Abstract
Purpose
This study was to elucidate the potential benefit of beta blockers on cancer survival.
Methods
We comprehensively searched PubMed, Embase, and the Cochrane Library from their inception to April 2013. Two authors independently screened and reviewed the eligibility of each study and coded the participants, treatment, and outcome characteristics. The primary outcomes were overall survival (OS) and disease-free survival (DFS).
Results
Twelve studies published between 1993 and 2013 were included in the final analysis. Four papers reported results from 10 independent groups, resulting in a total of 18 comparisons based on data obtained from 20,898 subjects. Effect sizes (hazard ratios, HR) were heterogeneous, and random-effects models were used in the analyses. The meta-analysis demonstrated that beta blocker use is associated with improved OS (HR 0.79; 95 % CI 0.67–0.93; p = 0.004) and DFS (HR 0.69; 95 % CI 0.53–0.91; p = 0.009). Although statistically not significant, the effect size was greater in patients with low-stage cancer or cancer treated primarily with surgery than in patients with high-stage cancer or cancer treated primarily without surgery (HR 0.60 vs. 0.78, and 0.60 vs. 0.80, respectively). Although only two study codes were analyzed, the studies using nonselective beta blockers showed that there was no overall effect on OS (HR 0.52, 95 % CI 0.09–3.04).
Conclusion
This meta-analysis provides evidence that beta blocker use can be associated with the prolonged survival of cancer patients, especially patients with early-stage cancer treated primarily with surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Previous studies have shown that stress may be linked with the onset of cancer and cancer progression. Catecholamines are the major transmitters of the stress response (Antoni et al. 2006; Ben-Eliyahu 2003; Justice 1985; McEwen 1998; Reiche et al. 2004), and interestingly, beta adrenergic receptors have been identified on nasopharyngeal (Yang et al. 2006), pancreatic (Weddle et al. 2001), breast (Vandewalle et al. 1990), and ovarian (Thaker et al. 2006) cancer cells. In addition, beta adrenergic stimulation is known to lead to increased angiogenesis, tumor invasion (Thaker et al. 2006), resistance to anoikis (Sood et al. 2010), and integrin-mediated cell adhesion (Rangarajan et al. 2003), and these phenotypes are abrogated by a beta adrenergic antagonist.
Several retrospective cohort studies have examined the impact of beta blockers on long-term cancer outcomes. In triple-negative breast cancer patients, the use of beta blockers before surgery was associated with improved recurrence-free survival (Melhem-Bertrandt et al. 2011); moreover, in malignant melanoma patients, beta blocker treatment significantly predicted a reduced mortality (Lemeshow et al. 2011).
Although the benefit of beta blockers is clear in some malignancies, the clinical value of the drug in cancers overall remains unclear. To our knowledge, there are no clinical trials that directly test the impact of beta blockers on survival from cancer. Two clinical trials are currently investigating the role of beta blockers in cancer recurrence and progression in patients with breast (NCT00502684) and colorectal (NCT 00888797) cancer undergoing surgery with curative intent. Despite the ongoing trials, we do not know which patients are the best candidates for this intervention.
The present meta-analysis was performed to assess whether adding beta blockers to the treatment regimen of patients with various types of cancer had an impact on survival. In addition, the study will compare how the study population, such as patients with early-/late-stage cancer or patients treated with/without surgery, influence the effect size.
Materials and methods
The review was planned and conducted in accordance with the PRISMA guidelines for meta-analysis and the recommendations of the Cochrane Collaboration (Furlan et al. 2009). This was a meta-analysis of published summary data and therefore did not require ethics approval.
Search strategy
We performed electronic searches in PubMed/MEDLINE (1960–2013), Embase (1980–2013), and the Cochrane Library. The literature search was constructed using key words such as “neoplasm” or “cancer” for disease; “beta blocker” for intervention; and “progression,” “recurrence,” “survival,” or “mortality” for outcome. The complete search strategy for PubMed was as follows: (“neoplasm” OR “tumor” OR “cancer” OR “carcinoma” OR “adenocarcinoma”) AND (“beta blocker” OR “atenolol” OR “propranolol” OR “metoprolol” OR “arotinolol” OR “betaxolol” OR “bevantolol” OR “bisoprolol” OR “carteolol” OR “carvedilol” OR “celiprolol”) AND (“progression” OR “recurrence” OR “metastasis” OR “survival” OR “mortality”), and the search strategy was adapted for each database as necessary. Reference lists and conference proceedings were also searched to identify additional potential studies. The last systematic search was dated May 14, 2013.
Study selection
A study that met the following conditions was eligible for inclusion in the study: (1) participants must have a cancer diagnosis; (2) beta blockers must be used in the treatment group; (3) studies must be a comparative study; (4) outcome variable must be the survival time; and (5) it must be possible to calculate a hazard ratio (HR) for survival with an associated variance between the treatment and control group. If this was not directly reported in the primary report, it must be possible to obtain it by other means.
A study was excluded from this meta-analysis under the following conditions: (1) if two or more studies were reported by the same institution and/or authors and showed an overlap between the results; (2) if multi-center studies contained data which were already included in a single-center study; and (3) if a HR with 95 % confidence interval (CI) for survival was not directly reported, or it was impossible to calculate that from the paper.
Coding variables
The following variables were used for coding the study: publication year, number of subjects in the control and intervention groups, mean age, type of cancer, metastatic/nonmetastatic cancer dominant, and whether surgery was done or not. The name of the first author and the year of publication of the article were used for identification purposes. When there were subgroups in the article, they were coded independently for the subgroup analysis. Two reviewers (CHC and TS) independently extracted the data from all included studies. A third reviewer (BGK) was consulted to resolve disagreements.
Type of effect size
The primary outcome analyzed was overall survival (OS). Another point of interest was disease-free survival (DFS). For each study, a log hazard ratio (lnHR) with its standard error was calculated. In some of the primary reports, these parameters were reported directly. In other studies, we had to estimate them from other reported data using the methods provided (Parmar et al. 1998).
Predefined subgroup analysis was conducted to assess the role of beta blocker in different cancers, different stages, and different primary treatment modalities (surgery or not). Most cancer types can be staged using one of the following classification systems: the overall stage groupings (I–IV) and the TNM staging. In the present study, we divided studies into either “early stage dominant” or “late stage dominant” with more or <50 % of cases having stage I/II or T1/T2, respectively. For the treatment history, surgery was the important variable in the assessment of beta blocker efficacy, and studies were divided into either “surgery done” or “surgery not done.”
Statistical analysis
Because we compared the effect on a wide range of different cancers, we decided to use random-effects modeling overall. The analyses were done using RevMan version 5.2 (Cochrane Collaboration, Oxford, UK). The lnHR and its variance were pooled using an inverse variance-weighted average, and the results were presented as HR and 95 % CI. Statistical heterogeneity in the results of the trials was assessed by the chi-square test (DerSimonian and Laird 1986) and was expressed as the I 2 index, as described by Higgins et al. (Higgins et al. 2003). A funnel plot was produced to assess the possibility of publication bias. A sensitivity analysis was performed to test the stability of our conclusions, and it was prespecified. The treatment effect was examined based on the time to publication and sample size.
Results
Literature search
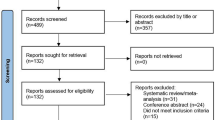
The flow chart of the systematic search is summarized in Fig. 1. A total of 181 citations were identified from the MEDLINE and Embase database. After review by all of the authors, a total of 12 papers fulfilled the inclusion criteria and were eligible for this study (Barron et al. 2011; De Giorgi et al. 2011; Diaz et al. 2012; Ganz et al. 2011; Grytli et al. 2013; Grytli et al. 2014; Heitz et al. 2013; Hole et al. 1993; Lemeshow et al. 2011; Melhem-Bertrandt et al. 2011; Powe et al. 2010; Wang et al. 2013). In one of the studies excluded, beta blocker was compared with other antihypertensive drugs, and so, we judged the groups in this study to not qualify as proper treatment and control groups (Shah et al. 2011). Four papers reported results from 10 independent groups with different types of cancer (Barron et al. 2011; Grytli et al. 2013; Higgins and Thompson 2002). This resulted in a total of 18 comparisons based on the data obtained from 20,898 subjects.
Reporting of information
A detailed description of all included studies is presented in Table 1. Of the 12 trials, four were done on breast cancer, two on ovarian cancer, two on melanoma, two on prostate cancer, one on NSCLC, and one on mixed cancers. Of the 18 study codes, nine were classified as “low-stage dominant,” and seven as “high-stage dominant,” according to the definitions. Similarly, ten were classified as “surgery done,” and six “surgery not done.” The beta blockers used were diverse and mixed in many studies. Of them, beta 1 selective blocker was used mainly in 11 study codes, and only 2 study codes analyzed nonselective beta blocker separately.
A funnel plot showed the existence of possible publication bias as there was a relative lack of studies in the lower right portion (Fig. 2). Because of the low number of studies, this plot was not very informative, but it is regarded as good practice to routinely publish such plots in meta-analyses.
Funnel plot of comparison: beta blocker versus control group for all studies. Outcome: OS for all patients. There may be some factors accounting for the small amount of asymmetry, such as the exclusion of small trials with negative results that were not published, differences in trial quality or true study heterogeneity
Survival
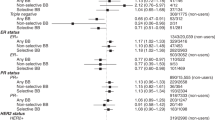
The meta-analysis for the total sample of studies shows a significant treatment effect. Beta blocker usage was associated with a significant improvement in OS (HR 0.79; 95 % CI 0.67–0.93; p = 0.004) (Fig. 3). The sample was marginally heterogeneous (χ 2 = 41.97, df = 17, p = 0.0007, I 2 = 59 %).
A subgroup analysis demonstrated that OS was significantly improved even when the meta-analysis was restricted to low-stage dominant cancer (HR 0.60; 95 % CI 0.40–0.90; p = 0.01) or high-stage dominant (HR 0.78; 95 % CI 0.66–0.91; p = 0.002) (Fig. 4) cancer. Although statistically not significant, the effect sizes were greater in low-stage dominant subjects. Similarly, beta blocker showed a more pronounced positive impact on OS for patients treated with surgery (HR 0.60; 95 % CI 0.42–0.86; p = 0.005) than patients not treated with surgery (HR 0.80; 95 % CI 0.69–0.93; p = 0.003) (Fig. 5).
Analysis according to cancer type showed significant effects in breast cancer (HR 0.58; 95 % CI 0.35–0.99; p = 0.04), ovarian cancer (HR 0.66; 95 % CI 0.48–0.92; p = 0.01), and NSCLC (HR 0.78; 95 % CI 0.63–0.97; p = 0.03), but not in prostate cancer (HR 0.61; 95 % CI 0.23–1.60; p = 0.31) and melanoma (HR 0.76; 95 % CI 0.43–1.33; p = 0.34) (Supplemental Fig. 1). Of interest, although only 2 study codes were analyzed, the studies using nonselective beta blocker were found not to have an overall effect on OS (HR 0.52, 95 % CI 0.09–3.04) (Supplemental Fig. 2).
Trials after the year 2000 only and trials with larger samples only (more than 300 patients) were examined separately for the sensitivity analysis, and no significant difference was found in this subset analysis (Table 2).
Data on DFS could be extracted from seven papers, accounting for 4,878 patients. The patients receiving beta blocker had a significantly longer DFS than those not receiving beta blocker, with a benefit percentage of 31 % (95 % CI 9–47 %, p = 0.009) (Fig. 6).
Discussion
To the best of our knowledge, this study is the first attempt at quantifying the effects of beta blockers on cancer survival. A total of twelve papers were investigated, and most of these papers had recently been published. When all the outcomes from each study were pooled together, there was a significant benefit with the addition of beta blocker not only for OS but also for DFS. Although there was a marginal heterogeneity between the studies included, the results of this meta-analysis are important because the data were gathered from more than 20,000 various cancer patients who were followed up for a sufficient duration of time. Another main finding from the present analysis is that beta blocker had more dramatic association effects in patients who had low-stage cancer and/or who had received surgical treatment for their cancer than in patients with high stage and/or in whom surgery was not the primary treatment. This result can be particularly important in designing trials investigating the role of beta blocker in cancer survival.
The results of this meta-analysis are supported by in vitro and in vivo animal studies using a variety of human tumor lines. They indicated that activation of tumor beta adrenergic receptors can (1) enhance the production of several metastasis-promoting factors, including VEGF, matrix metalloproteinase 2 (MMP-2), and MMP-9, interleukin 6 (IL-6), and IL-8, and (2) facilitate tumor angiogenesis, survival, migration, proliferation, and resistance to anoikis—effects that are all blocked by beta blockers (Bernabe et al. 2011; Masur et al. 2001; Sood et al. 2006, 2010; Thaker et al. 2006; Yang et al. 2006, 2009).
Interestingly, there was a trend for low-stage subjects to benefit more dramatically from using a beta blocker than high-stage subjects. This suggests that the treatment is effective in controlling the initial stages of the metastatic process, rather than treating established metastatic foci. Some epidemiologic studies have shown that beta blocker was associated with reduction in cancer rates (Barron et al. 2011; Powe et al. 2010), which is evidence for the role of beta blocker in blocking cancer initiation and progression. In addition, beta adrenergic stimulation is known to lead to resistance to anoikis, which is a hallmark of malignant transformation that allows detached cells to survive (Sood et al. 2010).
Similar to stage of cancer, patients treated with surgery showed a more pronounced impact with beta blocker, although this finding was not statistically significant. Because early-stage patients tend to undergo surgery, we do not know whether a surgical factor or a stage factor was associated with the effect size of beta blocker. Surgery has been suspected to facilitate the progression of preexisting micrometastasis and the initiation of new metastases via several mechanisms such as increased shedding of tumor cells (Yamaguchi et al. 2000), increased levels of growth factors (Abramovitch et al. 1999), and decreased antiangiogenic factors (e.g., endostatin) (O’Reilly et al. 1997). And one of the mechanisms is through the increase of catecholamines which are known to act directly on malignant cells, activating several processes that are critical for tumor metastatic activity, including tumor cell proliferation (Bernabe et al. 2011; Mathew et al. 2011), extracellular matrix invasion capacity (Mathew et al. 2011), resistance to apoptosis (Kerros et al. 2010; Roche-Nagle et al. 2004; Sood et al. 2010), and secretion of proangiogenic factors (Thaker et al. 2006; Wei et al. 2004; Yang et al. 2009). Lee et al. have shown that, in mice with ovarian cancer, propranolol mitigated the effects of surgical stress on tumor growth and angiogenesis (Lee et al. 2009). According to the preclinical data and the results of this study, the perioperative period would theoretically present an opportunity to eradicate cancer or successfully arrest its progression.
There are some limitations in this study. First, although retrospective cohort studies have many methodological shortcomings, they are included in this study because there are no randomized controlled trials yet investigating this subject. Second, for the evaluation of surgical factors, extensive surgeries such as colon, hepatobiliary, or stomach cancer can give more information than breast cancer or melanoma, but studies examining those cancers were not researched in this analysis. Third, there is a variation in effect sizes (heterogeneity), and it may be due to systematic differences among the studies. The studies used various beta blockers with different types of subjects in different settings. Another limitation is the possible existence of some unpublished studies, which could lead to potential publication bias in this study, with the favoring of published positive studies. Lastly, we could not analyze the type of beta blocker that produced these effects because most studies combined beta blockers in their analyses.
In summary, though there are some limitations in this study, beta blocker usage was associated with prolonged survival of cancer patients in this meta-analysis, especially in patients with early-stage cancer that had been treated primarily with surgery. Therefore, beta blocker can be considered a standard approach for adjuvant therapy in various types of cancer. In addition, this approach is also advantageous in that it uses commonly administered medications that are relatively safe and inexpensive. Further trials must be explored with larger patient cohorts.
References
Abramovitch R, Marikovsky M, Meir G, Neeman M (1999) Stimulation of tumour growth by wound-derived growth factors. Br J Cancer 79:1392–1398
Antoni MH, Lutgendorf SK, Cole SW, Dhabhar FS, Sephton SE, McDonald PG, Stefanek M, Sood AK (2006) The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer 6:240–248
Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K (2011) Beta blockers and breast cancer mortality: a population-based study. J Clin Oncol 29:2635–2644
Ben-Eliyahu S (2003) The promotion of tumor metastasis by surgery and stress: immunological basis and implications for psychoneuroimmunology. Brain Behav Immun 17(Suppl 1):S27–S36
Bernabe DG, Tamae AC, Biasoli ER, Oliveira SH (2011) Stress hormones increase cell proliferation and regulates interleukin-6 secretion in human oral squamous cell carcinoma cells. Brain Behav Immun 25:574–583
De Giorgi V, Grazzini M, Gandini S, Benemei S, Lotti T, Marchionni N, Geppetti P (2011) Treatment with beta-blockers and reduced disease progression in patients with thick melanoma. Arch Intern Med 171:779–781
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Diaz ES, Karlan BY, Li AJ (2012) Impact of beta blockers on epithelial ovarian cancer survival. Gynecol Oncol 127:375–378
Furlan AD, Pennick V, Bombardier C, van Tulder M (2009) 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine (Phila Pa 1976) 34:1929–1941
Ganz PA, Habel LA, Weltzien EK, Caan BJ, Cole SW (2011) Examining the influence of beta blockers and ACE inhibitors on the risk for breast cancer recurrence: results from the LACE cohort. Breast Cancer Res Treat 129:549–556
Grytli HH, Fagerland MW, Fossa SD, Tasken KA, Haheim LL (2013) Use of beta-blockers is associated with prostate cancer-specific survival in prostate cancer patients on androgen deprivation therapy. Prostate 73:250–260
Grytli HH, Fagerland MW, Fossa SD, Tasken KA (2014) Association between use of beta-blockers and prostate cancer-specific survival: a cohort study of 3561 prostate cancer patients with high-risk or metastatic disease. Eur Urol 65(3):635–641
Heitz F, du Bois A, Harter P, Lubbe D, Kurzeder C, Vergote I, Plante M, Pfisterer J (2013) Impact of beta blocker medication in patients with platinum sensitive recurrent ovarian cancer—a combined analysis of 2 prospective multicenter trials by the AGO Study Group, NCIC-CTG and EORTC-GCG. Gynecol Oncol 129:463–466
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Hole DJ, Hawthorne VM, Isles CG, McGhee SM, Robertson JW, Gillis CR, Wapshaw JA, Lever AF (1993) Incidence of and mortality from cancer in hypertensive patients. BMJ 306:609–611
Justice A (1985) Review of the effects of stress on cancer in laboratory animals: importance of time of stress application and type of tumor. Psychol Bull 98:108–138
Kerros C, Brood I, Sola B, Jauzac P, Allouche S (2010) Reduction of cell proliferation and potentiation of Fas-induced apoptosis by the selective kappa-opioid receptor agonist U50 488 in the multiple myeloma LP-1 cells. J Neuroimmunol 220:69–78
Lee JW, Shahzad MM, Lin YG, Armaiz-Pena G, Mangala LS, Han HD, Kim HS, Nam EJ, Jennings NB, Halder J, Nick AM, Stone RL, Lu C, Lutgendorf SK, Cole SW, Lokshin AE, Sood AK (2009) Surgical stress promotes tumor growth in ovarian carcinoma. Clin Cancer Res 15:2695–2702
Lemeshow S, Sorensen HT, Phillips G, Yang EV, Antonsen S, Riis AH, Lesinski GB, Jackson R, Glaser R (2011) beta-Blockers and survival among Danish patients with malignant melanoma: a population-based cohort study. Cancer Epidemiol Biomark Prev 20:2273–2279
Masur K, Niggemann B, Zanker KS, Entschladen F (2001) Norepinephrine-induced migration of SW 480 colon carcinoma cells is inhibited by beta-blockers. Cancer Res 61:2866–2869
Mathew B, Lennon FE, Siegler J, Mirzapoiazova T, Mambetsariev N, Sammani S, Gerhold LM, LaRiviere PJ, Chen CT, Garcia JG, Salgia R, Moss J, Singleton PA (2011) The novel role of the mu opioid receptor in lung cancer progression: a laboratory investigation. Anesth Analg 112:558–567
McEwen BS (1998) Protective and damaging effects of stress mediators. N Engl J Med 338:171–179
Melhem-Bertrandt A, Chavez-Macgregor M, Lei X, Brown EN, Lee RT, Meric-Bernstam F, Sood AK, Conzen SD, Hortobagyi GN, Gonzalez-Angulo AM (2011) Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol 29:2645–2652
O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J (1997) Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 88:277–285
Parmar MK, Torri V, Stewart L (1998) Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 17:2815–2834
Powe DG, Voss MJ, Zanker KS, Habashy HO, Green AR, Ellis IO, Entschladen F (2010) Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget 1:628–638
Rangarajan S, Enserink JM, Kuiperij HB, de Rooij J, Price LS, Schwede F, Bos JL (2003) Cyclic AMP induces integrin-mediated cell adhesion through Epac and Rap1 upon stimulation of the beta 2-adrenergic receptor. J Cell Biol 160:487–493
Reiche EM, Nunes SO, Morimoto HK (2004) Stress, depression, the immune system, and cancer. Lancet Oncol 5:617–625
Roche-Nagle G, Connolly EM, Eng M, Bouchier-Hayes DJ, Harmey JH (2004) Antimetastatic activity of a cyclooxygenase-2 inhibitor. Br J Cancer 91:359–365
Shah SM, Carey IM, Owen CG, Harris T, Dewilde S, Cook DG (2011) Does beta-adrenoceptor blocker therapy improve cancer survival? Findings from a population-based retrospective cohort study. Br J Clin Pharmacol 72:157–161
Sood AK, Bhatty R, Kamat AA, Landen CN, Han L, Thaker PH, Li Y, Gershenson DM, Lutgendorf S, Cole SW (2006) Stress hormone-mediated invasion of ovarian cancer cells. Clin Cancer Res 12:369–375
Sood AK, Armaiz-Pena GN, Halder J, Nick AM, Stone RL, Hu W, Carroll AR, Spannuth WA, Deavers MT, Allen JK, Han LY, Kamat AA, Shahzad MM, McIntyre BW, Diaz-Montero CM, Jennings NB, Lin YG, Merritt WM, DeGeest K, Vivas-Mejia PE, Lopez-Berestein G, Schaller MD, Cole SW, Lutgendorf SK (2010) Adrenergic modulation of focal adhesion kinase protects human ovarian cancer cells from anoikis. J Clin Invest 120:1515–1523
Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, Jennings NB, Armaiz-Pena G, Bankson JA, Ravoori M, Merritt WM, Lin YG, Mangala LS, Kim TJ, Coleman RL, Landen CN, Li Y, Felix E, Sanguino AM, Newman RA, Lloyd M, Gershenson DM, Kundra V, Lopez-Berestein G, Lutgendorf SK, Cole SW, Sood AK (2006) Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med 12:939–944
Vandewalle B, Revillion F, Lefebvre J (1990) Functional beta-adrenergic receptors in breast cancer cells. J Cancer Res Clin Oncol 116:303–306
Wang HM, Liao ZX, Komaki R, Welsh JW, O’Reilly MS, Chang JY, Zhuang Y, Levy LB, Lu C, Gomez DR (2013) Improved survival outcomes with the incidental use of beta-blockers among patients with non-small-cell lung cancer treated with definitive radiation therapy. Ann Oncol 24:1312–1319
Weddle DL, Tithoff P, Williams M, Schuller HM (2001) Beta-adrenergic growth regulation of human cancer cell lines derived from pancreatic ductal carcinomas. Carcinogenesis 22:473–479
Wei D, Wang L, He Y, Xiong HQ, Abbruzzese JL, Xie K (2004) Celecoxib inhibits vascular endothelial growth factor expression in and reduces angiogenesis and metastasis of human pancreatic cancer via suppression of Sp1 transcription factor activity. Cancer Res 64:2030–2038
Yamaguchi K, Takagi Y, Aoki S, Futamura M, Saji S (2000) Significant detection of circulating cancer cells in the blood by reverse transcriptase–polymerase chain reaction during colorectal cancer resection. Ann Surg 232:58–65
Yang EV, Sood AK, Chen M, Li Y, Eubank TD, Marsh CB, Jewell S, Flavahan NA, Morrison C, Yeh PE, Lemeshow S, Glaser R (2006) Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res 66:10357–10364
Yang EV, Kim SJ, Donovan EL, Chen M, Gross AC, Webster Marketon JI, Barsky SH, Glaser R (2009) Norepinephrine upregulates VEGF, IL-8, and IL-6 expression in human melanoma tumor cell lines: implications for stress-related enhancement of tumor progression. Brain Behav Immun 23:267–275
Acknowledgments
Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2013R1A1A2013629).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Chel Hun Choi, Taejong Song, Jeong-Won Lee, and Byoung-Gie Kim have contributed equally to this paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Choi, C.H., Song, T., Kim, T.H. et al. Meta-analysis of the effects of beta blocker on survival time in cancer patients. J Cancer Res Clin Oncol 140, 1179–1188 (2014). https://doi.org/10.1007/s00432-014-1658-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-014-1658-7