Abstract
Purpose
The expression levels of human antioxidant genes (HAGs) and oxidative markers were investigated in light of lung adenocarcinoma aggressiveness and patient outcome.
Methods
We assayed in vitro the tumoral invasiveness and multidrug resistance in human lung adenocarcinoma (AdC) cell lines (EKVX and A549). Data were associated with several redox parameters and differential expression levels of HAG network. The clinicopathological significance of these findings was investigated using microarray analysis of tumor tissue and by immunohistochemistry in archival collection of biopsies.
Results
An overall increased activity (expression) of selected HAG components in the most aggressive cell line (EKVX cells) was observed by bootstrap and gene set enrichment analysis (GSEA). In vitro validation of oxidative markers revealed that EKVX cells had high levels of oxidative stress markers. In AdC cohorts, GSEA of microarray datasets showed significantly high levels of HAG components in lung AdC samples in comparison with normal tissue, in advanced stage compared with early stage and in patients with poor outcome. Cox multivariate regression analysis in a cohort of early pathologic (p)-stage of AdC cases showed that patients with moderate levels of 4-hydroxynonenal, a specific and stable end product of lipid peroxidation, had a significantly less survival rate (hazard ratio of 8.87) (P < 0.05).
Conclusions
High levels of oxidative markers are related to tumor aggressiveness and can predict poor outcome of early-stage lung adenocarcinoma patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide, presenting in many countries a mortality rate that outranks prostate, colorectal and pancreatic cancer cases combined (Siegel et al. 2013). Despite recent advances in therapy protocols (Coate et al. 2009), 5-year survival rates of advanced stages remain as low as 2 % (Siegel et al. 2013). Thus, it is essential to uncover any biological processes or molecular mechanisms associated with the initiation and progress of the aggressive malignant phenotype of lung cancer cells in order to identify potential targets for novel interventional strategies.
Lung tumor cells are able to grow in a highly oxidative environment (Melloni et al. 1996; Brennan et al. 2000; Ho et al. 2007; Esme et al. 2008; Chan et al. 2009), which is believed to contribute with tumor progression and metastasis (Sotgia et al. 2011). Lung tumors are especially exposed to a pro-oxidative milieu due to the factors such as tobacco smoke, high atmospheric oxygen pressure (Rahman et al. 2006) and the bulk amount of reactive species (RS) generated by pro-inflammatory cells in the pulmonary circulation (Ilonen et al. 2009). Moreover, clinical data have shown that lung cancer patients have increased oxidative stress markers in peripheral blood (Esme et al. 2008), erythrocytes (Kaynar et al. 2005; Ho et al. 2007), epithelium lining fluid (Melloni et al. 1996), breath condensate (Chan et al. 2009) and tumor biopsies (Jaruga et al. 1994; Coursin et al. 1996; Blair et al. 1997), and the inadequate ingest of antioxidants constitutes a risk factor for lung cancer development (Brennan et al. 2000). Although pulmonary cells have these reduction/oxidation (redox) particularities, the influence of oxidative stress in lung cancer biology is poorly understood.
The present study focused on redox state and cancer cell growth in vitro, comparing a more aggressive human lung AdC cell line (EKVX) to a less aggressive one (A549). We found that the most aggressive AdC cell line presents high levels of oxidative stress markers. Follow-up experiments indicate that this oxidative imbalance may support malignant features of AdC cells and has clinical significance, since we found that human antioxidant gene (HAG) components and 4-hydroxynonenal (4-HNE) levels have prognostic value in predicting lung AdC patient outcome.
Materials and methods
Cell lines and chemicals
The human lung adenocarcinoma cell lines A549 and EKVX were obtained from NCI-Frederick cell line repository. Exponentially growing cells were maintained in RPMI 1640 medium (Invitrogen®) containing 10 % fetal bovine serum, 1 μg/mL of amphotericin B and 50 μg/L of garamycin at 37 °C in a humidified atmosphere of 5 % of CO2. Protein concentration was determined by Lowry’s method. Chemicals were obtained from Sigma® Chemical Co.
Cellular aggressiveness and redox parameters
The BioCoat Matrigel Invasion Chamber System (BD Bioscience®) was used to access the invasion index. Briefly, cells were seeded in the upper wells, while the chemoattractant (medium RPMI with 10 % of SFB) was added to the lower wells. After 22-h incubation, the trans-well movement of cells through the pore was determined. Cells that penetrated to the underside surfaces of the inserts were fixed and stained with HEMA 3 staining kit (Fisher Scientific®) and counted under the microscope. Cells were considered migratory when moved through uncoated pores and invasive when moved through Matrigel-coated pores. Data are expressed as the percentage of invasive/migratory and expressed as “invasion index.”
Multidrug resistance was determined based on drug dose–response curves of Cisplatin, Carboplatin, Daunorubicin, Doxorubicin, 5-Fluorouracil, Hydroxyurea and Taxol (Sigma® Chemical Co.) using the sulforhodamine B (SRB) assay, following NCI-60 protocol (Vichai and Kirtikara 2006).
Superoxide dismutase (SOD) (E.C. 1.15.1.1) activity was measured by inhibition of superoxide-dependent epinephrine auto-oxidation at 480 nm (Misra and Fridovich 1972). Catalase (CAT) (E.C. 1.11.1.6) activity was measured by H2O2 consumption at 240 nm. Glutathione peroxidase (GPX) (E.C. 1.11.1.9) activity was measured by NADPH oxidation at 340 nm (Wendel 1981).
Nonenzymatic antioxidant potential was determined by total radical-trapping antioxidant potential (TRAP) assay (Dresch et al. 2009). Elman’s sulfhydryl group (–SH) level was determined with 5-thio-2-nitrobenzoic acid at 412 nm (ε412 nm = 27,200/M cm) and expressed as nmol –SH/mg protein. Thiobarbituric acid reactive species (TBARS) assay was used as a lipoperoxidation index. TBARS were assayed at 532 nm and expressed as nmol MDA equivalents/mg protein. DCF-DA (2′,7′-dichlorodihydrofluorescein diacetate) oxidation and Amplex Red® were used to determine intracellular generation of RS in a 96-well plate reader (Spectra Max GEMINI XPS, Molecular Devices®).
Proliferation and growth inhibition assays
Cells were treated with active or heat-inactivated Catalase, Trolox® or N-acetylcysteine (NAC). Cell growth inhibition was evaluated for 72 h with SRB assay. To investigate whether the growth inhibition by CAT was reversible, the assay was repeated removing CAT. The effect of bolus amount of H2O2 addition or CAT inhibition with aminotriazole on cell growth was also evaluated.
Human antioxidant gene (HAG) network and microarray datasets
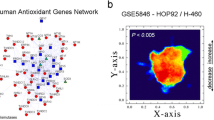
The HAG was designed to cluster functional gene network to facilitate high-throughput analysis of redox processes (Gelain et al. 2009). HAG is composed of 63 genes whose products are thiol-containing proteins or enzymes that react directly with RS and was subclassified into three functional groups: peroxidases, superoxide dismutases and thiol-containing redox proteins (Fig. 1a).
Differential gene expression of HAG network in human lung AdC cell lines. a STRING representation of HAG network gene interactions. b Two-state landscape analysis of HAG expression between cell lines (GSE5846 dataset). Coordinates (X- and Y-axis) represent normalized values of the input network topology. Color gradient (Z-axis) represents the relative functional state mapped onto graph according to the data input from the adenocarcinomas EKVX-a versus A549-b, where z = a/(a + b). The landscape was generated with ViaComplex® V1.0
Microarray expression profiles were extracted from the gene expression omnibus (GEO) (http://www.ncbi.nlm.nih.gov/geo/). For the human lung AdC cell lines, we used GSE5846 dataset. For cohort analysis, we used three microarray datasets: 21 surgically resected tumor tissue and adjacent normal tissue from primary NSCLC patients, with histological and staging details (GSE21933); 269 NSCLC specimens containing histological, staging, survival and recurrence details (GSE41271); and 174 NSCLC specimens containing histological, staging and survival details (GSE42127).
Differential gene expression and enrichment analysis
Differential gene expression was evaluated using ViaComplex® software (Castro et al. 2009, 2010). Gene set enrichment analysis (GSEA) was used to identify genes that contribute individually to global changes in expression levels in a given microarray dataset (Subramanian et al. 2005).
Retrospective cohort and immunohistochemistry
Formalin-fixed paraffin-embedded lung AdC tumors from patients diagnosed between 1998 and 2004 were obtained from the Pathology Service at the Santa Casa de Misericórdia de Porto Alegre (Porto Alegre, Brazil) (Sánchez et al. 2006). The pathological diagnoses were reviewed and classified by two independent pathologists, according to World Health Organization criteria. Inclusion criteria were lung adenocarcinomas as primary tumor and clinical follow-up data of at least 5 years available. Gender, age, height, weight, histology, pathological stage, smoking history and lung function information were collected. The research program was approved by the local Research Ethics Committee (#1852/08). The Helsinki Declaration of Human Rights was observed when performing these experiments, and written informed consent was provided.
Sections of 4 μm were deparaffinized and rehydrated, antigen retrieval was performed by pepsin (Zytonvision®), endogenous peroxidase was blocked with 5 % H2O2 in methanol, and nonspecific blocking was done with 1 % bovine serum albumin (BSA). The slides were incubated overnight at 4 °C with rabbit polyclonal antibody against 4-hydroxynonenal (4-HNE) (Abcam ab46545) 1:1,200 in 1 % BSA and rinsed and incubated with HRP-labeled-polymer-conjugated kit (Invitrogen®). Sections were counterstained with hematoxylin. Negative control was obtained performing the same protocol without the primary antibody. The assessment of immunostaining intensity was performed semiquantitatively and in a blinded fashion (0 = no staining; 1 = weak staining; 2 = moderate staining; and 3 = intense staining) (Rahman et al. 2002).
Statistical analysis
Data are expressed as mean ± SEM of at least three independent experiments carried out in triplicate, and Student’s t test was used (P < 0.05) (GraphPad® Software 5.0). Multivariate Cox proportional hazards regression models were used to test the independent contribution of each variable on mortality, and the results were summarized by calculating hazard ratios (HR) and corresponding 95 % confidence intervals. Chi-squared test was used to assess the independence of the staining groups related to the cohort baseline characteristics. A chi-squared approximation for low-frequency groups was obtained using Monte Carlo simulated P values, based on 2,000 replicates in R (http://www.R-project.org/).
Results
Cellular aggressiveness, HAG activity and oxidative stress markers
In vitro analysis of basal invasion index and multidrug resistance was used to establish the aggressiveness between two human lung adenocarcinoma cell lines (Table 1). Comparing cells, EKVX presents fivefold higher invasive potential (P = 0.0004) and a significant cross-resistance to all seven drugs evaluated (range of 1.61- to 68.66-fold increase in drug GI50 values) and was established as the most aggressive cell line.
In addition, we assayed the cellular redox status of both cell lines by analyzing HAG network activities. To do that, landscape maps of gene expression were built (Fig. 1a), and using the open-source software ViaComplex®, we observe that EKVX cell line has increased expression of HAG components (P = 0.016) (Fig. 1b). The specific genes that contribute to this difference, obtained by GSEA analysis, are summarized in Supplementary Table 1. As an example, metallothioneins (1E/F/H/X and 2A), the mitochondrial SOD (SOD2) and components of the thioredoxin system (TXN2/TXNIP/TXNRD2) were found to be up-regulated in the most aggressive phenotype. To further validate the differences obtained with microarray data, several redox parameters were accessed in vitro in our AdC cell panel (Table 1). We found a significant imbalance in antioxidant enzyme (AOE) activities between cell lines, more specifically an increased SOD activity with a concomitant decrease in CAT/GPX activities, suggesting H2O2 accumulation in EKVX cells. Consistent with this, EKVX cell line generates higher steady-state levels of RS and H2O2 (Table 1). In addition, regarding nonenzymatic parameters, basal lipoperoxidation (TBARS levels) is twofold higher and there is a significant decrease in total antioxidant capacity (TRAP) and reduced sulfhydryl levels (–SH) in EKVX cells. Therefore, high levels of intracellular oxidative markers might be associated with cellular aggressiveness in AdC cells, possibly due to the combination of imbalance in AOE activities and high RS generation.
Intracellular oxidative state modulates the proliferative rates of lung AdC cell lines
To deepen the question whether oxidative state influences cellular aggressiveness or is a by-product, we evaluated the effect of antioxidant treatments in the growth rates of human lung AdC cells. Exogenous addition of CAT specifically decreases H2O2 levels since this oxidant diffuses through membranes (Policastro et al. 2004) and caused a dose-dependent inhibition of both lung AdC cells’ growth (Fig. 2a). CAT washout restored proliferation rate of cells (Fig. 2b), arguing that CAT addition caused a cytostatic (not cytotoxic) effect, since there was no decrease in cell viability (data not shown). This phenomenon seems to be specifically related to H2O2 scavenging and not to a general antioxidant effect, since Trolox® (synthetic analog of alpha-tocopherol) and N-acetyl-cysteine (NAC, a glutathione precursor) treatment did not inhibit cell proliferation (Fig. 2a). Although the proliferative rates of A549 and EKVX were equally inhibited by CAT treatment, a dose–response curve showed that the most aggressive AdC cell line presents a significantly higher resistance to H2O2 toxicity (increase in GI50 value) (P < 0.05) (Fig. 2c). Moreover, sublethal doses of H2O2 (<40 μM) and aminotriazole (a specific catalase inhibitor) can consistently enhance the proliferative rates in EKVX cells (Fig. 2d). Collectively, these data suggest that an intracellular pro-oxidative state accompanies tumor progression and H2O2 plays a major role in cellular aggressiveness in lung AdC.
Cell growth is dependent on H2O2 in human lung adenocarcinoma cell lines. a Exogenous addition of catalase (125–1,000 U/mL) for 72 h causes dose-dependent inhibition in cell proliferation of AdC cell lines. b CAT washout after 48 h of incubation allowed cells to return to its original proliferation rate. c Dose–response curve against H2O2 lung AdC cell lines. d Sublethal doses (<40 μM) of H2O2 and aminotriazole stimulate cell growth on EKVX. Data represent mean ± SEM of at least three independent experiments (n = 3), performed in triplicate *different from respective control group (P < 0.05) (Student’s t test)
HAG is up-regulated in clinical lung adenocarcinoma samples
In order to access the clinical value of the redox imbalance found in vitro, we analyzed differential gene expression levels of HAG components using several microarray datasets derived from human cohorts of lung AdC samples (Table 2). Even though we found collectively a significant difference in HAG activity between groups, the contributions of each subgroup of HAG components (e.g., peroxidases, thiol-containing proteins and superoxide dismutases) were specific to each comparison performed (Table 2). As an example, the peroxidase set of genes was enriched only in tumor samples as compared to healthy tissues (P = 0.022). In contrast, increases in thiol-containing gene set were found to be significantly up-regulated in advanced (III–IV) stages as compared to early (I–II) stages (P = 0.016), and in patient with poor prognosis (death) as compared to good prognosis (alive) (P = 0.005). SOD gene set was found to be up-regulated only in advanced stage of disease. All in all, our metadata analysis showed that HAG components are significantly altered in different aspects of lung tumor cells and have a strong prognostic impact for AdC patients.
Imbalance in redox marker has prognostic value for lung adenocarcinoma patient
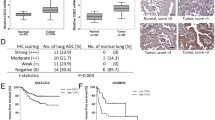
The immunostaining of 4-HNE (a specific end product of lipoperoxidation) was evaluated in a cohort of patients with early-stage lung AdC (Fig. 3a). Table 3 shows the complete description of the patient cohort baseline characteristics, which are well balanced among the staining groups. All baseline conditions are independent from the HNE levels. The moderate immunostaining of 4-HNE was the only parameter significantly associated with higher risk of poor outcome (HR 8.87; 95 % CI 1.04–75.35; P < 0.05) (Fig. 3b).
4-Hydroxynonenal levels in human lung adenocarcinoma samples. a Representative immunostaining of 4-HNE in AdC biopsies is shown. IHC images represent negative controls (absence of primary antibody) (grade 0), weak (grade 1), moderate (grade 2) and strong stain (grade 3). Images are at ×400 magnification. b Cox multivariate regression analysis was used to estimate HR for cohort clinical covariates and 4-HNE levels. CI confidence interval, BMI body mass index, FEV1 forced expiratory volume in 1 s, FVC forced vital capacity
Discussion
In many malignancies, footprints of oxidative damage, the markers of the imbalance between oxidants and antioxidants, have been detected and associated with several cancer-related processes, such as resistance to chemotherapy (Yoo et al. 2008; Wang et al. 2009), angiogenesis (Marikovsky et al. 2002), cellular immortalization (Pani et al. 2010) and cell death (Klamt and Shacter 2005; Circu and Aw 2010). Additionally, we recently described that patients with p53 mutations, the most common mutational status of human cancer and the underlying defect of Li–Fraumeni syndrome (LFS), present high levels of oxidative markers (Macedo et al. 2012). Nonetheless, it is surprising that no antioxidant drug or intervention has been successfully translated to the oncologic clinical setting (Sotgia et al. 2011). So, the specific role played by oxidative stress in human lung adenocarcinoma biology is currently unknown and under investigation. In the present study, we took the advantage of an in vitro cell system to establish an association between tumoral aggressiveness and cellular redox imbalance. Despite intrinsic limitations of in vitro studies (e.g., lack of intercellular interactions), cell lines are reliable experimental models for cancer research since they retain relevant properties of primary tumors (Wistuba et al. 1999). Moreover, we used a bioinformatics tool, the HAG network, to explore the expression levels of major antioxidant system in cell systems and in clinical samples. Finally, we demonstrated that quantification of the oxidative marker 4-hydroxynonenal (an end product of lipoperoxidation) has prognostic value for lung AdC patient outcome.
The redox state in the lung is controlled by complex and cell-specific antioxidant mechanisms. In addition to classical antioxidant enzymes (AOEs) (e.g., SOD, CAT, GPX), human lung tissue expresses several thiol-containing proteins and small molecules, including thioredoxins (TRX1 and TRX2), metallothioneins (MTs 1–4), glutathione (GSH) and peroxidases such as thioredoxin reductases (TRXR1 and TRXR2) and peroxiredoxins (also called thioredoxin peroxidases) (PRXs I–VI), which all contain the amino acid cysteine in their active centers (Blair et al. 1997; Kinnula et al. 2004; Ho et al. 2007). These molecules collectively participate not only in reactions to break down or scavenge H2O2, but also in the regulation of signal transduction pathways. Although dysregulation in these redox processes can be hypothesized to have fundamental role in carcinogenesis, tumor progression and drug resistance, very little is known about their in vivo properties, especially with respect to alteration in their expression and functions in human lung AdC (Lehtonen et al. 2004). As an example, the multifunctional protein thioredoxin (TRX) is responsible for catalyzing protein disulfide reductions. In tumors, TRX increases cell proliferation and resistance of various cells to oxidants and drugs. Moreover, metallothioneins (MTs) are proteins involved in metal binding and free radical scavenging activities, being associated with drug resistance and associated with lung cancer progression and poor patient outcome (Cherian et al. 2003). Corroborating with these, in our study we found both protein families to be enriched in the most aggressive AdC cell line, which could be related to the multidrug resistance presented by EKVX cells, and involved clinically in tumor progression and poor patient outcome. Our data also pointed to the involvement of peroxidase gene set in the initiation of carcinogenesis (tumor vs. normal tissue). The previous study suggests that, in general, human lung AdC may contain increased levels of PRXs, specifically in PRXI, II, IV and VI (Lehtonen et al. 2004). Moreover, in the same study, PRX II expression was shown to be associated with advanced tumor stage (IIB–IV) in lung AdC, corroborating with our data that showed enrichment in PRXII expression in the most aggressive cell line.
To overcome the oxidizing microenvironment of lung tissue, it was thought that malignant cells have overexpression of antioxidant defenses. However, our data and from others (Laurent et al. 2005; Myung et al. 2010) show that high levels of oxidative markers are present in cancer cells and are associated with tumoral aggressiveness (Chaiswing et al. 2007; Jorgenson et al. 2013). Chemically, oxidative stress is associated with increased production of oxidizing species or a significant decrease in the effectiveness of antioxidant defenses and repair systems. Moreover, some RS act as cellular messengers (e.g., H2O2). Thus, oxidative stress can cause disruptions in normal mechanisms of cellular signaling. In our study, the stimulatory effect of RS in tumor growth seems to be specifically related to H2O2, because we could not find any effects of the supplementation of NAC and Trolox® on the proliferation rates of AdC cells. In this context, it was already suggested that tumors could not properly detoxify H2O2 (Coursin et al. 1996). This specific physiological oxidant can stimulate cell proliferation (Burhans and Heintz 2009), migration and invasion (Polytarchou et al. 2005; Connor et al. 2007) and is involved with the resistance against chemotherapy (Yoo et al. 2008; Wang et al. 2009). We showed that both lung AdC cell lines are highly dependent on H2O2 to proliferate, since we could not find any differences between the two cell lines in relation to the growth inhibition in response to CAT treatment and the stimulatory effect of the CAT inhibitor AMT. Moreover, the most aggressive cell line (EKVX) has increased levels of oxidative markers, generates higher steady-state levels of H2O2 and, more importantly, presents an additional stimulatory effect in the proliferation rate in response to sublethal doses of H2O2. All in all, collectively our data support that an imbalance in redox status is important for the pathological homeostasis of lung AdC and is associated with tumor progression. So, in fully developed cancer cells, the generation of high rates of reactive oxygen species may act as a driving force to induce oxidative damage to lipids, mutations in DNA bases and posttranslational modifications in proteins, contributing so to the genetic instability and metastatic potential of tumor cells (Cairns et al. 2011).
Despite the positive correlation between tumor aggressiveness and oxidative stress demonstrated here in vitro, we found a bell-shape curve effect of the oxidative stress marker 4-HNE in predicting the outcome of early-stage lung AdC patients. Only moderate levels of 4-HNE were significantly associated with poor patient outcome. The biological effects of oxidative stress depend upon the size of these changes, with a cell system being able to overcome small perturbations by inducing the expression of AOE and regain its original state, a process known as cellular adaptation. However, more severe oxidative stress or chronic exposure to oxidants can cause extensive cellular damage, leading to cell death by apoptosis or necrosis (Klamt et al. 2009) (Englert and Shacter 2002). Possibly, strong 4-HNE levels in tumors reflect an extremely increased ROS level that reaches toxic effect in cellular functions. On the other hand, moderate levels of 4-HNE possibly reflect the stimulatory level where high amount of RS fuels malignant features specifically in tumor cells (Lisanti et al. 2011). Even though, to our knowledge, this is the first demonstration of the prognostic role of 4-HNE levels in lung AdC, unfortunately, the determination of RS levels and oxidative markers does not provide mechanistic insight concerning cancer development and progression.
A recent meta-analysis of randomized controlled trials that evaluated the efficacy of antioxidant supplementation in cancer indicated that there is no clinical evidence to support preventive effect of antioxidant supplementation (Myung et al. 2010). Moreover, the relationship of redox imbalances with different aspects of cancer biology can be systematically studied with the use of high-throughput experimental tools, such as redox proteome (Klamt et al. 2009) or differential gene expression levels of the HAG network with microarray data (Gelain et al. 2009). Along with other studies, we support the idea that compounds with H2O2-scavenging capacity might be a good approach for cancer management. As already shown in the literature, catalase overexpression reverted malignant features in different cell lines (Policastro et al. 2004) and prevented tumor growth and metastasis in mouse lung (Nishikawa et al. 2009). Moreover, mitochondrial-targeted catalase suppresses invasive breast cancer in mice (Goh et al. 2011), and the role of catalase has already been established for in vivo models (Nishikawa et al. 2009) and should be further considered for human clinical trials in lung AdC patients.
In summary, we demonstrated an association between redox imbalance and tumor aggressiveness in human lung adenocarcinoma samples. To our knowledge, this is the first study suggesting 4-HNE as a possible prognostic marker. Thereby, it seems plausible that imbalance in redox metabolism is pivotal to tumor malignancy, and besides consistent evidence of increased oxidative stress exists for lung cancer patients, future studies should focus on the specific mechanism of redox imbalances that mediates different aspects of tumor aggressiveness for the improvement of cancer therapy.
References
Blair SL, Heerdt P, Sachar S et al (1997) Glutathione metabolism in patients with non-small cell lung cancers. Cancer Res 57:152–155
Brennan P, Fortes C, Butler J et al (2000) A multicenter case–control study of diet and lung cancer among non-smokers. Cancer Causes Control 11:49–58
Burhans WC, Heintz NH (2009) The cell cycle is a redox cycle: linking phase-specific targets to cell fate. Free Radic Biol Med 47:1282–1293. doi:10.1016/j.freeradbiomed.2009.05.026
Cairns RA, Harris IS, Mak TW (2011) Regulation of cancer cell metabolism. Nat Rev Cancer 11:85–95. doi:10.1038/nrc2981
Castro MAA, Filho JLR, Dalmolin RJS et al (2009) ViaComplex: software for landscape analysis of gene expression networks in genomic context. Bioinformatics 25:1468–1469. doi:10.1093/bioinformatics/btp246
Castro MAA, Dal-Pizzol F, Zdanov S et al (2010) CFL1 expression levels as a prognostic and drug resistance marker in nonsmall cell lung cancer. Cancer 116:3645–3655. doi:10.1002/cncr.25125
Chaiswing L, Bourdeau-Heller JM, Zhong W, Oberley TD (2007) Characterization of redox state of two human prostate carcinoma cell lines with different degrees of aggressiveness. Free Radic Biol Med 43:202–215. doi:10.1016/j.freeradbiomed.2007.03.031
Chan HP, Tran V, Lewis C, Thomas PS (2009) Elevated levels of oxidative stress markers in exhaled breath condensate. J Thorac Oncol 4:172–178. doi:10.1097/JTO.0b013e3181949eb9
Cherian MG, Jayasurya A, Bay B-H (2003) Metallothioneins in human tumors and potential roles in carcinogenesis. Mut Res 533:201–209. doi:10.1016/j.mrfmmm.2003.07.013
Circu ML, Aw TY (2010) Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med 48:749–762. doi:10.1016/j.freeradbiomed.2009.12.022
Coate LE, John T, Tsao M-S, Shepherd FA (2009) Molecular predictive and prognostic markers in non-small-cell lung cancer. Lancet Oncol 10:1001–1010. doi:10.1016/S1470-2045(09)70155-X
Connor KM, Hempel N, Nelson KK et al (2007) Manganese superoxide dismutase enhances the invasive and migratory activity of tumor cells. Cancer Res 67:10260–10267. doi:10.1158/0008-5472.CAN-07-1204
Coursin DB, Cihla HP, Sempf J et al (1996) An immunohistochemical analysis of antioxidant and glutathione S-transferase enzyme levels in normal and neoplastic human lung. Histol Histopathol 11:851–860
Dresch MTK, Rossato SB, Kappel VD et al (2009) Optimization and validation of an alternative method to evaluate total reactive antioxidant potential. Anal Biochem 385:107–114. doi:10.1016/j.ab.2008.10.036
Englert RP, Shacter E (2002) Distinct modes of cell death induced by different reactive oxygen species: amino acyl chloramines mediate hypochlorous acid-induced apoptosis. J Biol Chem 277(23):20518–20526. doi:10.1074/jbc.M200212200
Esme H, Cemek M, Sezer M et al (2008) High levels of oxidative stress in patients with advanced lung cancer. Respirology 13:112–116. doi:10.1111/j.1440-1843.2007.01212.x
Gelain DP, Dalmolin RJS, Belau VL et al (2009) A systematic review of human antioxidant genes. Front Biosci (Landmark Ed) 14:4457–4463
Goh J, Enns L, Fatemie S et al (2011) Mitochondrial targeted catalase suppresses invasive breast cancer in mice. BMC Cancer 11:191. doi:10.1186/1471-2407-11-191
Ho JC, Chan-Yeung M, Ho SP et al (2007) Disturbance of systemic antioxidant profile in nonsmall cell lung carcinoma. Eur Respir J 29:273–278. doi:10.1183/09031936.00000106
Ilonen IK, Räsänen JV, Sihvo EI et al (2009) Oxidative stress in non-small cell lung cancer: role of nicotinamide adenine dinucleotide phosphate oxidase and glutathione. Acta Oncol 48:1054–1061. doi:10.1080/02841860902824909
Jaruga P, Zastawny TH, Skokowski J et al (1994) Oxidative DNA base damage and antioxidant enzyme activities in human lung cancer. FEBS Lett 341:59–64
Jorgenson TC, Zhong W, Oberley TD (2013) Redox imbalance and biochemical changes in cancer. Cancer Res 73:6118–6123. doi:10.1158/0008-5472.CAN-13-1117
Kaynar H, Meral M, Turhan H et al (2005) Glutathione peroxidase, glutathione-S-transferase, catalase, xanthine oxidase, Cu–Zn superoxide dismutase activities, total glutathione, nitric oxide, and malondialdehyde levels in erythrocytes of patients with small cell and non-small cell lung cancer. Cancer Lett 227:133–139. doi:10.1016/j.canlet.2004.12.005
Kinnula VL, Pääkkö P, Soini Y (2004) Antioxidant enzymes and redox regulating thiol proteins in malignancies of human lung. FEBS Lett 569:1–6. doi:10.1016/j.febslet.2004.05.045
Klamt F, Shacter E (2005) Taurine chloramine, an oxidant derived from neutrophils, induces apoptosis in human B lymphoma cells through mitochondrial damage. J Biol Chem 280:21346–21352. doi:10.1074/jbc.M501170200
Klamt F, Zdanov S, Levine RL et al (2009) Oxidant-induced apoptosis is mediated by oxidation of the actin-regulatory protein cofilin. Nat Cell Biol 11:1241–1246. doi:10.1038/ncb1968
Laurent A, Nicco C, Chereau C et al (2005) Controlling tumor growth by modulating endogenous production of reactive oxygen species. Cancer Res 65:948–956
Lehtonen ST, Svensk A-M, Soini Y et al (2004) Peroxiredoxins, a novel protein family in lung cancer. Int J Cancer 111:514–521. doi:10.1002/ijc.20294
Lisanti MP, Martinez-Outschoorn UE, Lin Z et al (2011) Hydrogen peroxide fuels aging, inflammation, cancer metabolism and metastasis: the seed and soil also needs “fertilizer”. Cell Cycle 10:2440–2449
Macedo GS, Lisbôa da Motta L, Giacomazzi J et al (2012) Increased oxidative damage in carriers of the germline TP53 p. R337H mutation. PLoS One 7:e47010. doi:10.1371/journal.pone.0047010
Marikovsky M, Nevo N, Vadai E, Harris-Cerruti C (2002) Cu/Zn superoxide dismutase plays a role in angiogenesis. Int J Cancer 97:34–41. doi:10.1002/ijc.1565
Melloni B, Lefebvre MA, Bonnaud F et al (1996) Antioxidant activity in bronchoalveolar lavage fluid from patients with lung cancer. Am J Respir Crit Care Med 154:1706–1711. doi:10.1164/ajrccm.154.6.8970359
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Myung S-K, Kim Y, Ju W et al (2010) Effects of antioxidant supplements on cancer prevention: meta-analysis of randomized controlled trials. Ann Oncol 21:166–179. doi:10.1093/annonc/mdp286
Nishikawa M, Hashida M, Takakura Y (2009) Catalase delivery for inhibiting ROS-mediated tissue injury and tumor metastasis. Adv Drug Deliv Rev 61:319–326. doi:10.1016/j.addr.2009.01.001
Pani G, Galeotti T, Chiarugi P (2010) Metastasis: cancer cell’s escape from oxidative stress. Cancer Metastasis Rev 29:351–378. doi:10.1007/s10555-010-9225-4
Policastro L, Molinari B, Larcher F et al (2004) Imbalance of antioxidant enzymes in tumor cells and inhibition of proliferation and malignant features by scavenging hydrogen peroxide. Mol Carcinog 39:103–113. doi:10.1002/mc.20001
Polytarchou C, Hatziapostolou M, Papadimitriou E (2005) Hydrogen peroxide stimulates proliferation and migration of human prostate cancer cells through activation of activator protein-1 and up-regulation of the heparin affin regulatory peptide gene. J Biol Chem 280:40428–40435. doi:10.1074/jbc.M505120200
Rahman I, van Schadewijk AAM, Crowther AJL et al (2002) 4-Hydroxy-2-nonenal, a specific lipid peroxidation product, is elevated in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 166:490–495. doi:10.1164/rccm.2110101
Rahman I, Biswas SK, Kode A (2006) Oxidant and antioxidant balance in the airways and airway diseases. Eur J Pharmacol 533:222–239. doi:10.1016/j.ejphar.2005.12.087
Sánchez PG, Vendrame GS, Madke GR et al (2006) Lobectomy for treating bronchial carcinoma: analysis of comorbidities and their impact on postoperative morbidity and mortality. J Bras Pneumol 32:495–504. doi:10.1590/S1806-37132006000600005
Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63:11–30. doi:10.3322/caac.21166
Sotgia F, Martinez-Outschoorn UE, Lisanti MP (2011) Mitochondrial oxidative stress drives tumor progression and metastasis: should we use antioxidants as a key component of cancer treatment and prevention? BMC Med 9:62. doi:10.1186/1741-7015-9-62
Subramanian A, Tamayo P, Mootha VK et al (2005) Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 102:15545–15550. doi:10.1073/pnas.0506580102
Vichai V, Kirtikara K (2006) Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc 1:1112–1116. doi:10.1038/nprot.2006.179
Wang D, Xiang D-B, Yang X-Q et al (2009) APE1 overexpression is associated with cisplatin resistance in non-small cell lung cancer and targeted inhibition of APE1 enhances the activity of cisplatin in A549 cells. Lung Cancer 66:298–304. doi:10.1016/j.lungcan.2009.02.019
Wendel A (1981) Glutathione peroxidase. Methods Enzymol 77:325–333
Wistuba II, Bryant D, Behrens C et al (1999) Comparison of features of human lung cancer cell lines and their corresponding tumors. Clin Cancer Res 5:991–1000
Yoo DG, Song YJ, Cho EJ et al (2008) Alteration of APE1/ref-1 expression in non-small cell lung cancer: the implications of impaired extracellular superoxide dismutase and catalase antioxidant systems. Lung Cancer 60:277–284. doi:10.1016/j.lungcan.2007.10.015
Acknowledgments
Brazilian funds MCT/CNPq Universal (470306/2011-4), PRONEX/FAPERGS (1000274), PRONEM/FAPERGS (11/2032-5), PqG/FAPERGS (2414-2551/12-8) and MCT/CNPq INCT-TM (573671/2008-7) provided the financial support without interference in the ongoing work.
Conflict of interest
The authors declare none.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lisbôa da Motta, L., Müller, C.B., De Bastiani, M.A. et al. Imbalance in redox status is associated with tumor aggressiveness and poor outcome in lung adenocarcinoma patients. J Cancer Res Clin Oncol 140, 461–470 (2014). https://doi.org/10.1007/s00432-014-1586-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-014-1586-6