Abstract
Objectives
While the predictive value of programmed cell death ligand-1 (PD-L1) protein expression for immune checkpoint inhibitor therapy of lung cancer has been extensively studied, the impact of standard platinum-based chemotherapy on PD-L1 or programmed cell death-1 (PD-1) expression is unknown. The aim of this study was to determine the changes in PD-L1 expression of tumor cells (TC) and immune cells (IC), in PD-1 expression of IC, and in the amount of stromal mononuclear cell infiltration after platinum-based chemotherapy in patients with lung cancer.
Materials and methods
We determined the amount of stromal mononuclear cells and PD-L1/PD-1 expressions by immunohistochemistry in bronchoscopic biopsy samples including 20 adenocarcinomas (ADC), 15 squamous cell carcinomas (SCC), 2 other types of non-small cell lung cancer, and 4 small cell lung cancers together with their corresponding surgical resection tissues after platinum-based chemotherapy.
Results
PD-L1 expression of TC decreased in ten patients (24.4%) and increased in three patients (7.32%) after neoadjuvant chemotherapy (p = 0.051). The decrease in PD-L1 expression, however, was significant only in patients who received cisplatin–gemcitabine combination (p = 0.020), while in the carboplatin–paclitaxel group, no similar tendency could be observed (p = 0.432). There was no difference between ADC and SCC groups. Neither PD-1 expression nor the amount of stromal IC infiltration showed significant changes after chemotherapy.
Conclusions
This is the first study, in which both PD-L1 and PD-1 expression were analyzed together with the amount of stromal IC infiltration in different histological subtypes of lung cancer before and after platinum-based chemotherapy. Our results confirm that chemotherapy decreases PD-L1 expression of TC in a subset of patients, therefore, rebiopsy and re-evaluation of PD-L1 expression may be necessary for the indication of immune checkpoint inhibitor therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
First-line platinum-based chemotherapy is the most commonly used therapeutic modality in the treatment of lung cancer. Worldwide, there are 1.8 million new lung cancer cases per year, of which about 80% are diagnosed at a late stage when surgery is not possible to perform, therefore, this drug combination is applied in more than 1.4 million new lung cancer patients yearly (Cheng et al. 2016). Platinum-based chemotherapy is also used in adjuvant and neoadjuvant settings.
In the clinical practice, the therapeutic effect of various chemotherapeutic agents is mainly determined by the extent of tumor shrinkage visualized by chest X-ray or CT scan, however, their effects on the expression of tissue biomarkers within the tumor are far less known. It is possible to study such effects of platinum-based chemotherapy in the setting of neoadjuvant treatment in lung cancer, when both chemotherapy-naive preoperative tumor tissue biopsies and surgically resected tumors after chemotherapy are available.
Immune checkpoint inhibitor therapy is a revolutionary new treatment option for lung cancer patients, which is still mainly used today as second- or third-line treatment after the failure of platinum-based chemotherapy. Unlike in the case of molecularly targeted therapies, robust validated patient selection criteria have not yet been established, although some studies indicate the predictive value of programmed cell death ligand-1 (PD-L1) immunopositivity of tumor cells, especially in lung adenocarcinoma (ADC) (Passiglia et al. 2016).
It has already been shown that chemotherapy can alter the expression of PD-L1 and PD-1 (programmed cell death-1) in cell lines but the direction of change depended on the cell line and chemotherapeutic agent investigated (Chacon et al. 2016; Ghebeh et al. 2010; Peng et al. 2015; Zhang et al. 2008). Recently, Sheng et al. investigated the effect of chemotherapy in the clinical setting comparing 32 pre- and post-treatment NSCLC tumor pairs. They found downregulation of PD-L1 expression of tumor cells (TC) and no significant change in PD-L1 expression of immune cells (IC) after chemotherapy. They, however, did not study the effect of platinum-based chemotherapy on PD-1 expression of IC or on the amount of stromal IC (Sheng et al. 2016).
The aim of our present work was to extend these observations and study the amount of tumor-infiltrating IC and the expression of PD-L1 and PD-1 in lung cancer patients before and after platinum-containing chemotherapy using immunohistochemistry (IHC) on diagnostic bronchoscopic biopsy materials compared to the corresponding surgical tumor tissue samples.
Materials and methods
Formalin-fixed paraffin-embedded paired lung cancer tissue samples obtained before and after platinum-based neoadjuvant chemotherapy of 41 patients were studied from the archive of the National Korányi Institute of Pulmonology, Budapest, Hungary, and the 1st Department of Pathology and Experimental Cancer Research, Semmelweis University, Budapest, Hungary. Permissions to use the archived tissue have been obtained from the Regional Ethics Committees (Scientific and Research Ethics Committee of the Medical Research Council, ETT-TUKEB Nos: 510/2013, 86/2015, and Semmelweis University Regional and Institutional Committee of Science and Research Ethics, No 241/2016). Tumors were classified according to the IASLC/ATS/ERS classification (Travis et al. 2011). According to the tumor histology 20 ADC, 15 squamous cell carcinomas (SCC), 1 mucoepidermoid carcinoma (MEC), 1 adenosquamous carcinoma (ADSQ), and 4 small cell lung cancers (SCLC) were involved in this study. All patients received 1–4 cycles of neoadjuvant chemotherapy before the surgical resection. We grouped the applied chemotherapies as follows: (i) cisplatin–gemcitabine (ii) carboplatin–paclitaxel and (iii) other combinations.
We compared the paired samples of each patient from several aspects, including the amount of stromal mononuclear infiltration, PD-L1 expression of TC and IC, as well as PD-1 expression of IC. In an attempt to investigate even more homogenous cohorts, patients were divided into subgroups based on several clinical characteristics, including gender, tumor histology (ADC and SCC), postoperative disease stage and composition of neoadjuvant chemotherapy.
The amount of stromal mononuclear IC, including lymphocytes, histiocytes and plasma cells was determined on hematoxylin- and eosin-stained sections by two independent pathologists, and was recorded by a semi-quantitative method as follows: (i) < 20% or (ii) ≥ 20% of the tumor stroma contained IC (Salgado et al. 2015; Teglasi et al. 2017).
IHC for PD-L1 (SP142, Spring Bioscience; dilution 1:100) and PD-1 (ab52587, Abcam; dilution 1:100) was performed on 3-µm-thick sections of bronchoscopic tissue biopsy and of tissue microarray blocks from surgical resection material. Each case was represented by 3 cores. The amount of positive TC and IC were determined by a semi-quantitative method as percentage of positive cells. For TC 1, 5, 10 and 50%, while for IC 1, 5 and 10% cut-off levels were recorded, which are the most commonly used thresholds (Teglasi et al. 2017; Festino et al. 2016; Yang et al. 1990). We used a second scoring system for the evaluation of PD-L1 expression of TC: less than 1, 1–5, 6–10, 11–20, 21–30, 31–40, 41–50, 51–60, 61–70, 71–80, 81–90 and 91–100%, to explore significant changes within ranges of higher cut-off values.
Statistical analysis
As our main objective was to determine the direction of changes caused by chemotherapy in different parameters regardless of their absolute value, cases with any increment in the investigated variable were represented with the value of + 1, decrements with the value of − 1 and the cases with no observed change with the value of 0. As all analyzed parameters were measured on a semi-quantitative scale, only changes large enough to result in a switch between different categories were considered.
The mean value of the changes was tested with a T test for the null hypothesis that chemotherapy does not affect the investigated variables. Correlation between the directions of changes was determined by calculating the Spearman R-value for the appropriate datasets.
Throughout the analysis, the significance level was set to α = 0.05. Given the relatively low number of cases, Bonferroni corrections were omitted from the pipeline, thus accordingly all presented results should be considered more as strong tendencies rather than well-established facts.
All statistical analysis was performed using the computing environment Python with the open-source software package of SciPy.
Results
Patients’ characteristics
Clinicopathological data including tumor histology, smoking status, chemotherapeutic regimen, response to treatment and postoperative disease stage are summarized in Table 1.
The effect of neoadjuvant chemotherapy on PD-L1 expression of TC
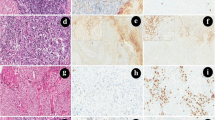
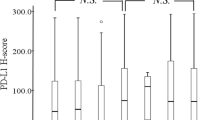
In the whole cohort, nine patients (22%) showed reduction, three patients (7.3%) showed increase, and 29 patients (70.7%) showed no change in the PD-L1 expression of TC after neoadjuvant chemotherapy (p = 0.083) (Fig. 1). Using the more detailed scoring system, 10 patients (24.4%) showed reduced PD-L1 expression, 3 patients (7.3%) showed increase, and 28 patients (68.3%) showed no change in the PD-L1 expression of TC (p = 0.051) (Fig. 1). The most striking decrease was observed in an ADC patient, in whom 70% of TC showed PD-L1 positivity in the bronchoscopic sample, whereas it decreased to < 1% after three cycles of carboplatin–paclitaxel chemotherapy (Fig. 2). The decrease in PD-L1 expression of TC was significant in those 16 patients who received cisplatin–gemcitabine combination (p = 0.02 both with the classical and in the more detailed scoring systems), meanwhile in the carboplatin–paclitaxel group, no such tendency could be observed for any scoring system (p = 0.669 and p = 0.432, respectively). There were no significant changes in PD-L1 expression of TC in either the ADC or the SCC subgroups (p = 0.428 and p = 0.189, respectively). Similarly, there were no changes in either male or female patients (p = 0.328 and p = 0.163, respectively). No significant results were observed in the subgroups based on other clinicopathological parameters (data not shown).
Comparison of the changes of the parameters before and after platinum-based chemotherapy. Color codes show the direction of changes in the studied parameters including PD-L1 expression of TC and IC, PD-1 expression of IC and the amount of stromal IC after platinum-based chemotherapy. The first column shows the changes in the PD-L1 expression of TC determined by the first scoring system (1, 5, 10, 50%), meanwhile the second column shows the changes determined by the more detailed scoring system (less than 1, 1–5, 6–10, 11–20, 21–30, 31–40, 41–50, 51–60, 61–70, 71–80, 81–90 and 91–100%). In the second column, we also indicated the percentage of PD-L1-positive TC before and after chemotherapy in those cases where we observed a decrease
The effect of neoadjuvant chemotherapy on PD-L1 expression of IC
In the whole cohort, 10 patients (24.4%) showed increase, 8 patients (19.5%) showed decrease and 23 patients (56.1%) showed no change in the PD-L1 expression of IC after neoadjuvant chemotherapy (Fig. 1). Based on these results, no significant impact of neoadjuvant chemotherapy could be established on PD-L1 expression of IC (p = 0.643). The direction of changes in the PD-L1 expression of IC remained insignificant in the group of patients with ADC (p = 0.541) or SCC (p = 0.104). Similarly, the direction of changes in the PD-L1 expression of IC remained insignificant in the group of patients treated with cisplatin–gemcitabine (p = 0.432) or carboplatin–paclitaxel (p = 0.751). No significant results were observed in the subgroups based on other clinicopathological parameters (data not shown).
The effect of neoadjuvant chemotherapy on PD-1 expression of IC
In the whole cohort, 12 patients (29.3%) showed increase, 16 patients (39%) showed decrease and 13 patients (31.7%) showed no change in the PD-1 expression of IC after neoadjuvant chemotherapy (Fig. 1). Based solely on these results, neoadjuvant chemotherapy seemed to have no significant impact on PD-1 expression of IC (p = 0.456). The direction of changes in the PD-1 expression of IC remained insignificant in the group of patients with ADC (p = 0.577) or SCC (p = 1.0). Similarly, the direction of changes in the PD-L1 expression of IC remained insignificant in the group of patients treated with cisplatin–gemcitabine (p = 0.383) or carboplatin–paclitaxel (p = 0.136). No significant results were observed in the subgroups based on other clinicopathological parameters (data not shown).
The effect of neoadjuvant chemotherapy on the amount of stromal IC
In the whole cohort, 38 tumor samples were eligible for evaluation of stromal IC. Four patients (10.5%) showed increase, 6 patients (15.8%) showed decrease and 28 patients (73.7%) showed no change in the amount of stromal IC after neoadjuvant chemotherapy (Fig. 1). These results suggest no significant impact of neoadjuvant chemotherapy on the amount of stromal IC (p = 0.534). The direction of changes remained insignificant in the group of patients with ADC (p = 0.331) or SCC (p = 0.671). Similarly, the direction of changes in the PD-L1 expression of IC remained insignificant in the group of patients treated with cisplatin–gemcitabine (p = 0.67) or carboplatin–paclitaxel (p = 1.0). No significant results were observed in the subgroups based on other clinicopathological parameters (data not shown).
Correlation of the changes among the different histological parameters
Regarding the direction of changes after neoadjuvant chemotherapy, there was a positive correlation between both PD-1 and PD-L1 expressions of IC and the amount of stromal IC (p = 0.002, Spearman R = 0.491 and p = 0.004, Spearman R = 0.454 respectively), and consequently between PD-1 and PD-L1 expressions of IC (p = 0.035, Spearman R = 0.331) (Fig. 1). This means that an increase in the amount of stromal IC is likely to be accompanied with an increase in both PD-1 and PD-L1 expressions of IC.
Discussion
In this study, we presented a comprehensive analysis of the changes in the amount of stromal IC, in the PD-L1 expression of TC and IC, and PD-1 expression of IC after platinum-based neoadjuvant chemotherapy in a cohort of lung cancer patients. We observed decrease in PD-L1 expression of TC in 10 cases out of 41, and increase only in 3 tumor pairs. This is somewhat in line with the results of Sheng et al., who examined 32 NSCLC tumor pairs including 26 ADC cases. Similarly to our result, they observed downregulation of PD-L1 expression of TC and no significant change in PD-L1 expression of IC after chemotherapy. They, however, did not study PD-1 expression of IC or the amount of stromal IC as described in the present work (Sheng et al. 2016). Recently, Zhang et al., who also focused only on the PD-L1 expression of TC, also observed generally decreased protein expression after neoadjuvant treatment in their cohort of 30 NSCLC pairs, but similarly to our results, they also described cases with increased PD-L1 protein expression (Zhang et al. 2016). They examined the correlation of PD-L1 immunopositivity and chemotherapy resistance, and stated that high PD-L1 expression of TC after neoadjuvant chemotherapy could be an indication of therapeutic resistance and poor prognosis in NSCLC. Their results were confirmed by in vivo experiments. Based on this one might speculate that in case of chemosensitive NSCLC, chemotherapy selectively kills PD-L1-positive TC resulting in a decrease in PD-L1 expression. In our cohort, out of nine patients with decreased PD-L1 expression and available therapeutic response data, eight patients showed partial regression after neoadjuvant chemotherapy, however, all three patients with increased PD-L1 expression after chemotherapy also showed partial regression.
A comparison between bronchoscopic excision and surgical resection tumor samples is always challenging. Meert et al. studied the reliability of diagnostic biopsies as compared to the corresponding surgically resected tumors regarding the expression of different biomarkers (Meert et al. 2004). When evaluated immunohistochemically, the expression of p53, EGFR, c-erbB-2 and Ki-67 in 28 lung cancer patients, they found concordant results in 85%, concluding that biopsies may provide reliable information.
Another significant challenge in lung cancer biomarker research is tumor heterogeneity. In a recent study of Munari et al., reliability of PD-L1 expression in small biopsies, such as core biopsy of lung cancer, was studied (Munari et al. 2017). They observed a discordance rate of 20 and 7.9% for ≥ 1 and ≥ 50% cutoffs, respectively. They suggest that caution must be taken when evaluating single biopsies from patients with advanced NSCLC eligible for immunotherapy, and concluded that at least four biopsy samples are necessary to minimize the risk of tumor misclassification. In line with this, considering that our results, like most other observations available in the literature, are based on single-sample evaluations, extreme care should be taken when interpreting their biological and clinical significance. A more comprehensive study of biopsies consistently obtained from multiple sites of the tumor both before and after chemotherapy would be needed to establish the observed tendencies with a larger statistical power. Albeit, the sizes of the preoperative bronchoscopic biopsy materials or transthoracic core biopsy samples make it difficult to carry out such an examination.
Immunotherapy is a new hope for lung cancer patients, however, criteria of patient selection are not clarified yet. PD-L1 expression of TC seems to be the most promising in predicting response to pembrolizumab treatment, especially when 50% cut-off value is used (Reck et al. 2016). Besides, combination of PD-L1 expression of TC and IC as predictive marker is also intensively studied (Spira et al. 2015). Immunotherapy is often used in second- or third-line setting after platinum-based chemotherapy or at tumor recurrence after surgical resection combined with neoadjuvant or adjuvant treatment.
Immunotherapy appears to give a new hope also in the management of small cell lung cancer, therefore, the investigation of predictive markers in this high-grade malignancy is also very important (Li et al. 2016). In our cohort, all four SCLC patients had low PD-L1 expression of TC (< 1%) before neoadjuvant chemotherapy, which remained unchanged in the surgical specimen.
We observed differences in changes of PD-L1 expression of TC regarding chemotherapy components, as we demonstrated significant decrease when cisplatin–gemcitabine combination was applied and observed no similar tendency for carboplatin–paclitaxel-treated cases. In cisplatin–gemcitabine-treated patients, we found no tumor pairs with increase in PD-L1 expression of TC after chemotherapy, whereas 3/16 cases with carboplatin–paclitaxel chemotherapy showed increase in PD-L1 expression of TC. This latter observation might be in line with the results of Peng et al., who studied ovarian cancer cell lines and mouse model of ovarian cancer and demonstrated upregulation of PD-L1 expression when carboplatin–paclitaxel treatment was applied (Peng et al. 2015). Interestingly enough, upregulation of PD-L1 expression was also observed when gemcitabine treatment was used. Similarly, both protein expression enhancement and mRNA expression enhancement were observed in human pancreatic cell lines when treated with gemcitabine or paclitaxel (Doi et al. 2017).
According to our result, PD-L1 expression decreases after platinum-based chemotherapy in about a quarter of lung cancer patients, and according to the present patient selection criteria for immunotherapy, this decrease risks the presence of this important tissue biomarker in every seventh patient resulting in the abandonment of a promising therapy (Kerr and Nicolson 2016).
We observed no significant trends in the general directionality of changes in PD-L1 and PD-1 expression of IC and the amount of stromal IC after neoadjuvant chemotherapy. Interestingly, however, the changes of these parameters in certain tumor pairs were parallel (Fig. 1). These results somewhat agree with our recent observation, when we compared brain metastases from lung ADC with and without chemotherapy and found no correlation between PD-1 expression of IC and the amount of stromal IC and the application of chemotherapy (Teglasi et al. 2017).
In summary, this is the first study, in which both PD-L1 and PD-1 expression were analyzed together with the amount of stromal IC infiltration in lung cancer cases before and after platinum-based chemotherapy. Our results bring us a step closer to confirming that chemotherapy decreases PD-L1 expression of TC in a certain proportion of patients. As this may reduce the applicability of immunotherapy, rebiopsy and re-evaluation of PD-L1 expression is proposed before immunotherapy. Based on this observation, first-line immunotherapy may also be considered.
Notwithstanding, our results might suffer from the limitations of having a relatively small number of cases. Undoubtedly, a significantly larger cohort would give more reliable insights into the changes of the described biomarkers due to different types of chemotherapies. Given, however, that such groups of samples analyzed with a consistent and controlled pipeline are extremely hard to establish, our results still represent a huge step forward to the understanding of application criteria of different immunotherapies.
Abbreviations
- ADC:
-
Adenocarcinoma
- ADSQ:
-
Adenosquamous carcinoma
- IC:
-
Immune cells
- IHC:
-
Immunohistochemistry
- MEC:
-
Mucoepidermoid carcinoma
- NSCLC:
-
Non-small cell lung cancer
- PD-L1:
-
Programmed cell death ligand-1
- PD-1:
-
Programmed cell death-1
- SCC:
-
Squamous cell carcinoma
- SCLC:
-
Small cell lung cancer
- TC:
-
Tumor cells
References
Chacon JA, Schutsky K, Powell DJ (2016) The impact of chemotherapy, radiation and epigenetic modifiers in cancer cell expression of immune inhibitory and stimulatory molecules and anti-tumor efficacy. Vaccines 4(4): 43. https://doi.org/10.3390/vaccines4040043
Cheng TY, Cramb SM, Baade PD, Youlden DR, Nwogu C, Reid ME,, and Characteristics T (2016) The international epidemiology of lung cancer: latest trends, disparities. J Thorac Oncol 11(10):1653–1671. https://doi.org/10.1016/j.jtho.2016.05.021
Doi T, Ishikawa T, Okayama T, Oka K, Mizushima K, Yasuda T, Sakamoto N, Katada K, Kamada K, Uchiyama K, Handa O, Takagi T, Naito Y, Itoh Y (2017) The JAK/STAT pathway is involved in the upregulation of PD-L1 expression in pancreatic cancer cell lines. Oncol Rep 37(3):1545–1554. https://doi.org/10.3892/or.2017.5399
Festino L, Botti G, Lorigan P, Masucci GV, Hipp JD, Horak CE, Melero I, Ascierto PA, Cancer treatment with anti-PD-1/PD-L1 agents: is PD-L1 expression a biomarker for patient selection? Drugs 76(9) (2016) 925–45. https://doi.org/10.1007/s40265-016-0588-x
Ghebeh H, Lehe C, Barhoush E, Al-Romaih K, Tulbah A, Al-Alwan M, Hendrayani SF, Manogaran P, Alaiya A, Al-Tweigeri T, Aboussekhra A, Dermime S (2010) Doxorubicin downregulates cell surface B7-H1 expression and upregulates its nuclear expression in breast cancer cells: role of B7-H1 as an anti-apoptotic molecule. Breast Cancer Res BCR 12(4):R48. https://doi.org/10.1186/bcr2605
Kerr KM, Nicolson MC, Non-small cell lung cancer, PD-L1, and the pathologist. Arch Pathol Lab Med 140(3) (2016) 249–54. https://doi.org/10.5858/arpa.2015-0303-SA
Li Q, Yuan D, Ma C, Liu Y, Ma L, Lv T, Song Y, A new hope: the immunotherapy in small cell lung cancer. Neoplasma 63(3) (2016) 342–350. https://doi.org/10.4149/302_151001n511
Meert AP, Martin B, Verdebout JM, Paesmans M, Berghmans T, Ninane V, Sculier JP (2004) Correlation of different markers (p53, EGF-R, c-erbB-2, Ki-67) expression in the diagnostic biopsies and the corresponding resected tumors in non-small cell lung cancer. Lung Cancer (Amsterdam Netherlands) 44(3):295–301. https://doi.org/10.1016/j.lungcan.2003.12.009
Munari E, Zamboni G, Marconi M, Sommaggio M, Brunelli M, Martignoni G, Netto GJ, Moretta F, Mingari MC, Salgarello M, Terzi A, Picece V, Pomari C, Lunardi G, Cavazza A, Rossi G, Moretta L, Bogina G (2017) PD-L1 expression heterogeneity in non-small cell lung cancer: evaluation of small biopsies reliability. Oncotarget 8(52):90123–90131. https://doi.org/10.18632/oncotarget.21485
Passiglia F, Bronte G, Bazan V, Natoli C, Rizzo S, Galvano A, Listi A, Cicero G, Rolfo C, Santini D, Russo A (2016) PD-L1 expression as predictive biomarker in patients with NSCLC: a pooled analysis. Oncotarget 7(15):19738-47. https://doi.org/10.18632/oncotarget.7582
Peng J, Hamanishi J, Matsumura N, Abiko K, Murat K, Baba T, Yamaguchi K, Horikawa N, Hosoe Y, Murphy SK, Konishi I, Mandai M (2015) Chemotherapy induces programmed cell death-ligand 1 overexpression via the nuclear factor-kappaB to foster an immunosuppressive tumor microenvironment in ovarian cancer. Cancer Res 75(23):5034-5045. https://doi.org/10.1158/0008-5472.can-14-3098
Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. New Engl J Med 375(19):1823–1833. https://doi.org/10.1056/NEJMoa1606774
Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL, Penault-Llorca F, Perez EA, Thompson EA, Symmans WF, Richardson AL, Brock J, Criscitiello C, Bailey H, Ignatiadis M, Floris G, Sparano J, Kos Z, Nielsen T, Rimm DL, Allison KH, Reis-Filho JS, Loibl S, Sotiriou C, Viale G, Badve S, Adams S, Willard-Gallo K, Loi S, The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 26(2) (2015) 259–271. https://doi.org/10.1093/annonc/mdu450
Sheng J, Fang W, Yu J, Chen N, Zhan J, Ma Y, Yang Y, Huang Y, Zhao H, Zhang L (2016) Expression of programmed death ligand-1 on tumor cells varies pre and post chemotherapy in non-small cell lung cancer. Sci Rep 6:20090. https://doi.org/10.1038/srep20090
Spira AI, Park K, Mazières J, Vansteenkiste JF, Rittmeyer A, Ballinger M, Waterkamp D, Kowanetz M, Mokatrin A, Fehrenbacher L (2015) Efficacy, safety and predictive biomarker results from a randomized phase II study comparing MPDL3280A vs docetaxel in 2L/3L NSCLC (POPLAR). J Clin Oncol 33(15_suppl): 8010–8010. https://doi.org/10.1200/jco.2015.33.15_suppl.8010
Teglasi V, Reiniger L, Fabian K, Pipek O, Csala I, Bago AG, Varallyai P, Vizkeleti L, Rojko L, Timar J, Dome B, Szallasi Z, Swanton C, Moldvay J (2017) Evaluating the significance of density, localization, and PD-1/PD-L1 immunopositivity of mononuclear cells in the clinical course of lung adenocarcinoma patients with brain metastasis. Neuro Oncol. https://doi.org/10.1093/neuonc/now309
Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ, Van Schil PE, Garg K, Austin JH, Asamura H, Rusch VW, Hirsch FR, Scagliotti G, Mitsudomi T, Huber RM, Ishikawa Y, Jett J, Sanchez-Cespedes M, Sculier JP, Takahashi T, Tsuboi M, Vansteenkiste J, Wistuba I, Yang PC, Aberle D, Brambilla C, Flieder D, Franklin W, Gazdar A, Gould M, Hasleton P, Henderson D, Johnson B, Johnson D, Kerr K, Kuriyama K, Lee JS, Miller VA, Petersen I, Roggli V, Rosell R, Saijo N, Thunnissen E, Tsao M, Yankelewitz D (2011) International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 6(2) 244–285. https://doi.org/10.1097/JTO.0b013e318206a221
Yang CY, Lin MW, Chang YL, Wu CT, Yang PC (1990) Programmed cell death-ligand 1 expression is associated with a favourable immune microenvironment and better overall survival in stage I pulmonary squamous cell carcinoma. Eur J Cancer (Oxford England) 57:(2016) 91–103. https://doi.org/10.1016/j.ejca.2015.12.033
Zhang P, Su DM, Liang M, Fu J (2008) Chemopreventive agents induce programmed death-1-ligand 1 (PD-L1) surface expression in breast cancer cells and promote PD-L1-mediated T cell apoptosis. Mol Immunol 45(5):1470-6. https://doi.org/10.1016/j.molimm.2007.08.013
Zhang P, Ma Y, Lv C, Huang M, Li M, Dong B, Liu X, An G, Zhang W, Zhang J, Zhang L, Zhang S, Yang Y (2016) Upregulation of programmed cell death ligand 1 promotes resistance response in non-small-cell lung cancer patients treated with neo-adjuvant chemotherapy. Cancer Sci 107(11):1563–1571. https://doi.org/10.1111/cas.13072
Acknowledgements
We thank Zsuzsanna Kaminszky, Anna Tamási and Mónika Szilágyiné Paulusz for their excellent technical assistance, and Ildikó Krencz for constructing the tissue microarray (TMA) blocks.
Funding
This work was supported by the Research and Technology Innovation Fund (KTIA_NAP_13-2014-0021 to L.R., Z.S., J.M.); Hungarian Science Foundation (OTKA-PD115792 to L.R., OTKA-K116151 to L.R., J.T., B.D., OTKA-K112371 to J.T.); Breast Cancer Research Foundation and the Novo Nordisk Foundation Interdisciplinary Synergy Programme Grant (NNF15OC0016584 to Z.S.). For the remaining authors none were declared.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Permissions to use the archived tissue have been obtained from the Regional Ethical Committee (Nos: 510/2013, 86/2015, 241/2016).
Rights and permissions
About this article
Cite this article
Rojkó, L., Reiniger, L., Téglási, V. et al. Chemotherapy treatment is associated with altered PD-L1 expression in lung cancer patients. J Cancer Res Clin Oncol 144, 1219–1226 (2018). https://doi.org/10.1007/s00432-018-2642-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-018-2642-4