 0.836 (range 0.105–2.168) ng/mL (p < 0.001)
Serum sPD-L1 levels and prognosis
Based on the ROC curve (not shown), the optimal cutoff value for sPD-L1 in predicting high risk for death was 2.825 ng/mL, with an AUC of 0.650 (p = 0.029). Using this cutoff value, 59 (73%) patients in the study group were classified as being at high risk for death.
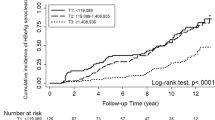
In univariate analyses, the following were significantly associated with DFS and OS, respectively: maximum tumor diameter ≤ 50 mm, tumor number = 1, no LN metastasis, AFP concentration < 400 ng/mL, BCLC stage 0 or A, and TNM stage 1 or 2 (Table 2). Surgical therapy was significantly associated with OS (p = 0.027), but not DFS (p = 0.081). Also, patients with sPD-L1 concentrations < 2.825 ng/mL had significantly longer DFS and OS than those with sPD-L1 concentrations ≥ 2.825 ng/mL (p = 0.011 and p = 0.015, respectively) (Table 2; Fig. 3).
Kaplan–Meier and disease-free survival (DFS) and overall survival (OS) analyses in 81 patients with hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC), diagnosed in 2010–2012 at Sun Yat-sen University Cancer Center. Based on receiver operating characteristic (ROC) curve analysis, the optimal cutoff value for serum-soluble programmed death-ligand 1 (sPD-L1) concentrations in predicting high risk for death was 2.825 ng/mL. Patients with low sPD-L1 concentrations (< 2.825 ng/mL) had significantly longer DFS (p = 0.011) and longer OS (p = 0.015). The median DFS and OS of patients with high sPD-L1 concentrations were 15.73 (95% CI 4.59–26.88) months and 25.73 (95% CI 14.77–36.70) months, respectively
Covariates with significant DFS and OS differences on univariate analysis were subsequently entered into multivariate analysis (Table 2). The following were significant independent predictors of DFS: maximum tumor diameter ≤ 50 mm, tumor number = 1, no LN metastasis, AFP concentration < 400 ng/mL, and sPD-L1 concentration < 2.825 ng/mL (HR 3.503; 95% CI 1.559–7.871; p = 0.002). Similarly, the following were significant independent predictors of OS: maximum tumor diameter ≤ 50 mm, tumor number = 1, AFP concentration < 400 ng/mL, BCLC stage 0 or A, TNM stage 1 or 2, surgical therapy, and sPD-L1 concentration < 2.825 ng/mL (HR 3.399; 95% CI 1.308–8.831; p = 0.012).
Correlation of serum sPD-L1 and tumor PD-L1 expression
In the smaller study group of 20 patients with HBV-related HCC, expression of PD-L1 (relative to total protein) in tumor (T), peritumor (P), and normal (N) tissues differed significantly (p = 0.015) (Fig. 4a). Relative PD-L1 expression was higher in tumor tissue than in peritumor tissue in 15 (75%) patients, and higher than in normal liver tissue in 14 (70%) patients (Fig. 4b).
Relative expression of programmed death-ligand 1 (PD-L1) in tumor, peritumor, and normal tissue in 20 patients with hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC) diagnosed in 2016 at Sun Yat-sen University Cancer Center: (a) significant differences in expression of PD-L1 (relative to total protein) in 20 patients, between normal (N), peritumor (P), and tumor (T) liver tissues, as measured by enzyme-linked immunosorbent assay (ELISA) (p = 0.015); (b) differences in 20 patients in PD-L1 expression (relative to total protein) between tumor and peritumor tissues, and between tumor and normal tissues. Relative PD-L1 expression higher in tumor than in peritumor tissue in 15 (75%) patients, and higher in tumor than normal tissue in 14 (70%) patients. (c) Correlation analysis results in 20 patients between serum-soluble PD-L1 (sPD-L1) concentration and PD-L1 expression (relative to total protein) in tumor tissue. Serum sPD-L1 concentration positively correlated with relative tumor PD-L1 expression (Spearman r = 0.527, p = 0.017), but there was no correlation between serum sPD-L1 concentration and relative peritumor or normal tissue PD-L1 expression. The relative expression of PD-L1 in tumor tissue was significantly higher in patients in high serum sPD-L1 group (concentration above median serum sPD-L1 concentration of 5.47 ng/ml) than in low serum sPD-L1 group (concentration below median) (p = 0.012)
Correlation analysis was performed on paired serum sPD-L1 concentrations and tumor PD-L1 expression in the same 20 patients. A moderate correlation (r = 0.527, p = 0.017) between serum sPD-L1 and tumor PD-L1 was observed. Patients were also classified into low and high serum sPD-L1 groups, based on having sPD-L1 levels below or above the median serum sPD-L1 concentration of 5.471 ng/ml. The expression of PD-L1 in tumor tissue was significantly higher in the patients who were in the high serum sPD-L1 group (p = 0.012) (Fig. 4c). However, the expression of PD-L1 in peritumor and normal tissues did not differ significantly between those in the low and high serum sPD-L1 groups.
Discussion
Hepatitis B virus infection is often involved in the development of HCC in Asia. During the “inflammation–fibrosis–cancer” process, immune tolerance has been observed, manifested by an increased expression of PD-1 in infiltrating lymphocytes and of PD-L1 in tumor cells. Because of this, the levels of expression of PD-1 and PD-L1 within tumors have been used to predict prognosis in some studies. More recently, a soluble form of PD-L1 has also been identified, similar to that identified in other members of the B7 family of immune-regulatory ligands. Soluble ligands can bind receptors in a manner similar to their membrane-bound counterparts, and as a result may play an important role in the regulation of receptor activity (Chen et al. 2011). The existence of sPD-L1 in human peripheral blood suggests that the PD-1/PD-L1 co-inhibitory signal in immune regulation may be more complex than originally thought. However, the actual role played by sPD-L1, particularly in HCC, and specifically in HBV-related HCC, is unclear.
In our study, significantly higher serum sPD-L1 concentrations were observed in patients with HBV-related HCC, compared to healthy controls. To our knowledge, this is the first report of high serum sPD-L1 levels in HBV-related HCC, and it is consistent with similar reports involving several other tumors as well as chronic hepatitis C (Rossille et al. 2014; Wang et al. 2015; Takahashi et al. 2016; Yamagiwa et al. 2017). In contrast, another group has reported a median serum sPD-L1 level of 0.5 ng/mL in patients with HCC, and this was significantly lower than the median level of 0.78 ng/mL that they found in healthy persons (Finkelmeier et al. 2016). Yet this appears to be the only report of serum sPD-L1 concentrations being lower in patients with malignancy than in healthy controls. One of the reasons for these contradictory results may be that their study population was comprised of patients with both primary and recurrent HCC, and some of them may have had their sPD-L1 levels measured after treatment. And it has been demonstrated by others that treatment results in a reduction in sPD-L1 levels in patients with multiple myeloma (Wang et al. 2016). We also found that higher serum sPD-L1 concentrations were observed in larger HCC (maximum tumor diameters ≥ 50 mm), which implied sPD-L1 was relevant to tumor load.
The prognostic value of pre-treatment serum sPD-L1 levels in HBV-related HCC is unknown. In this study, we determined the recurrence and survival rates of patients with HBV-related HCC in relation to their pre-treatment serum levels of sPD-L1. We found that higher pre-treatment sPD-L1 levels predicted both shorter DFS and shorter OS. As opposed to the prognostic role of serum sPD-L1, the prognostic role of PD-L1 expression in tissue has been studied extensively and in many types of tumors (Konishi et al. 2004; Hino et al. 2010; Shi et al. 2013; Yang et al. 2014). The dominant view in these reports has been that local overexpression of PD-L1 is associated with early tumor progression and death. Similar findings involving PD-L1 tumor tissue expression have also been observed in two independent studies specifically involving patients with HCC (Gao et al. 2009; Jung et al. 2017).
However, other researchers have come up with different results. One study found that higher PD-L1 levels expressed by HCC tumor cells were predictive of early recurrence, but that higher levels were not an independent predictor on multivariate analysis (Calderaro et al. 2016). Another study failed to find any correlation between tissue PD-L1 expression and prognosis in 80 patients with HCC (Umemoto et al. 2015). Furthermore, on an even more contradictory note, others have reported that higher PD-L1 expression was actually associated with longer DFS and OS in patients with HCC (Xie et al. 2016). In this and other studies, however, PD-L1 expression was measured primarily with immunohistochemical (IHC) techniques. The variability of the methods and primary antibodies used in IHC may impact the reliability of IHC-based PD-L1 expression results. In addition, the heterogeneity of PD-L1 expression in various tissues in the same patient may also contribute to inconsistent results, such that levels detected in one site may not accurately reflect the levels present at other sites. Given this reasoning, we hypothesize that ELISA-based serum sPD-L1 levels might represent a less invasive and more accurate prognostic measure than IHC-based tissue PD-L1 expression for HCC.
In our study, higher PD-L1 expression was detected in tumor tissue than in peritumor tissue in 75% of patients, and in tumor tissue than in normal liver tissue in 70% of patients. And few studies have reported on the quantitative measurement results of PD-L1 in matched tumor, peritumor, and liver tissue within the same patients. These results are consistent with previous observations that PD-L1 was highly expressed in tumor cells and in tumor-infiltrating lymphocytes (Topalian et al. 2015).
We also demonstrated that patients with higher serum sPD-L1 concentrations had higher tumor tissue PD-L1 expression. These findings are consistent with an in vivo study which revealed that pre-treatment serum sPD-L1 levels were positively correlated with the percentage of total malignant cells expressing PD-L1 (Wang et al. 2016). They are also consistent with studies showing that PD-L1 was detectable in tissue lysates and in serum (Simon et al. 2006); that sPD-L1 was detectable in supernatants from PD-L1-membrane-positive, but not PD-L1-membrane-negative, cell lines (Jung et al. 2017); and that immune cells, such as activated mature dendritic cells, both produced and released sPD-L1 (Frigola et al. 2012). Our findings suggest that PD-L1 expressed on the tumor cell surface may be a source of sPD-L1.
However, we observed only a moderate positive level of correlation between serum sPD-L1 levels and tumor PD-L1 expression. This could be attributable to bias caused by the limited sample size. Another potential reason is that PD-L1 is expressed not only in tumor tissue, but also in peripheral blood mononuclear cell-derived T cells and monocytes (Frigola et al. 2012). This weak correlation could be one of the explanations for the limited value of tumor tissue PD-L1 expression in predicting responses to anti-PD-1/PD-L1 therapies. On the other hand, high serum sPD-L1 levels have been shown to be predictive of treatment responses in first-line therapy for multiple myeloma (Wang et al. 2015). Looking forward, it may be worthwhile to investigate whether anti-PD-1/PD-L1 immunotherapies may be particularly beneficial for patients with HCC who have higher sPD-L1 levels.
Limitations
This study is limited by a relatively small study population, particularly for the portion involving the correlation analysis. It is suggested that studies with larger sample sizes can be performed to confirm our results. In particular, a study including tumor tissue samples from a larger cohort of patients with HBV-related HCC should be done to confirm the association between serum sPD-L1 levels and PD-L1 expression in tumor tissue.
Conclusions
In patients with HBV-related HCC, serum sPD-L1 concentrations were elevated and were positively correlated with tumor tissue PD-L1 expression. Furthermore, lower pre-treatment serum sPD-L1 levels were associated with more favorable DFS and OS in these patients. Based on our findings, serum sPD-L1 testing has the potential to play a role in HBV-related HCC disease assessment, systemic therapy choices, and survival prediction.
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- PD-1:
-
Programmed cell death-1
- PD-L1:
-
Programmed cell death-ligand 1
- sPD-L1:
-
Soluble PD-L1
- HBV:
-
Hepatitis B virus
- OS:
-
Overall survival
- DFS:
-
Disease-free survival
- mRECIST:
-
Modified response evaluation criteria in solid tumors
- ELISA:
-
Enzyme-linked immunosorbent assay
- EASL:
-
European Association for the Study of the Liver
- CEA:
-
Carcinoembryonic antigen
- AFP:
-
Alpha-fetoprotein
- TNM:
-
Tumor, node, metastases
- BCLC:
-
Barcelona Clinic Liver Cancer
- CLIP:
-
Cancer of the Liver Italian Program
References
Calderaro J, Rousseau B, Amaddeo G, Mercey M, Charpy C, Costentin C, Luciani A, Zafrani ES, Laurent A, Azoulay D, Lafdil F, Pawlotsky JM (2016) Programmed death ligand 1 expression in hepatocellular carcinoma: relationship with clinical and pathological features. Hepatology 64(6):2038–2046
Chen Y, Wang Q, Shi B, Xu P, Hu Z, Bai L, Zhang X (2011) Development of a sandwich ELISA for evaluating soluble PD-L1 (CD274) in human sera of different ages as well as supernatants of PD-L1 + cell lines. Cytokine 56(2):231–238
El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela CC, Lang L, Neely J, Tang H, Dastani HB, Melero I (2017) Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 389(10088):2492–2502
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136(5):E359–E386
Finkelmeier F, Canli Ö, Tal A, Pleli T, Trojan J, Schmidt M, Kronenberger B, Zeuzem S, Piiper A, Greten FR, Waidmann O (2016) High levels of the soluble programmed death-ligand (sPD-L1) identify hepatocellular carcinoma patients with a poor prognosis. Eur J Cancer 59(12):152–159
Frigola X, Inman BA, Krco CJ, Liu X, Harrington SM, Bulur PA, Dietz AB, Dong H, Kwon ED (2012) Soluble B7-H1: differences in production between dendritic cells and T cells. Immunol Lett 142(1–2):78–82
Galle PR, Forner A, Llovet JM, Mazzaferro V, Piscaglia F, Raoul JL, Schirmacher P, Vilgrain V (2018) EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 69(1):182–236
Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS, Xu Y, Fan J (2009) Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res 15(3):971–979
Hino R, Kabashima K, Kato Y, Yagi H, Nakamura M, Honjo T, Okazaki T, Tokura Y (2010) Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer 116(7):1757–1766
Jung HI, Jeong D, Ji S, Ahn TS, Bae SH, Chin S, Chung JC, Kim HC, Lee MS, Baek MJ (2017) Overexpression of PD-L1 and PD-L2 Is Associated with poor prognosis in patients with hepatocellular carcinoma. Cancer Res Treat 49(1):246–254
Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M (2004) B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res 10(15):5094–5100
Kudo M (2017) Immune checkpoint inhibition in hepatocellular carcinoma: basics and ongoing clinical trials. Oncology 92(Suppl 1(1):50–62
Nara S, Shimada K, Sakamoto Y, Esaki M, Kishi Y, Kosuge T, Ojima H (2012) Prognostic impact of marginal resection for patients with solitary hepatocellular carcinoma: evidence from 570 hepatectomies. Surgery 151(4):526–536
Rossille D, Gressier M, Damotte D, Maucort-Boulch D, Pangault C, Semana G, Le GS, Haioun C, Tarte K, Lamy T, Milpied N, Fest T (2014) High level of soluble programmed cell death ligand 1 in blood impacts overall survival in aggressive diffuse large B-Cell lymphoma: results from a French multicenter clinical trial. Leukemia 28(12):2367–2375
Shi SJ, Wang LJ, Wang GD, Guo ZY, Wei M, Meng YL, Yang AG, Wen WH (2013) B7-H1 expression is associated with poor prognosis in colorectal carcinoma and regulates the proliferation and invasion of HCT116 colorectal cancer cells. PLoS One 8(10):e76012
Simon I, Zhuo S, Corral L, Diamandis EP, Sarno MJ, Wolfert RL, Kim NW (2006) B7-h4 is a novel membrane-bound protein and a candidate serum and tissue biomarker for ovarian cancer. Cancer Res 66(3):1570–1575
Takahashi N, Iwasa S, Sasaki Y, Shoji H, Honma Y, Takashima A, Okita NT, Kato K, Hamaguchi T, Yamada Y (2016) Serum levels of soluble programmed cell death ligand 1 as a prognostic factor on the first-line treatment of metastatic or recurrent gastric cancer. J Cancer Res Clin Oncol 142(8):1727–1738
Topalian SL, Drake CG, Pardoll DM (2015) Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 27(4):450–461
Umemoto Y, Okano S, Matsumoto Y, Nakagawara H, Matono R, Yoshiya S, Yamashita Y, Yoshizumi T, Ikegami T, Soejima Y, Harada M, Aishima S, Oda Y, Shirabe K, Maehara Y (2015) Prognostic impact of programmed cell death 1 ligand 1 expression in human leukocyte antigen class I-positive hepatocellular carcinoma after curative hepatectomy. J Gastroenterol 50(1):65–75
Wang L, Wang H, Chen H, Wang WD, Chen XQ, Geng QR, Xia ZJ, Lu Y (2015) Serum levels of soluble programmed death ligand 1 predict treatment response and progression free survival in multiple myeloma. Oncotarget 6(38):41228–41236
Wang H, Wang L, Liu WJ, Xia ZJ, Huang HQ, Jiang WQ, Li ZM, Lu Y (2016) High post-treatment serum levels of soluble programmed cell death ligand 1 predict early relapse and poor prognosis in extranodal NK/T cell lymphoma patients. Oncotarget 7(22):33035–33045
Xie QK, Zhao YJ, Pan T, Lyu N, Mu LW, Li SL, Shi MD, Zhang ZF, Zhou PH, Zhao M (2016) Programmed death ligand 1 as an indicator of pre-existing adaptive immune responses in human hepatocellular carcinoma. Oncoimmunology 5(7):e1181252
Yamagiwa S, Ishikawa T, Waguri N, Sugitani S, Kamimura K, Tsuchiya A, Takamura M, Kawai H, Terai S (2017) Increase of soluble programmed cell death ligand 1 in patients with chronic hepatitis C. Int J Med Sci 14(5):403–411
Yang CY, Lin MW, Chang YL, Wu CT, Yang PC (2014) Programmed cell death-ligand 1 expression in surgically resected stage I pulmonary adenocarcinoma and its correlation with driver mutations and clinical outcomes. Eur J Cancer 50(7):1361–1369
Zhou J, Sun HC, Wang Z, Cong WM, Wang JH, Zeng MS, Yang JM, Bie P, Liu LX, Wen TF (2018) Guidelines for diagnosis and treatment of primary liver cancer in China (2017 Edition). Liver Cancer 7(7):235–260
Funding
The design of the study was supported by the National Natural Science Foundation of China under Grant [no. 81771955]; the Guangzhou Science and Technology Program (key projects of collaborative innovation of health medicine) under Grant [no. 201704020228]; and the Guangzhou Science and Technology Program (key projects of collaborative innovation of production, learning and research) under Grant [no. 201704020134]; Sun Yat-sen University Clinical Trial 5010 Project under Grant [no. 2016002].
Author information
Authors and Affiliations
Contributions
JH, QC, and YG contributed to the conception and design of the study. XH, MZ and JH drafted and reviewed the manuscript. XH, SL, HC and HD participated in data collection and performed the experiments. Min-shan Chen provided the patients’ specimen. YG and XH contributed to the data analysis and interpretation. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the Institutional Review Committee of Sun Yat-sen University Cancer Centre in Guangzhou, China (YB2015-018-01). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent for procedures was obtained from patients and families. All procedures were performed after obtaining informed consent in accordance with the approved protocol.
Rights and permissions
About this article
Cite this article
Han, X., Gu, Yk., Li, Sl. et al. Pre-treatment serum levels of soluble programmed cell death-ligand 1 predict prognosis in patients with hepatitis B-related hepatocellular carcinoma. J Cancer Res Clin Oncol 145, 303–312 (2019). https://doi.org/10.1007/s00432-018-2758-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-018-2758-6