Abstract
Objective
Patients with pathologic N2 non-small cell lung cancer comprise a heterogeneous group. The objective of this study was to evaluate which subgroup of patients with pathologic N2 benefit from surgery in terms of survival probability.
Methods
This retrospective study included 141 patients who had undergone major resection with pathologically proven N2 from 1990 to 2006 (103 with adenocarcinoma, 38 with squamous cell carcinoma). Patients undergoing preoperative induction therapy were excluded. Records were examined for age, gender, tumor size, surgical procedure, surgical side, clinical N status, primary tumor lobe, curative resection, and metastatic N2 stations.
Results
In patients with adenocarcinoma, surgical procedure, clinical N status, curative resection, and metastatic N2 stations were significant prognostic factors in univariate analysis. Age and curative resection were significant factors in patients with squamous cell carcinoma. In multivariate analysis, clinical N2 (P = 0.003), incomplete resection (P = 0.04), and multi-station N2 (P = 0.004) were significant adverse prognostic factors in patients with adenocarcinoma, whereas only incomplete resection (P = 0.002) was significant in patients with squamous cell carcinoma. For adenocarcinoma patients with pathologic N2, the 5-year survival rates were 58.8% for clinical N0-1 and single-station N2, 50% for clinical N2 and single-station N2, 23.9% for clinical N0-1 and multi-station N2, and 0% for clinical N2 and multi-station N2.
Conclusions
Prognosis of patients with pathologic N2 can be grouped according to clinical N status and metastatic N2 stations in adenocarcinoma, but not in squamous cell carcinoma. Only adenocarcinoma patients with pathologic N2 appear to have heterogeneous subgroups with different prognoses.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Patients with pathologic N2 non-small cell lung carcinoma (NSCLC) are considered to have a poor prognosis. The role of surgery in the treatment of these patients remains ambiguous. However, patients with pathologic N2 comprise a heterogeneous group, with different patients having a range of different prognoses (Ratto et al. 2009; Robinson et al. 2007; Suzuki et al. 1999; Andre et al. 2000; Inoue et al. 2004; Sakao et al. 2010), and as such, some of these patients are candidates for curative surgery. Several prognostic factors have been defined in clinical studies, which have shown that complete resection, single mediastinal lymph node metastasis, clinical N0-1, pathologic T1-2, and small tumor size are predictors of a favorable prognosis in patients with N2 NSCLC (Suzuki et al. 1999; Andre et al. 2000; Inoue et al. 2004). Patients with preoperatively diagnosed N2 appear to have a significantly worse prognosis than those whose N2 is diagnosed intraoperatively. In particular, multi-station N2 is one of the adverse prognostic factors for N2 patients (Andre et al. 2000). Although many reports have investigated factors affecting the prognosis of patients with pathologic N2, the area remains controversial and the impact of tumor histology on prognosis for patients with pathologic N2 remains unclear.
To investigate the prognostic heterogeneity that may exist among patients with pathologic N2 NSCLC, we retrospectively investigated several clinicopathologic prognostic factors according to tumor histology.
Patients and methods
Patient selection
This retrospective analysis was based on records collected in a database of patients with primary lung cancer, who had been histologically diagnosed and had received thoracotomy at National Hospital Organization Toneyama Hospital in Japan. Between 1990 and 2006, a total of 1,247 consecutive patients underwent thoracotomy for primary lung cancer. Among them, 194 patients were diagnosed with pathologic N2. Of these, 141 patients were enrolled in this study, excluding 53 patients who were diagnosed preoperatively pathologically proven N2 with induction therapy. In principle, we considered only tumors with single-station N2 in the upper lobe to be resectable and those with extranodal or bulky mediastinal node involvement or multi-station N2 to be inoperable (Inoue et al. 2004; Keller et al. 2004). These 141 patients had initially undergone lobectomy or pneumonectomy with systemic nodal dissection of the mediastinum and hilum. Mediastinal lymph node dissection of stations 2R, 4R, and 7 was required during a right thoracotomy and of stations 4L, 5, 6, and 7 during a left thoracotomy. In case of lower lobectomy, dissection of stations 8 and 9 was also performed. This subgroup included 103 patients with adenocarcinoma and 38 patients with squamous cell carcinoma. In our institution, we adopted adjuvant chemotherapy from 2005. Of these 141 patients, 19 patients had received adjuvant chemotherapy mainly using carboplatin–paclitaxel and 6 had adjuvant radiotherapy.
Methods
The clinicopathologic records were categorized according to age (≤70 years old vs. ≥71 years old), gender (male vs. female), tumor size (≤3.0 vs. ≥3.1 cm), surgical procedure (lobectomy vs. pneumonectomy), surgical side (right vs. left), clinical N status (clinical N0-1 vs. clinical N2), primary tumor lobe (upper vs. middle, lower lobe), curative resection (complete resection vs. incomplete resection), and metastatic N2 stations (single-station vs. multi-station). We retrospectively compared these factors with patient survival and tumor histology.
All patients underwent staging according to the 7th UICC TNM classification criteria (Detterbeck et al. 2009). Clinical N status is based on the size of the lymph node as determined by computed tomography (CT) scan. All patients had thoracic CT scans preoperatively, with 10-mm-thick contiguous sections to evaluate nodal status. If lymph nodes were detected, 2-mm-thick sections were further assessed to detect lymph node involvement. All CT scans were reviewed by a radiologist, thoracic surgeons, and pulmonologists preoperatively. Mediastinal or hilar lymph nodes 1 cm or larger in their shortest axis were diagnosed as metastatic (Silvestri et al. 2007). Lymph node stations were classified according to IASLC map (Rusch et al. 2009). When mediastinal nodal involvements were found in two or more stations, cases were classified as multi-station N2. Mediastinoscopy was performed in 124 patients and transbronchial fine needle aspiration was performed in 138 patients during this period to evaluate N2 status. Among them, 53 patients undergoing mediastinoscopy and 49 patients undergoing transbronchial fine needle aspiration were actually diagnosed to have pathologic N2 disease. Among these 102 patients, 53 patients received preoperative induction therapy followed by surgery and residual 49 patients received only non-surgical therapy. Twelve patients undergoing mediastinoscopy and 5 patients undergoing transbronchial fine needle aspiration had false-negative results. Positron emission tomography was used since 2005. An institutional review board approved this retrospective study, and written informed consent for the surgical intervention was obtained from each patients.
Statistical analysis
The chi-square method was used to compare differences between two groups. The Mann–Whitney U test was used to analyze continuous variables. Survival was defined as the time between the date of operation and death. The mean follow-up period was 121.9 months. Survival rates were calculated using the Kaplan–Meier method and compared by log-rank test. The relative importance of various prognostic factors for postoperative survival as identified by multivariate analysis was performed with Cox’s proportional hazards model. A P-value of <0.05 was considered statistically significant.
Results
The clinical characteristics of patients enrolled in the study are presented in Table 1. A total of 103 patients had adenocarcinoma, and 38 patients had squamous cell carcinoma. The mean age of these two groups was 62.7 ± 9.7 years and 64.9 ± 7.8 years, respectively (P = 0.21). Over 90% of patients with squamous cell carcinoma were male (P < 0.0001). The mean tumor size of squamous cell carcinoma was significantly greater than that of adenocarcinoma (4.4 ± 1.8 cm vs. 3.2 ± 1.3 cm; P < 0.0001). The distributions of clinical and pathologic T status were significantly different. In adenocarcinoma, 78% of patients with pathologic N2 were upgraded from clinical N0-1, and in squamous cell carcinoma, 66% of those with pathologic N2 were upgraded from clinical N0-1. The distribution of clinical stage was significantly different (P = 0.03); adenocarcinoma patients had lower clinical stage than squamous cell carcinoma patients. Squamous cell carcinoma patients were more likely to undergo pneumonectomy than patients with adenocarcinoma (P = 0.03).
In univariate survival analysis of patients with pathologic N2, surgical procedure (P = 0.008), clinical N status (P = 0.0002), curative resection (P < 0.0001), and metastatic N2 stations (P = 0.0004) were significant prognostic factors in patients with adenocarcinoma (Table 2). Age (P = 0.02) and curative resection (P = 0.0002) were significant prognostic factors in patients with squamous cell carcinoma. Clinical N status and metastatic N2 stations were not significant prognostic factors (Table 3). In multivariate survival analysis of patients with pathologic N2, clinical N2 (P = 0.003), incomplete resection (P = 0.02), and multi-station N2 (P = 0.03) were significant adverse prognostic factors in patients with adenocarcinoma, whereas only incomplete resection (P = 0.002) was a significant adverse prognostic factor in patients with squamous cell carcinoma (Table 4).
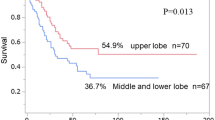
Survival rates of patients with pathologic N2 according to clinical N status are shown in Fig. 1a and b. Adenocarcinoma patients with clinical N0-1 had a significantly better survival rate than those with clinical N2 (5-year survival: 43.2 vs. 15.0%; P = 0.0002), although this was not the case for patients with squamous cell carcinoma (5-year survival: 28.7 vs. 30.8%; P = 0.65). Survival rates of patients with pathologic N2 according to metastatic N2 stations are shown in Fig. 2a and b. Adenocarcinoma patients with single-station N2 had a significantly better survival rate than those with multi-station N2 (5-year survival: 62.8 vs. 14.7%; P = 0.004), although this was not the case for patients with squamous cell carcinoma (5-year survival: 32.7 vs. 26.9%; P = 0.18). Finally, survivals of adenocarcinoma patients with pathologic N2 were compared based on clinical N status and metastatic N2 stations. The 5-year survival rates were 58.8% for clinical N0-1 and single-station N2, 50% for clinical N2 and single-station N2, 23.9% for clinical N0-1 and multi-station N2, and 0% for clinical N2 and multi-station N2 (Fig. 3a). Patients with pathologic N2 were divided into 4 subgroups with different prognoses. The worst survival rates were observed for patients with both clinical N2 and multi-station N2. Conversely, the prognosis was similar in squamous cell carcinoma patients regardless of clinical N status or metastatic N2 stations (Fig. 3b).
Survival rate of patients with pathologic N2 according to metastatic N2 levels. Adenocarcinoma patients with single-level N2 had a significantly better survival rate than those with multi-level N2 (P = 0.004) (a), although this was not the case for patients with squamous cell carcinoma (P = 0.18) (b)
Survival rate of patients according to clinical N status and metastatic N2 levels. Patients with pathologic N2 were divided into 4 subgroups with different prognoses (a). Conversely, the prognosis was similar in squamous cell carcinoma patients regardless of clinical N status or metastatic N2 level (b)
Discussion
Patients with pathologic N2 NSCLC comprise a heterogeneous group in terms of their prognosis (Ratto et al. 2009; Robinson et al. 2007; Suzuki et al. 1999; Andre et al. 2000, Inoue et al. 2004; Sakao et al. 2010), which stems from multiple adverse factors such as clinical N2, incomplete resection, large tumor size, and number of involved mediastinal lymph nodes. In a previous study, Andre et al. (2000) reported four negative prognostic factors: clinical N2, number of involved mediastinal lymph nodes, pathologic T3-4, and lack of preoperative chemotherapy. Vansteenkiste et al. (1997) also noted that survival was poor in patients with higher T status, lower performance status, involvement of multi-station N2, non-squamous histology, and clinical N2. However, few have considered whether the prognosis of patients with pathologic N2 depends on tumor histology.
Clinical N status is one of the most significant prognostic factors in surgically resected N2 NSCLC (Suzuki et al. 1999; Andre et al. 2000). Occult N2 (preoperatively undetectable N2; clinical N0-1) and clinical N2 are two distinct stages of NSCLC (Andre et al. 2000). In the study by Andre et al. (2000), the 5-year survival rates of patients with pathologic N2 were 29, and 7% for clinical N0-1 and clinical N2 patients, respectively. As many as one quarter of patients with pathologic N2 are found in surgery to have occult N2 metastatic disease (Goldstraw et al. 1994; Misthos et al.2004). The prognosis of patients with occult N2 metastasis (clinical N0-1) is better than for patients with clinical N2. Thus, patients with incidental N2 at surgery have the best chance of survival (Andre et al.2000; Goldstraw et al. 1994). In the present study, however, clinical N status was a significant prognostic factor only in patients with pathologic N2 adenocarcinoma. Thus, clinical N0-1 indicates a better prognosis than clinical N2 only in adenocarcinoma patients, and not in squamous cell carcinoma patients.
It has been shown that multi-station N2 patients have much poorer prognoses than those with single-station N2 (Andre et al. 2000; Goldstraw et al. 1994), although Keller et al. (2004) documented that this was the case only where the tumor was detected in the left upper lobe. Ichinose et al. (2001) also reported that single-station N2 is a good prognostic factor except for patients with a primary tumor in the left lower lobe. Misthos et al. (2008) reported that skip metastasis, regional N2 spread, and single-station N2 all correlated with good survival rates, although multivariate analysis established single-station N2 as the only independent favorable prognostic factor. Patients with the involvement of single-station N2 fared significantly better than patients with multi-station N2 (Ratto et al. 2009; Inoue et al. 2004; Misthos et al. 2008). Andre et al. (2000) also reported that the number of involved mediastinal lymph node levels was an independent prognostic factor. In this study, multi-station N2 was a significant prognostic factor only for patients with pathologic N2 adenocarcinoma, not for squamous cell carcinoma patients. Squamous cell carcinoma patients with pathologic N2 had a similar prognosis even if they had clinical N0-1 or single-station N2.
A preoperative N2 status and the number of involved lymph node stations are the most relevant prognostic factors in N2 NSCLC (Andre et al. 2000). Sakao et al. (2010) also reported that among patients with multi-station N2, the prognoses were different between those with clinical N0-1 and those with clinical N2. Andre et al. (2000) stated that surgery was mandatory in patients with clinical N0-1 and single-station N2. In this study, multivariate analyses indicated that clinical N2, incomplete resection, and multi-station N2 were significant prognostic factors for patients with adenocarcinoma. In adenocarcinoma, patients can be classified into 4 distinct prognostic subgroups according to clinical N status and metastasis N2 stations. Clinical N status and metastasis N2 stations were associated with prognosis in adenocarcinoma. In our study, the 5-year survival rate of adenocarcinoma patients with clinical N0-1 and single-station N2 was relatively good: 58.8%. On the contrary, in squamous cell carcinoma, it is interesting that patients cannot be classified into these subgroups. The prognosis was similar in squamous cell carcinoma patients regardless of clinical N status or metastatic N2 stations. This result may be due to the different metastatic properties of the two tumor histologies. We supposed that patients who discovered surgically positive N2 nodes or those with single-station N2 in adenocarcinoma patients could benefit from surgical resection. On the contrary, surgery was not beneficial to patients with both clinical N2 and multi-station N2 in either histologic subgroup.
Limitations of this study, however, are that the analysis was retrospective and that the exclusion of unresectable N2 led to a bias in the subclassification. In addition, routine adjuvant chemotherapy for N2 patients was started in 2005. Therefore, it was difficult to evaluate the effect of adjuvant chemotherapy on prognosis in this study. Furthermore, the sample of patients with N2, especially that of squamous cell carcinoma patients, was small, which restricted our ability to generalize the results. A greater number of patients with N2 should be investigated further in future studies to determine the prognostic heterogeneity of tumor histology. Finally, irrespective of prior CT or PET scan finding, transbronchial or endobronchial ultrasound fine needle aspiration for mediastinal small lymph nodes was warranted, which might have a substantial impact on further management.
In conclusion, adenocarcinoma patients can be grouped according to their prognoses by clinical N status and metastatic N2 stations. This was not the case in squamous cell carcinoma. Tumor histology affects the prognostic factors of patients with pathologic N2 NSCLC.
References
Andre F, Grunenwald D, Pignon JP et al (2000) Survival of patients with resected N2 non-small-cell lung cancer: Evidence for a subclassification and implications. JCO 18:2981–2989
Detterbeck FC, Boffa DJ, Tanoue LT (2009) The new lung cancer staging system. Chest 136:260–271
Goldstraw P, Mannam GC, Kaplan DK et al (1994) Surgical management of non-small-cell lung cancer with ipsilateral mediastinal node metastasis (N2 disease). J Thorac Cardiovasc Surg 107:19–28
Ichinose Y, Kato H, Koike T et al (2001) Completely resected stage IIIA non–small cell lung cancer: the significance of primary tumor location and N2 station. J Thorac Cardiovasc Surg 122:803–808
Inoue M, Sawabata N, Takeda S et al (2004) Results of surgical intervention for p-stage IIIA (N2) non–small cell lung cancer: acceptable prognosis predicted by complete resection in patients with single N2 disease with primary tumor in the upper lobe. J Thorac Cardiovasc Surg 127:1100–1106
Keller SM, Vangel MG, Wagner H et al (2004) Prolonged survival in patients with resected non–small cell lung cancer and single-level N2 disease. J Thorac Cardiovasc Surg 128:130–137
Misthos P, Sepsas E, Athanassiadi K et al (2004) Skip metastases: analysis of their clinical significance and prognosis in the IIIA stage of non-small cell lung cancer. Eur J Cardiothorac Surg 25:502–508
Misthos P, Sepsas E, Kokotsakis J et al (2008) The significance of one-station N2 disease in the prognosis of patients with non small-cell lung cancer. Ann Thorac Surg 86:1626–1631
Ratto GB, Costa R, Maineri P et al (2009) Is there a subset of patients with preoperatively diagnosed N2 non–small cell lung cancer who might benefit from surgical resection? J Thorac Cardiovasc Surg 138:849–858
Robinson LA, Ruckdeschel JC, Wagner RH Jr et al (2007) Treatment of non-small cell lung cancer-stage IIIA: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 132:243S–265S
Rusch VW, Asamura H, Watanabe H et al (2009) The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 4:568–577
Sakao Y, Okumura S, Mun M et al (2010) Prognostic heterogeneity in multilevel N2 non-small cell lung cancer patients: importance of lymphadenopathy and occult intrapulmonary metastases. Ann Thorac Surg 89:1060–1063
Silvestri GA, Gould MK, Margolis ML et al (2007) Noninvasive staging of non-small cell lung cancer: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest 132:178S–201S
Suzuki K, Nagai K, Yoshida J et al (1999) The prognosis of surgically resected N2 non-small cell lung cancer. J Thorac Cardiovasc Surg 118:145–153
Vansteenkiste JF, De Leyn PR, Deneffe GJ et al (1997) Survival and prognostic factors in resected N2 non-small cell lung cancer: a study of 140 cases. Ann Thorac Surg 63:1441–1450
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Funakoshi, Y., Takeuchi, Y., Kusumoto, H. et al. Which subgroup of patients with pathologic N2 non-small cell lung cancer benefit from surgery?. J Cancer Res Clin Oncol 138, 1027–1033 (2012). https://doi.org/10.1007/s00432-012-1175-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-012-1175-5