Abstract
Purpose
Hypertension, hyperglycemia, and overweight are considered associated with the development and prognosis of prostate cancer (PCa). This study is aimed at investigating the association between pre-existing hypertension, hyperglycemia, and overweight and the overall survival (OS) of PCa patients receiving androgen deprivation therapy (ADT).
Methods
We studied the clinical data of 323 patients of PCa receiving ADT in our hospital from January 2003 to August 2012 aged 50–91. The association between OS and hypertension, hyperglycemia, or overweight, both separately and together, was analyzed via Kaplan–Meier method. The distributions of clinicopathological features among groups were evaluated using Fisher’s exact or chi-square test.
Results
23 men (7.12 %) were lost to follow-up during this study. During a median follow-up for 43 months (range 3–119 months), 122 deaths (40.67 %) were confirmed. The five-year OS rate of men with both hypertension and overweight (28.57 %) was significantly lower than that of control group (48.33 %, P = 0.024). It was also moderately lower than that of men just with hypertension (50.00 %, P = 0.095) or overweight (55.56 %, P = 0.088). Men with both hyperglycemia and overweight had significantly shorter survival time than control group (P = 0.037). The distributions of clinical information were similar among all the groups except that overweight patients had a lower proportion of PSA level over 20 ng/mL (65.38 %) than control group (84.95 %, P = 0.026).
Conclusions
Pre-existing hypertension, hyperglycemia, and overweight were associated with poor prognosis of PCa patients. Men with both hypertension and overweight, or with both hyperglycemia and overweight had significantly shorter survival time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (PCa) is one of the most frequently diagnosed malignant tumors among men worldwide (Siegel et al. 2012). The American Cancer Society estimates that in 2011, 240,890 men were diagnosed with PCa and 33,720 men died of it in United States (Brawley 2012). Because of westernization in life style and dietary habits, the prevalence of PCa has been increasing in China and it has become a major public health problem (Gu 2003). The prevalence of metabolic diseases, like hypertension, diabetes mellitus, and obesity, are also increasing in China (Lao et al. 2012; Yang et al. 2010; Wang et al. 2007). Some studies suggested that hypertension, hyperglycemia, and overweight were associated with an elevated risk and the developing of PCa (Fitzpatrick et al. 2001; Lee et al. 2012; Beebe-Dimmer et al. 2007). Besides, obesity and diabetes mellitus were reported to increase the overall mortality of PCa individually (Efstathiou et al. 2007; Smith et al. 2008). Thus, hypertension, hyperglycemia, or overweight might be closely associated with PCa, acting as not only a risk factor, but also a negative prognostic factor.
Early detection of PCa is limited in China since prostate-specific antigen (PSA) screening is not commonly practiced, resulting in that a large portion of patients are found with relatively high-grade or advanced-stage PCa upon diagnosis. Allowing for the fact that a large portion of these patients are beyond the indication of radical prostatectomy, androgen deprivation therapy (ADT) is more commonly practiced in China than that in western countries among patients suffering from locally advanced or metastatic PCa. ADT is effective at suppressing PSA production, stabilizing disease, alleviating symptoms, and potentially prolonging survival. However, previous studies indicated that ADT would contribute to metabolic disorder, like hypertension, hyperglycemia, and overweight, which make the overall survival (OS) of ADT patients equal to those without ADT (Braga-Basaria et al. 2006; Saylor and Smith 2009). This unpleasant fact brought us in a dilemma that patients would have a decreased risk of dying from PCa after ADT, while they would have an elevated risk of dying from hypertension, hyperglycemia, or overweight. However, it is unclear about the association between OS and pre-existing hypertension, hyperglycemia, or overweight, both separately and together, among PCa patients receiving ADT.
The objective of this study was to investigate the association between OS and pre-existing hypertension, hyperglycemia, and overweight, both separately and together, among PCa patients receiving ADT.
Materials and methods
Study population
We thoroughly studied the 393 patients of PCa receiving ADT at the Department of Urology of Huashan Hospital, Fudan University, Shanghai, PR China, from January 2003 to August 2012. All cases were diagnosed with PCa through needle biopsy (ultrasound-guided transperineal needle biopsy of prostate, 10-core biopsy). A total of 323 patients with integrated clinical information, such as serum PSA level, Gleason score, imaging data of ultrasonography, computed tomography (CT) or magnetic resonance imaging (MRI), and bone scan with emission computed tomography (ECT), were included in this study. All subjects were ethnic Han Chinese and permanent residents of Shanghai, Southern China. Each subject was informed about the aims and requirements of this study, and informed consent for participation was obtained in accordance with institutional guidance at Huashan Hospital, Fudan University. A structured questionnaire was completed by interviewers, in order to collect information on clinical data. Two independent pathologists blindly determined pathological grading of biopsied specimens simultaneously, and a consensus grading was reached for each score.
Clinical measurements
Clinical information of patients, such as height, weight, blood pressure, fasting serum plasma glucose, history of hypertension, and diabetes mellitus, were collected on admission. The most recent serum PSA levels were selected in analysis. Hyperglycemia is defined as elevated fasting plasma glucose level (≥5.6 mmol/L) or pharmacological treatment for type 2 diabetes mellitus. Hypertension is defined as elevated blood pressure (≥130/85 mmHg) or pharmacological treatment for hypertension. And men with BMI ≥ 25 kg/m2 are considered overweight, based on the definition established by the World Health Organization (WHO). Pathological profiles were classified according to the American Joint Committee on Cancer (AJCC 2009). The definition of organ-confined PCa is based on pathology of clinical tumor staging of T1 or T2 without nodal involvement or metastatic disease, while advanced-stage PCa is based on pathology of clinical tumor staging of locally to regionally advanced tumor (T3 or T4), nodal involvement, or metastatic disease.
Follow-up data collection
Follow-up started on the day of ADT and concluded on the date of death or the date of last follow-up for patients still alive. Patients were generally contacted by phone every three months. All deaths were confirmed using both the Shanghai Medical Insurance System and information from Centers for Disease Control (CDC).
Statistical analysis
Patients’ age and BMI were expressed as mean ± SD, and two-way analysis of variance was used for comparison among all the groups for parametric analysis. The differences in the distributions of clinicopathological features (disease grade stratified as poorly differentiated (Gleason > 7), moderately differentiated (Gleason = 7), and well differentiated (Gleason < 7); PSA level stratified as low group (≤20 or ≤50 ng/mL) and high group (>20 or >50 ng/mL); clinical stage stratified according to AJCC TNM system) among different groups were evaluated using Fisher’s exact or chi-square nonparametric analysis. OS was assessed with Kaplan–Meier method and the results compared with the logrank test. Statistical analyses were processed using SPSS version 17.0 for Windows (SPSS Inc., Chicago, IL, USA); P < 0.05 was considered statistically significant.
Results
Patient characteristics
Among the 323 eligible subjects, 23 men (7.12 %) were lost to follow-up. Table 1 presents the basic information of all the 300 men included in our study. The average age was 73.5 ± 7.7 on admission. Patients’ Gleason score ranged from 4 to 10 (median 7). Each patient had at least one of the three prostate imaging records of ultrasonography (n = 268, 89.33 %), MRI (n = 79, 26.33 %), or CT (n = 41, 13.67 %). Besides, all the patients had detailed records about ECT, PSA level, pathology from prostate needle biopsy, Gleason score, and clinical stage information. A total of 235 patients (78.33 %) had records about prostate volume with an average of 51.0 ± 27.3 mL.
Control group was defined as men without hyperglycemia, hypertension, or overweight (BMI ≥ 25). Cases were defined as men with hyperglycemia, hypertension, or overweight. Figure 1 illustrates the distribution of all the cases included. Eighty-four men (28.00 %) were identified as overweight, 76 men (25.33 %) with hyperglycemia, and 152 men (50.67 %) with hypertension. Among them, 69 men (23.00 %) had two metabolic abnormalities (including 8 men (2.67 %) with both overweight and hyperglycemia, 32 men (10.67 %) with both overweight and hypertension, and 29 men (9.67 %) with both hyperglycemia and hypertension). Additionally, 18 men (6.00 %) had all the three metabolic abnormalities.
PSA level, Gleason score and clinical stage
Table 2 shows patients’ information of PSA level, Gleason score and clinical stage on admission. Among all the 300 patients, 239 men (79.67 %) had PSA levels of over 20 ng/mL, and 172 men’s (57.33 %) PSA level exceeded 50 ng/mL. Thirty-seven men (12.33 %) had a Gleason score of 2–6, 115 men (38.33 %) had a Gleason score of 7, and nearly half (n = 148, 49.33 %) of all the patients had a Gleason score of 8–10. A total of 214 men (71.33 %) were identified as advanced stage based on pathology of clinical tumor staging of locally to regionally advanced tumor (T3 or T4), nodal involvement, or metastatic disease. Significant differences were rarely seen in different groups except that overweight patients had a lower proportion of PSA level over 20 ng/mL (65.38 %, P = 0.026) than controls (84.95 %) and patients with both hypertension and overweight (87.50 %, P = 0.045).
Overall survival
Our follow-up rate was 92.88 % during the whole follow-up with median of 43 months. A total of 122 men (40.67 %) died during this study from PCa, heart attack, stroke, respiratory failure, etc., including 32 men (10.67 %) in control group, 9 (3.00 %) in overweight group, 30 (10.00 %) in hypertension group, 9 (3.00 %) in hyperglycemia group, 16 (5.33 %) in hypertension and overweight group, 4 (1.33 %) in hyperglycemia and overweight group, 13 (4.33 %) in blood pressure abnormal and hyperglycemia group, and 9 (3.00 %) in all the three metabolic abnormalities group. As presented in Table 3, the total one-year OS rate was estimated at 96.80 % and the five-year OS rate was 47.17 %. No statistically significant difference of one-year OS rate was observed among different groups. However, the five-year OS rate of men with both hypertension and overweight (28.57 %) was significantly lower than that of control group (48.33 %, P = 0.024). It was also moderately lower than that of men just with hypertension (50.00 %, P = 0.095) or overweight (55.56 %, P = 0.088).
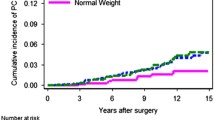
Figure 2a illustrates the OS curves for all the groups. Figure 2b illustrates the comparison of OS curves between hyperglycemia and overweight group and control group. Men with both hyperglycemia and overweight had significant shorter survival time than control group (P = 0.037). Figure 2c illustrates the comparison of OS curves between hyperglycemia and overweight group, hypertension and overweight group, and hyperglycemia and hypertension group. Men with both hyperglycemia and overweight had significantly shorter survival time than men with both hypertension and overweight (P = 0.009), or men with hypertension and hyperglycemia (P = 0.001). There was no significant difference among OS curves of all the three metabolic abnormalities group and other groups.
Overall survival curves of different groups. a Overall survival curves for all the groups containing metabolic disorder or not. b Comparison of overall survival curves between elevated glucose level and overweight group and control group c comparison of overall survival curves between elevated glucose level and overweight group, elevated blood pressure and overweight group, and elevated glucose level and elevated blood pressure group
Discussion
Development and prognosis of PCa is closely associated with hypertension, hyperglycemia, or overweight. In our research, we found that one-year OS after ADT was similar among different groups. However, the five-year OS rate of men with both hypertension and overweight (28.57 %) was significantly lower than that of control group (48.33 %, P = 0.024). It was also moderately lower than men just with hypertension (50.00 %, P = 0.095) or overweight (55.56 %, P = 0.088) with limited significance. Though pre-existing hypertension or overweight would not decrease five-year OS rate of PCa men individually, the combination of these two metabolic abnormalities was negatively associated with patients’ prognosis. Due to our limited population size, further study should be carried out. In survival analysis, we found that men with both hyperglycemia and overweight had significantly shorter survival time than control group (P = 0.037). What’s more, we compared the OS curves of men with both hyperglycemia and overweight and men with both hypertension and overweight, or men with both hypertension and hyperglycemia. Interestingly, we found that the combination of hyperglycemia and overweight was observed with the shortest survival time (P = 0.009 and P = 0.001 correspondingly).
It was noteworthy that we examined the association between OS of PCa patients and pre-existing hypertension, hyperglycemia, or overweight, both separately and together. Several previous studies examined the association of PCa mortality and overweight or diabetes mellitus individually. Rodriguez et al. found that overweight (relative risk (RR) 1.05, 95 % confidence interval (CI) 0.98–1.12), especially obesity (RR 1.21, CI 1.07–1.37) was associated with an increased PCa mortality (Rodriguez et al. 2001). The study by Smith et al. found an increased mortality among men with diabetes mellitus (hazard ratio: 1.77, 95 % CI 1.45–2.16). No studies examining the association between hypertension and survival of PCa patients were published before. There was an interesting finding in our study that pre-existing hypertension, hyperglycemia, and overweight had slightly longer survival time than that of control group individually. Further study is needed to investigate the association in larger scale.
Previous studies reported that patients after ADT were expected to have a higher prevalence of metabolic disorder (Braga-Basaria et al. 2006), which contribute to reduced expected benefits of ADT. Also an elevated risk of coronary heart disease, myocardial infarction, and life-threatening ventricular arrhythmia was expected, a significant increase in risk of sudden cardiac death and serious cardiovascular morbidity was observed after ADT (Saigal et al. 2007). Our findings suggested that pre-existing hypertension, hyperglycemia, or overweight were also significant risk factors for men’s prognosis after ADT, which further confirmed the close association between metabolic disorder and long-time survival of PCa patients receiving ADT.
Clinical information of cancer grade and stage were statistically non-significant among all the groups in our study. We found that men receiving ADT with pre-existing hypertension, hyperglycemia, or overweight had similar chances to develop aggressive PCa. However, several studies reported that some metabolic abnormalities might result in more aggressive PCa. Men with diabetes mellitus might have had a significantly higher percentage of high-grade tumors among patients undergoing biopsy (Moreira et al. 2011) and radical prostatectomy (Abdollah et al. 2011). Men with higher BMI were also found less likely to be diagnosed with localized or low-grade cancer, but were more likely to be diagnosed with localized high-grade disease or metastatic disease (Rodriguez et al. 2007; Wright et al. 2007; Gong et al. 2006). It was also confirmed in the previous meta-analysis that the risk of developing advanced PCa elevated with BMI (RR 1.12 per 5 kg/m2 increment, 95 % CI 1.01–1.23) (MacInnis and English 2006). PCa is one of clinically low-aggressive tumors. However, men having ADT usually had relatively high-grade tumors, excluding a large portion of low-grade ones. Different from these studies, men included in our study were generally confined to just part of the whole population, which were quite severer than those undergoing prostate needle biopsy or radical prostatectomy, contributing to our statistically non-significant finding.
In the study, we found that overweight men (BMI ≥ 25) had a significantly lower proportion of PSA level over 20 ng/mL than control group (P = 0.026). Similar findings were also reported in other studies (Baillargeon et al. 2005; Barqawi et al. 2005) with unclear reason so far. One potential reason is that less PSA production is due to lower testosterone levels among men with higher BMI, since PSA production is under direct testosterone control (Prins 2000). Besides, men with higher BMI have greater plasma volume, which may result in hemodilution, thus lowering the serum PSA levels (Banez et al. 2007). However, overweight men shared similar Gleason score or clinical stage with controls. So merely relatively lower PSA levels did not signify less aggressiveness, but might delay the detection of PCa since it would make men with higher BMI less likely to have an abnormal PSA test, and therefore, less prostate needle biopsy would be performed.
In conclusion, we found that pre-existing hypertension, hyperglycemia, or overweight would significantly affect patients’ OS after ADT separately. But men with both hypertension and overweight, or with both hyperglycemia and overweight had significantly shorter survival time than men without metabolic disorder.
There are two limitations of our study that should be taken into consideration. First, the sample size of our study is relatively small because of short follow-up period and missing data of patients. Our findings should be further discussed by multi-center study in large scale. Second, in this study, we discovered the phenomenon that metabolic disorders, like overweight, hypertension, and hyperglycemia were associated with poor overall survival of PCa patients after ADT. Potential mechanisms need to be uncovered and clarified. Further study should be carried out to investigate whether better control of metabolic status, as well as other medical therapies, will be helpful to the overall survival need.
References
Abdollah F, Briganti A, Suardi N, Gallina A, Capitanio U, Salonia A, Cestari A, Guazzoni G, Rigatti P, Montorsi F (2011) Does diabetes mellitus increase the risk of high-grade prostate cancer in patients undergoing radical prostatectomy? Prostate Cancer Prostatic Dis 14(1):74–78. doi:10.1038/pcan.2010.41
Baillargeon J, Pollock BH, Kristal AR, Bradshaw P, Hernandez J, Basler J, Higgins B, Lynch S, Rozanski T, Troyer D, Thompson I (2005) The association of body mass index and prostate-specific antigen in a population-based study. Cancer 103(5):1092–1095. doi:10.1002/cncr.20856
Banez LL, Hamilton RJ, Vollmer RT, Moul JW, Amling CL, Kane CJ, Aronson WJ, Terris MK, Presti JC, Freedland SJ (2007) Can hemodilution explain the lower PSA concentrations among obese men? J Urol 177(4):468
Barqawi AB, Golden BK, O’Donnell C, Brawer MK, Crawford ED (2005) Observed effect of age and body mass index on total and complexed PSA: analysis from a national screening program. Urology 65(4):708–712. doi:10.1016/j.urology.2004.10.074
Beebe-Dimmer JL, Dunn RL, Sarma AV, Montie JE, Cooney KA (2007) Features of the metabolic syndrome and prostate cancer in African-American men. Cancer 109(5):875–881. doi:10.1002/cncr.22461
Braga-Basaria M, Dobs AS, Muller DC, Carducci MA, John M, Egan J, Basaria S (2006) Metabolic syndrome in men with prostate cancer undergoing long-term androgen-deprivation therapy. J Clin Oncol 24(24):3979–3983. doi:10.1200/jco.2006.05.9741
Brawley OW (2012) Prostate cancer epidemiology in the United States. World J Urol 30(2):195–200. doi:10.1007/s00345-012-0824-2
Efstathiou JA, Bae K, Shipley WU, Hanks GE, Pilepich MV, Sandler HM, Smith MR (2007) Obesity and mortality in men with locally advanced prostate cancer—Analysis of RTOG 85–31. Cancer 110(12):2691–2699. doi:10.1002/cncr.23093
Fitzpatrick AL, Daling JR, Furberg CD, Kronmal RA, Weissfeld JL (2001) Hypertension, heart rate, use of antihypertensives, and incident prostate cancer. Ann Epidemiol 11(8):534–542. doi:10.1016/s1047-2797(01)00246-0
Gong Z, Neuhouser ML, Goodman PJ, Albanes D, Chi C, Hsing AW, Lippman SM, Platz EA, Pollak MN, Thompson IM, Kristal AR (2006) Obesity, diabetes, and risk of prostate cancer: results from the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev 15(10):1977–1983. doi:10.1158/1055-9965.EPI-06-0477
Gu FL (2003) Changing constituents of genitourinary cancer in recent 50 years in Beijing. Chin Med J 116(9):1391–1393
Lao XQ, Zhang YH, Wong MCS, Xu YJ, Xu HF, Nie SP, Ma WJ, Thomas GN, Yu ITS (2012) The prevalence of metabolic syndrome and cardiovascular risk factors in adults in southern China. BMC Public Health 12. doi:10.1186/1471-2458-12-64
Lee MY, Lin KD, Hsiao PJ, Shin SJ (2012) The association of diabetes mellitus with liver, colon, lung, and prostate cancer is independent of hypertension, hyperlipidemia, and gout in Taiwanese patients. Metabolism 61(2):242–249. doi:10.1016/j.metabol.2011.06.020
MacInnis RJ, English DR (2006) Body size and composition and prostate cancer risk: systematic review and meta-regression analysis. Cancer causes control 17(8):989–1003. doi:10.1007/s10552-006-0049-z
Moreira DM, Anderson T, Gerber L, Thomas JA, Banez LL, McKeever MG, Hoyo C, Grant D, Jayachandran J, Freedland SJ (2011) The association of diabetes mellitus and high-grade prostate cancer in a multiethnic biopsy series. Cancer Causes Control 22(7):977–983. doi:10.1007/s10552-011-9770-3
Prins GS (2000) Molecular biology of the androgen receptor. Mayo Clin Proc 75(Suppl):S32–S35
Rodriguez C, Patel AV, Calle EE, Jacobs EJ, Chao A, Thun MJ (2001) Body mass index, height, and prostate cancer mortality in two large cohorts of adult men in the United States. Cancer Epidemiol Biomark Prev 10(4):345–353
Rodriguez C, Freedland SJ, Deka A, Jacobs EJ, McCullough ML, Patel AV, Thun MJ, Calle EE (2007) Body mass index, weight change, and risk of prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev 16(1):63–69. doi:10.1158/1055-9965.EPI-06-0754
Saigal CS, Gore JL, Krupski TL, Hanley J, Schonlau M, Litwin MS (2007) Androgen deprivation therapy increases cardiovascular morbidity in men with prostate cancer. Cancer 110(7):1493–1500. doi:10.1002/cncr.22933
Saylor PJ, Smith MR (2009) Metabolic complications of androgen deprivation therapy for prostate cancer. J Urol 181(5):1998–2006. doi:10.1016/j.juro.2009.01.047
Siegel R, Naishadham D, Jemal A (2012) Cancer Statistics. CA Cancer J Clin 62(1):10–29. doi:10.3322/caac.20138
Smith MR, Bae K, Efstathiou JA, Hanks GE, Pilepich MV, Sandler HM, Shipley WU (2008) Diabetes and mortality in men with locally advanced prostate cancer: RTOG 92–02. J Clin Oncol 26(26):4333–4339. doi:10.1200/jco.2008.16.5845
Wang Y, Mi J, Shan XY, Wang QJ, Ge KY (2007) Is China facing an obesity epidemic and the consequences? The trends in obesity and chronic disease in China. Int J Obes 31(1):177–188. doi:10.1038/sj.ijo.0803354
Wright ME, Chang SC, Schatzkin A, Albanes D, Kipnis V, Mouw T, Hurwitz P, Hollenbeck A, Leitzmann MF (2007) Prospective study of adiposity and weight change in relation to prostate cancer incidence and mortality. Cancer 109(4):675–684. doi:10.1002/cncr.22443
Yang WY, Lu JM, Weng JP, Jia WP, Ji LN, Xiao JZ, Shan ZY, Liu J, Tian HM, Ji QH, Zhu DL, Ge JP, Lin LX, Chen L, Guo XH, Zhao ZG, Li Q, Zhou ZG, Shan GL, He J, China Natl Diabet Metab D (2010) Prevalence of diabetes among men and women in china. N Engl J Med 362(12):1090–1101. doi:10.1056/NEJMoa0908292
Acknowledgments
All authors declare there is no conflict of interest. This work received the grant from National Natural Science Foundation of China (NSFC), No. 81272835.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Hua Xu, Li-min Zhang and Jun Liu contributed to the manuscript equally.
Rights and permissions
About this article
Cite this article
Xu, H., Zhang, Lm., Liu, J. et al. The association between overall survival of prostate cancer patients and hypertension, hyperglycemia, and overweight in Southern China: a prospective cohort study. J Cancer Res Clin Oncol 139, 943–951 (2013). https://doi.org/10.1007/s00432-013-1407-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-013-1407-3