Abstract
Background
Our goal was to compare the survival advantage of tamoxifen (TAM) and aromatase inhibitor (AI) in female (FBC) and male breast cancer (MBC).
Patients and methods
We performed a retrospective study of 2785 FBC and 257 MBC patients treated with hormonal therapy.
Results
The median follow-up was 106 months (range 3–151 months) and 42 months (range 2–115 months) for FBC and MBC, respectively. The patients were divided into two groups according to the hormonal therapy used: TAM-treated and AI-treated. MBC was characterized by older age, advanced tumor stage, and higher rate of lymph node metastases, in comparison with FBC. Matching analysis was performed using six prognostic criteria: patient age, tumor stage, tumor grade, lymph node status, human epidermal growth factor receptor (HER2) status, and administration of chemotherapy. The female and male patients were matched 2:1. In this analysis, 316 women and 158 men treated with TAM, and 60 women and 30 men treated with AI, were included. The overall survival (OS) was estimated by the Kaplan–Meier method and was compared between FBC and MBC. TAM-treated FBC and MBC patients had similar 5-year OS, 85.1 and 89.2%, respectively (p = 0.972). Notably, FBC patients treated with AI had significantly greater 5-year OS (85.0%) in comparison with AI-treated MBC patients (5-year OS of 73.3%; p = 0.028).
Conclusions
The OS of TAM-treated patients with MBC was similar to the OS of TAM-treated FBC patients, whereas AI treatment is associated with poorer survival of MBC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Male breast cancer (MBC) is an uncommon disease and its rarity makes the performance of prospective randomised trials very difficult. As a result, the treatment concepts are based on limited retrospective studies and clinical management of the female breast cancer (FBC) (Losurdo et al. 2017). MBC appears to be hormone receptor positive in 80–90% of patients and endocrine therapy remains one valid treatment strategy. In the current literature, there is a lack of prospective randomised studies investigating the use of tamoxifen (TAM) and aromatase inhibitor (AI) in MBC. In a recent retrospective study of 257 MBC patients, we showed that adjuvant treatment with TAM was associated with a 1.4-fold decreased risk of cancer mortality compared to AI treatment (Eggemann et al. 2013). These data suggest that, similar to FBC, TAM treatment for 5 years should be recommended in the adjuvant setting for MBC. Based on the different physiologies of males and females, caution should be exercised when simply extrapolating data relative to management of FBC and applying this to the case of MBC. Unfortunately, there is a lack of data comparing the TAM and AI treatment between MBC and FBC.
In this large retrospective cohort study, we aimed to compare the benefit of TAM and AI treatment on overall survival (OS) among male and female patients with hormone-receptor (HR)-positive breast cancer. For this purpose, we used matching analysis.
Materials and methods
We investigated cases of FBC and MBC included in the local national cancer registry of Germany. This tumor register contains information about diagnosis, age at diagnosis, date of diagnosis, tumor histology, adjuvant therapy, and date of death (Eggemann et al. 2015). We analysed 5543 women and 743 men with primary breast cancer diagnosed between 2000 and 2009. We included only patients with non-metastatic invasive hormone-receptor breast cancer who were treated with tamoxifen (TAM) or aromatase inhibitor (AI) as adjuvant hormonal therapy. All patients were required to be hormonally treated for 5 years. Treatment less than 5 years was criteria for exclusion. A total of 257 male and 2785 female eligible ceases were identified and analysed. This study was approved by the Research and Ethical Committee of Otto-von-Guericke University, Magdeburg, Germany. Patients gave written informed consent for data transfer to the tumor registry before treatment. Additional individual consent for this analysis was not needed. The manuscript was prepared in accordance with the STROBE statement criteria (von Elm et al. 2007).
To avoid further selection bias, a matching analysis was performed. The matching process was based on six prognostic criteria: patient age, tumor stage, tumor grade, lymph node status, HER2 status, and administration of chemotherapy. Age as a criterion for matching was divided into four groups: under 40, 50, 51–60 years, and over 61 years. The matching procedure was conducted at random and without any information about patient outcome. FBC and MBC patients were matched 2:1. The primary outcome measure was OS, which was defined as the time from the date of diagnosis and the time of death from any cause. OS was used as the primary outcome, because information about a patient’s death and its cause is automatically recorded in the cancer registry via the civil registry office (Eggemann et al. 2015), leading to minimal loss of follow-up regarding overall survival, and thus keeping transfer bias to a minimum.
Statistical analysis
The statistical analyses were performed using SPSS Version 22.0 (SPSS, Chicago, IL, USA). Associations between tumor and treatment characteristics were analysed by cross tabulation and tested using the 2 test or Fisher’s exact test. All tests were two sided and determined statistically significant if the p value was ≤ 0.05. The OS probability distribution was examined using the Kaplan–Meier method. The equality of survival curves was tested using the log-rank test.
Results
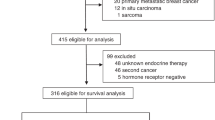
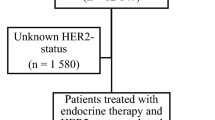
Between January 2000 and December 2009, 5543 women and 743 men with primary non-metastatic invasive breast cancer were identified. After exclusion of patients with unknown and negative hormone-receptor (HR) status, 2785 women and 257 men with HR-positive breast cancer were eligible for analysis (Fig. 1). The patients were divided into two groups according to the hormonal therapy used. In total, 1742 females (61.9%) were treated with TAM and 1061 (38.5%) with AI. Among MBC patients, 207 (80.1%) were treated with TAM and 50 (19.5%) received AI.
Patient characteristics are summarised in Table 1. In the group of TAM-treated patients, the females were younger, with a median age of 61 years (range 24–95 years), in comparison with their male counterparts who had a median age of 68 years (36–91 years). The TAM-treated MBC patients, in comparison with the TAM-treated FBC patients, were characterized by higher stage (p < 0.0001), higher rate of positive lymph nodes (p < 0.0001), and higher rate of HER2-negative cancers (p = 0.033), as well as lower incidence of high grade tumors (p = 0.009). The amount of patients who received chemotherapy was similar between FBC and MBC patients. The AI-treated MBC patients, in comparison with the AI-treated FBC patients, were characterized by older age at primary diagnosis [median age 68 years (range 44–84 years) versus 65 years (range 29–93 years), (p = 0.022)] and higher stage of disease (p < 0.0001). Interestingly, the rate of lymph node metastases, HER2 status, tumor grade, and chemotherapy received was similar between FBC and MBC patients (Table 1). Independent of the endocrine therapy, the follow-up period was significantly longer in the group of FBC patients.
Furthermore, we were interested to compare the survival of FBC and MBC patients. The OS rate during the follow-up period was 91.9 and 84.8% for patients with FBC and MBC, respectively. As demonstrated in Table 1, the basic characteristics of FBC and MBC patients were not well balanced, and the tumor and patients characteristics were unequally distributed. To avoid further confounder and selection bias caused by treatment choice, we carried out matching analysis based on six prognostic criteria. The female and male patients were matched 2:1. Based on this analysis, we identified 316 women and 158 men treated with TAM and 60 women and 30 men treated with AI (Fig. 1).
OS was estimated by the Kaplan–Meier method and was compared between FBC and MBC patients (Fig. 2). TAM-treated FBC and MBC patients had similar 5-year survival rates, 85.1 and 89.2%, respectively (Fig. 2a, p = 0.972). Notably, FBC patients treated with AI had significantly improved 5-year OS (85.0%) in comparison with AI-treated MBC patients (5-year OS of 73.3%; p = 0.028; see Fig. 2b).
Discussion
We determined the influence of gender on survival of endocrine treated female and male breast cancer patients. We found that an OS of TAM-treated FBC and MBC patient was quite similar, whereas the treatment of MBC with AI was associated with decreased OS, in comparison with their female counterparts. This study is the first demonstrating that the benefit of TAM treatment in MBC is comparable with the effect of TAM in FBC.
Data from studies comparing FBC and MBC suggest that MBC is largely hormone-receptor positive and the rates of ER and PR vary between 70 and 90% (Anderson et al. 2004; Losurdo et al. 2017). This is the basis for successful endocrine therapy. In accordance, TAM and AI are well established treatment options for FBC. However, the benefit of such endocrine therapy in MBC is relatively under investigated. There is a growing body of evidence regarding the ability of TAM to improve survival among MBC patients. In a recent study, Goss and colleagues demonstrated the significant positive influence of TAM treatment on OS of patients with MBC (Goss et al. 1999). Similar observations have been published in another trial with 39 MBC patients (Ribeiro and Swindell 1992). In these studies, the 5-year OS rate was 61 and 53%, respectively. Surprisingly, in our cohort of MBC patients, the observed 5-year OS was found to be 89.2%. The reason for this discrepancy could be explained by the duration of TAM treatment. In our cohort, the patients were treated for 5 years, whereas in the aforementioned studies, the duration of TAM treatment was 1 (Ribeiro and Swindell 1992) and 2 years (Goss et al. 1999), respectively. Nevertheless, the most notable observation in our present study is the fact that survival of TAM-treated MBC patients was comparable with the survival of their FBC counterparts. Although MBC was associated with poorer prognostic criteria, as previously described (Losurdo et al. 2017), the matching analysis clearly suggested that gender is not predictive of OS for breast cancer patients treated with TAM. These data again highlighted the use of TAM as a treatment of choice for HR-positive MBC.
In a recent report, we demonstrated that AI is inferior to TAM treatment for MBC (Eggemann et al. 2013). This observation was confirmed in the present study. Using matching analysis between patients with FBC and MBC, we were able to show that AI treatment of MBC is associated with significantly decreased survival. The estimated 5-year OS after AI treatment, for FBC and MBC, was 85.0 and 73.3%, respectively. Our findings could be explained by the different physiologies of males and females. In men, 80% of the estrogen is produced by utilisation of aromatase, and 20% direct in the testis, which might be the reason for AI’s ineffective suppression of estrogen level in men (Volm 2003). Moreover, increased levels of FSH and testosterone after AI treatment with increased aromatization are another possible explanation (Roselli and Resko 1997; Shetty et al. 1998). Nevertheless, our data clearly demonstrated that the TAM treatment effect in MBC is comparable with that in FBC. The lack of data from prospective randomised studies suggests that the optimal treatment strategy for MBC should be based on such retrospective analyses.
The most important limitations of the present study are its retrospective nature, and the relatively small number of MBC patients treated with AI. However, our study had several important strengths. First, it is the largest study of adjuvant hormonal therapy in male breast cancer patients. Second, the investigated cohorts of FBC and MBC patients were homogenous and included only patients with 5 years of endocrine treatment. Third, this study was population-based with high external validity.
Based on our data, we suggest the adjuvant use of TAM for five 5 years in the case of HR-positive MBC. The adjuvant use of AI in MBC should be discontinuation and further investigated.
References
Anderson WF, Althuis MD, Brinton LA, Devesa SS (2004) Is male breast cancer similar or different than female breast cancer? Breast Cancer Res Treat 83(1):77–86
Eggemann H, Ignatov A, Smith BJ, Altmann U, von Minckwitz G, Rohl FW, Jahn M, Costa SD (2013) Adjuvant therapy with tamoxifen compared to aromatase inhibitors for 257 male breast cancer patients. Breast Cancer Res Treat 137(2):465–470
Eggemann H, Ignatov T, Burger E, Kantelhardt EJ, Fettke F, Thomssen C, Costa SD, Ignatov A (2015) Moderate HER2 expression as a prognostic factor in hormone receptor positive breast cancer. Endocr Relat Cancer 22(5):725–733
Goss PE, Reid C, Pintilie M, Lim R, Miller N (1999) Male breast carcinoma: a review of 229 patients who presented to the Princess Margaret Hospital during 40 years: 1955–1996. Cancer 85(3):629–639
Losurdo A, Rota S, Gullo G, Masci G, Torrisi R, Bottai G, Zuradelli M, Gatzemeier W, Santoro A (2017) Controversies in clinicopathological characteristics and treatment strategies of male breast cancer: a review of the literature. Crit Rev Oncol Hematol 113:283–291
Ribeiro G, Swindell R (1992) Adjuvant tamoxifen for male breast cancer (MBC). Br J Cancer 65(2):252–254
Roselli CE, Resko JA (1997) Sex differences in androgen-regulated expression of cytochrome P450 aromatase in the rat brain. J Steroid Biochem Mol Biol 61(3–6):365–374
Shetty G, Krishnamurthy H, Krishnamurthy HN, Bhatnagar AS, Moudgal NR (1998) Effect of long-term treatment with aromatase inhibitor on testicular function of adult male bonnet monkeys (M. radiata). Steroids 63(7–8):414–420
Volm MD (2003) Male breast cancer. Curr Treat Options Oncol 4(2):159–164
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S (2007) The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370(9596):1453–1457
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Funding
This study was not funded.
Ethical approval
This article does not contain any studies with animals performed by any of the authors. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from all patients before treatment. An additional individual consent for this analysis was not needed.
Rights and permissions
About this article
Cite this article
Eggemann, H., Altmann, U., Costa, SD. et al. Survival benefit of tamoxifen and aromatase inhibitor in male and female breast cancer. J Cancer Res Clin Oncol 144, 337–341 (2018). https://doi.org/10.1007/s00432-017-2539-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-017-2539-7