Abstract
Purpose
Constitutive activation of JAK/STAT pathway is observed in various solid tumors and hematological malignancies. SOCS3 acts as a key negative regulator of JAK/STAT pathway and represents one of the candidate tumor suppressor genes. In the current study, we aimed to evaluate SOCS3 expression in breast carcinoma and to explore the prognostic significance of SOCS3.
Methods
The expression of SOCS3 was measured by Western blot and immunohistochemistry in breast carcinoma cells and a large cohort of tissue microarray, respectively.
Results
Among 367 human primary breast tumors, SOCS3 protein was detected in 103 patients. Deficient SOCS3 expression correlated significantly with lymph node metastasis (P = 0.003), blood vessel invasion (P = 0.029), VEGF (P = 0.001) and Ki-67 (P = 0.027). Univariate and multivariate analyses revealed that SOCS3 expression was an independent prognostic factor for disease-free survival (P < 0.0001). A positive SOCS3 protein expression correlated significantly with a low pSTAT3 protein expression in breast carcinoma (P = 0.015). The patients with a SOCS3 (+)/pSTAT3 (−) phenotype had a better prognosis than any other combination (DFI: P < 0.0001, BCSS: P = 0.013).
Conclusions
Deficient expression of SOCS3 is associated with an aggressive phenotype and portends a poor clinical outcome in breast carcinoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Accumulating evidence has indicated that constitutive activation of Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway occurs frequently in diverse types of tumor cells and contributes to malignant progression (Yu and Jove 2004). Among the members of STAT family, STAT3 plays a pivotal role in the oncogenesis. STAT3 is involved in many fundamental cellular processes with obvious links to cancer, such as cell-cycle progression, anti-apoptotic effects, proangiogenesis, immune evasion and tumor metastasis (Hirano et al. 2000; Niu et al. 2002; Abdulghani et al. 2008). Aberrant STAT3 signaling has been identified to be sufficient to induce tumor formation in a range of human malignancies (Weerasinghe et al. 2007; Li et al. 2006). There have been confirmed that constitutively activated STAT3 is frequently found in breast carcinoma cell lines and patients with advanced breast disease but is absent in normal breast epithelial cells (Garcia et al. 1997, 2001; Bowman et al. 2001). In particular, the phosphorylated form of STAT3 at tyrosine residue 705 (Y705) is frequently elevated in breast carcinogenesis and high expression of STAT3 in cancer lesions may be a useful biomarker for a poor prognosis in breast infiltrating ductal carcinoma (IDC) cases (Garcia et al. 1997; Sheen-Chen et al. 2008). Therefore, understanding the events leading to STAT3 suppression will provide critical insight for treating and preventing breast tumorigenesis.
Suppressor of cytokine signaling (SOCS) proteins are the classic negative counter regulatory inhibitors of JAK/STAT pathway activation and they also have a regulatory function on extracellular signal-regulated kinase/mitogen-activated protein kinase (ERK/MAPK) (Isobe et al. 2006) and nuclear factor-κB (NF-κB) signaling (Nakagawa et al. 2002). The SOCS family consists of eight members comprised of SOCS1–7 and CIS (cytokine-inducible SH2 domain proteins). As negative regulators of cytokine signaling via JAK/STAT pathway or as STAT-induced STAT inhibitors, the ability of SOCS to modulate signal transduction is based on two functional domains, an SH2 domain, which binds to phosphorylated tyrosine residues and a SOCS box, which serves as a recruiting site for ubiquitin ligases (Hilton. 1999). SOCS3 is one of the well-characterized members of SOCS family and is the key negative regulator of the STAT3 signaling pathway; it is also a potential tumor suppressor gene in specific cancers (Isomoto et al. 2007). An increasing number of studies have demonstrated the roles of SOCS3 in cytokine signaling, including the immune suppression and cytokine resistance of tumor cells. SOCS3 can bind both the cytokine receptor and JAK and is recruited to the tyrosine phosphorylated receptor, facilitating inhibition of JAK (Fujimoto and Naka 2003). Tumors often exhibit alterations in SOCS3 protein expression: CpG island methylation of SOCS3 gene loci and consequently, inhibition of SOCS3 expression was observed in a variety of solid tumors and hematological malignancies (Ogata et al. 2006; Tokita et al. 2007; Weber et al. 2005; Elliott et al. 2009). The fact that SOCS3 silencing by promoter methylation reveals an important epigenetic event during the development of tumor. Once SOCS3 is silenced by promoter hypermethylation, JAK activity and subsequent STAT3 activation is triggered. The inactivation of SOCS3 may result in cells becoming more sensitive to aberrant growth-stimulating signals that function through JAK/STAT pathway, leading to cell growth and survival. Previously, reports have indicated that hypermethylation of the SOCS3 promoter region correlates with silencing of SOCS3 in diverse cancer cell lines and SOCS3 is involved in the suppression of tumor growth and metastasis in lung cancer, malignant melanomas, HCC and neck and head squamous cell carcinoma (Ogata et al. 2006; Tokita et al. 2007; Weber et al. 2005; He et al. 2003). It has recently been reported that overexpression of SOCS3 markedly suppresses STAT3 expression and abrogates STAT5 phosphorylation, resulting in decreased epithelial cell proliferation, survival and anchorage-independent growth in breast carcinoma cells (Barclay et al. 2009). However, to the best of our knowledge, no study has yet been conducted regarding the expression of SOCS3 protein in surgical specimens for breast carcinoma; little is known about the association between SOCS3 expression and clinicopathological features, and its prognostic significance remains to be clarified. We, therefore, elucidated the expression of SOCS3 by immunohistochemistry (IHC) on tissue microarrays (TMAs) in 367 primary breast carcinoma and corresponding normal tissues. Our finding demonstrated for the first time that SOCS3 can be a biomarker of disease recurrence for breast carcinoma patients after surgery. The patients with a combined SOCS3 (+)/pSTAT3 (−) protein expression had a better prognosis that suggests a loss of SOCS3 function to be associated with an aggressive phenotype in breast carcinoma.
Materials and methods
Cell lines
The human breast carcinoma cell lines MCF-7, HuL100, BT474 and normal human mammary epithelial cells (HMECs) were purchased from the American Type Culture Collection (Manassas, VA). All cell lines were at 37°C and 5% CO2 humid atmosphere in Opti-Mem medium with 1% penicillin–streptomycin and 4% FBS (fetal bovine serum).
Tumor material, patient characteristics and preparation of TMAs
A total of 367 patients (median age, 52.8 years) with primary breast carcinomas who underwent curative surgery at Changhai Hospital in Shanghai, People’s Republic of China, from 1999 to 2005 were enrolled for this study. Histological confirmation of primary breast carcinoma was obtained from the Department of Pathology at Changhai Hospital. None of the patients received preoperative adjuvant chemotherapy. Of the 367 patients, paired non-neoplastic breast tissues which were sampled from the resection margins were available for 315. The following variables were recorded: patient age, TNM stage (UICC), tumor type, largest tumor diameter, lymph node status, blood vessel invasion, hormone receptor, Her-2, the histological grade (Bloom, Richardson, Elston-Ellis grading, BRE), VEGF and Ki-67. All of the tissue specimens were obtained for present study with patient informed consent and the use of the human specimens was approved by the Ethics Committee of Changhai Hospital.
TMA and immunohistochemistry
Seven paraffin-embedded TMA blocks of normal breast, breast cancer tissue specimens obtained from the patients were created using a manual arrayer (Beecher Instruments, Sun Prairie, WI, USA). Each block had at least one 1.5-mm core of non-neoplastic tissue and two 1.5-mm cores of primary tumor tissue and 4-µm paraffin sections were then made by standard technique. SOCS3 protein expression in the sections was detected with SOCS3 rabbit polyclonal antibody (dilution 1:100, sc-9023, Santa Cruz Biotechnology Inc., CA, USA), STAT3 rabbit polyclonal antibody (dilution 1:100, sc-7179, Santa Cruz Biotechnology Inc., CA, USA) and Phospho-Stat3 (Tyr-705) antibody (dilution 1:200, #9145, Cell Signaling Technology Inc., Danvers, MA, USA). An EnVision kit (Dako, Carpinteria, CA, USA) was used to visualize antibody binding, and slides were subsequently counterstained with hematoxylin.
Evaluation of immunostaining
Expression of SOCS3, STAT3 and pSTAT3 in the TMAs was evaluated by two individuals (Y.C. and M.X.H.) under an Olympus CX31 microscope (Olympus Optical, Tokyo, Japan). Discrepancies in the scores were resolved by discussion between the two evaluators. Briefly, a positive reaction was indicated by a reddish-brown precipitate in the cytoplasm. Two independent investigators scored the sections without the knowledge of patient outcome (double blinded). An average value of two independent scores was presented in the present study. Depending on the percentage of positive cells and staining intensity, SOCS3 staining positivity was classified into three groups: negative, weak positive, and strong positive. Specifically, the percentage of positive cells was divided into five grades (percentage scores):10% (0), 10–25% (1), 25–50% (2), 50–75% (3), and 75% (4). The intensity of staining was divided into four grades (intensity scores): no staining (0), light brown (1), brown (2), and dark brown (3). SOCS3 staining positivity was determined by the formula: overall scores = percentage score × intensity score. The overall score of ≤3 was defined as negative, of >3 to ≤6 as weak positive, and of >6 as strong positive.
Follow-up and survival analysis
The last date of follow-up was 31 December 2008 with a median observation time of 43.3 months (range from 24 to 77 months). No patients were lost to follow-up throughout the experiments. Data relating to survival were collated in a prospective manner including breast carcinoma-specific survival (BCSS) defined as the interval (in months) from the date of the primary treatment to the time of death due to breast carcinoma and disease-free interval (DFI) defined as the interval (in months) from the date of the primary treatment to the first loco-regional recurrence or distant metastasis. Patients were censored on the date of last contact if a treatment failure event had not been observed. Kaplan–Meier survival analyses were carried out for breast carcinoma-specific survival and DFI. The log-rank test was used to examine the statistical significance of the differences observed between the groups.
The principal subgroups of interest were defined by SOCS3 status (positive and negative) and combined SOCS3/pSTAT3 status: group I, both negative; group II, positive SOCS3 but negative pSTAT3; group III, negative SOCS3 but positive pSTAT3; and group IV, both positive status. To evaluate the effects of age, size, lymph node status, grade, hormone receptors and Her-2, a multivariate Cox regression model was used to compute relative ratios (RRs) and 95% confidence intervals (95% CIs). Estimates were considered statistically significant for two-tailed values of P < 0.05. All analyses were performed using the SPSS statistical software program 13.0 for Microsoft Windows (SPSS Inc., Chicago, IL, USA).
Western blot analysis
Whole cell extracts were prepared from pulverized breast cancer and normal adjacent tissue specimens. Standard Western blot was performed using rabbit polyclonal antibody anti-human SOCS3 (dilution 1:200, sc-9023, Santa Cruz Biotechnology Inc., CA, USA) and further incubated in anti-rabbit IR 800 secondary antibody (dilution 1:5,000, Rockland Immunochemicals, Gilbertsville, PA, USA). Equal protein sample loading was monitored by incubating the same membrane filter with an anti-β-actin antibody. The probe proteins were detected using a LICOR Odyssey Infrared Imager (LICOR, Biosciences, Lincoln, NB) according to the manufacturer’s instructions.
Statistical analysis
Associations between different variables were assessed by Pearson’s χ 2 test. Within-group correlations of continuous and ordinal variables were assessed using Pearson or Spearman rank correlation analysis where appropriate. Univariate survival analysis of DFI was assessed by the product-limit method (Kaplan–Meier) with the log-rank test for differences between categories of each variable. Cox regression analysis was used to estimate RRs and their 95% CIs. A factor was considered statistically significant if it had a two-sided P value of <0.05. All statistical analyses were performed using the SPSS 13.0 statistical software program for Microsoft Windows (SPSS Inc., Chicago, IL, USA).
Results
Down-regulation of SOCS3 is frequently detected in primary breast carcinoma
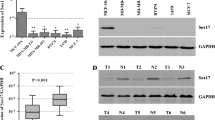
Western blot analysis revealed that breast carcinoma cell lines, including MCF-7, HuL100 and BT474, exhibited significantly lower levels of SOCS3 expression compared to levels in normal HMECs at protein levels (Fig. 1a). Comparative analysis indicated that SOCS3 was differentially down-regulated in ten breast carcinoma specimens paired with adjacent non-cancerous tissues (ANT) from the same patients which were confirmed by Western blot and IHC analysis of TMA (Figs. 1b, 2).
Expression of SOCS3 was reduced in breast carcinomas. a The expression of SOCS3 in four breast cell lines was determined by Western blot. One normal human mammary epithelial cell (HMEC) was used as a normal control; β-actin was used as internal control. Lane 1, HMEC; lane 2, MCF-7; lane 3, HuL100; lane 4, BT474. b Western blotting analysis of SOCS3 expression in each of the primary breast cancer tissue (T) and adjacent non-cancerous tissue (ANT) paired from the same patient
In clinical specimen test, the expression of SOCS3 protein was significantly higher in adjacent non-cancerous breast tissues than in breast cancer tissues (P < 0.0001, Pearson χ 2 test) (Table 1). The expression level of SOCS3 was significantly related with lymph node metastasis (P = 0.003), blood vessel invasion (P = 0.029), VEGF (P = 0.001) and Ki-67 (P = 0.027, Pearson χ 2 test) (Table 2). Taken together, our data suggest that SOCS3 was down-regulated in breast carcinomas.
Expression of SOCS3 is associated with phospho-STAT3
SOCS3 protein expression was deficient in 264 of 367 (71.93%) breast carcinoma cases, while the nuclear accumulation of pSTAT3 protein was detected in 253 of 367 (68.94%) cases. A significant correlation was observed between SOCS3 and pSTAT3 protein expression. A reduced SOCS3 protein expression correlated significantly with a high pSTAT3 protein expression (P = 0.015, Table 3).
Expression of SOCS3 is associated with early distant recurrence
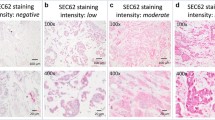
Tissues were scored on the basis of the intensity of SOCS3 cytoplasm labeling and percentage of SOCS3-positive tumor cells (Fig. 3a). An inverse correlation between N stage and SOCS3 expression was analyzed by Pearson χ 2 test (P < 0.0001) (Fig. 3b). Among these specimens, Kaplan–Meier survival analysis of 367 breast cancer specimens revealed a correlation between higher SOCS3 expression levels and longer DFIs (P < 0.0001) (Figs. 3c, 4). However, SOCS3 was not correlated with breast cancer-specific survival (P > 0.05). Moreover, multivariate Cox regression analysis including categorized SOCS3 expression, STAT3 phosphorylation and clinical parameters such as age, histology, lymph node metastasis, TNM stage and blood vessel invasion identified SOCS3 as an independent prognostic marker for early distant recurrence (P < 0.0001). N stage as an established prognostic factor was also confirmed in our set (P < 0.0001) (Table 4).
SOCS3 staining positivity is associated with N stage and early distant recurrence. A Three sets of consecutive tissue sections represented negative (a), weak (b) and strong (c) SOCS3 expression. Representative pictures were presented (×400). B Of note is that SOCS3 expression inversely correlated with N stage. C A log-rank test showed significant differences in DFI between groups
Expression of SOCS3/pSTAT3 has a prognostic relevance
Figures 5 and 6 show the survival curves stratified according to the combination of SOCS3/pSTAT3 protein expression. Univariate analyses indicated that patients with the combination of positive SOCS3/reduced pSTAT3 protein expression have a significantly better DFI (P < 0.0001) (Fig. 5) and breast cancer-specific survival (P = 0.013) (Fig. 6) than those with other combinations of the two protein expressions. Multivariate analyses of prognosis indicated that the combination of the positive SOCS3/reduced pSTAT3 protein expression is independently significant prognostic factors for DFI (P < 0.0001) (Table 4).
Discussion
In the present study, we offered clinical evidence for the first time that SOCS3 protein expression was significantly down-regulated in breast cancer specimens and altered expression of SOCS3 protein might directly influence the prognosis of breast cancer. We also confirmed that the SOCS3 expression was missing or dramatically under expressed in all three breast carcinoma cell lines tested but was detected in normal breast cell line, in keeping with literature data (He et al. 2003). In our series, the SOCS3 expression is associated with lymph node metastasis, blood vessel invasion, VEGF and Ki-67, suggesting SOCS3 is a valuable biomarker for the malignant potential in breast carcinoma. Survival analysis indicated that SOCS3 expression is a strong negative prognosticator of breast cancer relapse and the specific combined phenotypes of SOCS3/pSTAT3 can be a good predictor of postoperative survival in patients with breast cancer.
Previous studies have shown that JAK–STAT pathway alterations are believed to mediate neoplastic transformation and constitutive activation of STAT3 protein is correlated with cell proliferation and apoptosis in various solid tumors and hematological malignancies. As an established negative regulator of STATs, SOCS3 function has been examined in vitro and in vivo using diverse transformed cell lines. It has been identified that forced expression of SOCS3 resulted in growth inhibition of human lung adenocarcinoma A549 cells (He et al. 2003) and lung cancer xenografts in nude mice (Nakagawa et al. 2008). Mice deficient in SOCS3 die in utero as a consequence of uncontrolled leukemia inhibitory factor (LIF) signaling inactivation of the LIF receptor rescues the SOCS3−/− phenotype (Takahashi et al. 2008; Roberts et al. 2001). Conditional SOCS3 knockouts displayed sustained interleukin (IL)-6-mediated activation of STAT3, establishing SOCS3 as a crucial inhibitor of STAT3 in vivo (Takahashi et al. 2003). Furthermore, SOCS3 is part of a STAT3-inducible negative feedback loop and plays a role in determining the outcome of STAT3 signaling in the context of the DNA damage response. SOCS3 could support cell-cycle arrest by inhibiting p21 gene repression by STAT3. SOCS3 controls the DNA damage response by inhibiting STAT3-dependent radio-resistant DNA synthesis and promoting DNA repair by supporting p21 expression and G1 arrest. Thus, SOCS3 may be a key factor in the decision between cell-cycle arrest and radio-resistant DNA synthesis following DNA damage. Methylation silencing of SOCS3 is one of the most important mechanisms of constitutive activation of JAK–STAT pathway in cancer pathogenesis. It has been identified that the methylation of SOCS3 promoter may be involved in the pathogenesis of glioblastoma multiforme (GBM) (Martini et al. 2008), follicular lymphoma (Krishnadasan et al. 2006), neck and head squamous cell carcinoma (Weber et al. 2005), cholangiocarcinoma (Isomoto et al. 2007), malignant melanoma (Tokita et al. 2007) and hepatocellular carcinoma (Yang et al. 2008). SOCS3 itself may function as an important tumor suppressor gene and may be involved in the resistance of these neoplasms to conventional treatment. It was reported that SOCS3 deficiency enhances hepatocyte proliferation in association with enhanced STAT3 and ERK activation. SOCS3 h-KO mice develop hepatocellular carcinoma at an accelerated rate in the model of chemical-induced carcinogenesis (Riehle et al. 2008). In vitro, exogenous expression of SOCS3 in the highly aggressive anaplastic thyroid cancer cells reduces STAT3 phosphorylation and PI3K/Akt pathway activation resulting in alteration in the balance of proapoptotic and anti-apoptotic molecules and sensitization to chemotherapeutic drugs (Francipane et al. 2009). Likewise, exogenous expression of SOCS3 significantly reduces tumor growth and potently enhances the efficacy of chemotherapy in vivo. Restoration of SOCS3 in cancer cells where SOCS3 was methylation-silenced resulted in the down-regulation of active STAT3, induction of apoptosis and growth suppression in diverse types of cancer (Kim et al. 2008; Kinjyo et al. 2007). Given the biologic functions mentioned above, it is not surprising to find an inverse relationship between expression of SOCS3 and pSTAT3. Recent work on human breast carcinoma demonstrated that PRL could stimulate SOCS3 expression in T47D cells, SOCS3 overexpression markedly reduces activated STAT3 in the nucleus and decreases cell proliferation while SOCS3 siRNA knockdown increases proliferation in both T47D and MCF-7 cell lines (Barclay et al. 2009). Hypermethylation of the SOCS3 promoter region also correlates with silencing of SOCS3 in breast carcinoma cell lines. Collectively, these in vivo and in vitro data may suggest that SOCS3 modulates both neoplastic proliferative and anti-apoptotic processes and may act as a tumor suppressor in breast carcinoma.
Axillary lymph node status is vital for the staging of breast carcinoma. Whether or not prevention of regional recurrences has a true impact on survival remains unclear but nodal status is thought to be the most important independent prognostic indicator in breast cancer and plays an important role in adjuvant decision making (Quan and McCready 2009). Nakagawa et al. (2008) recently provided the first evidence that SOCS3 mRNA level is associated with lymph node status in breast cancer specimens. In our study, SOCS3 expression in the lymph node positive group was significantly lower than that in the negative group. Reduced expression of SOCS3 is closely related to lymph node metastasis. We also demonstrated that the expression of SOCS3 is associated with DFS but not BCSS, and DFS was strongly correlated to SOCS3 expression comparing no versus weak and strong expression. Our report suggested that the presence of SOCS3 in breast carcinoma may be protective against ipsilateral recurrence particularly in lymph node negative lesions and down-regulated SOCS3 expression is an independent predictor of increased risk of ipsilateral in breast recurrence. It is noteworthy that, for all investigated tissues, SOCS3 expression strictly correlated with the status of pSTAT3. Based on the above evidence that showed effects of SOCS3 on activation of STAT3 are negative, we speculated that the combined phenotype of SOCS3/pSTAT3 may have prognostic significance on breast cancer specimens. In our set, the patients with a combined SOCS3+/pSTAT3− protein expression had a better recurrence-free survival and breast cancer-specific survival suggesting targeting the STAT3–SOCS3 signaling loop may effectively modulate tumor biological behavior clinically.
Conclusions
In summary, our results have shown the first definitive evidence that low SOCS3 expression was an independent prognostic factor for recurrence of breast carcinoma patients after surgery. We also demonstrated that a significant correlation was observed between SOCS3 and pSTAT3 protein expression in breast carcinoma. The inverse correlation between SOCS3 expression and lymphatic and vascular invasion in breast carcinoma suggested that SOCS3 might be involved in angiogenesis and/or lymphangiogenesis and promote tumor metastasis. Further investigation of SOCS3 as a therapeutic target and as a clinical tumor marker appears to be warranted.
Abbreviations
- ANT:
-
Adjacent non-cancerous tissues
- BCSS:
-
Breast carcinoma-specific survival
- CI:
-
Confidence interval
- DFI:
-
Disease-free interval
- ER:
-
Estrogen receptor
- IDC:
-
Infiltrating ductal carcinoma
- IHC:
-
Immunohistochemistry
- LN:
-
Lymph node
- PR:
-
Progesterone receptor
- RR:
-
Relative risk
- SOCS3:
-
Suppressor of cytokine signaling 3
- STAT3:
-
Signal transducers and activators of transcription 3
- TSG:
-
Tumor suppressor gene
- TMA:
-
Tissue microarray
- SDS:
-
Sodium dodecyl sulfate
- VEGF:
-
Vascular endothelial growth factor
References
Abdulghani J, Gu L, Dagvadorj A, Lutz J, Leiby B, Bonuccelli G, Lisanti MP, Zellweger T, Alanen K, Mirtti T, Visakorpi T, Bubendorf L, Nevalainen MT (2008) Stat3 promotes metastatic progression of prostate cancer. Am J Pathol 172(6):1717–1728. doi:10.2353/ajpath.2008.071054
Barclay JL, Anderson ST, Waters MJ, Curlewis JD (2009) SOCS3 as a tumor suppressor in breast cancer cells, and its regulation by PRL. Int J Cancer 124(8):1756–1766. doi:10.1002/ijc.24172
Bowman T, Broome MA, Sinibaldi D, Wharton W, Pledger WJ, Sedivy JM, Irby R, Yeatman T, Courtneidge SA, Jove R (2001) Stat3-mediated Myc expression is required for Src transformation and PDGF-induced mitogenesis. Proc Natl Acad Sci USA 98(13):7319–7324. doi:10.1073/pnas.131568898
Elliott J, Suessmuth Y, Scott LM, Nahlik K, McMullin MF, Constantinescu SN, Green AR, Johnston JA (2009) SOCS3 tyrosine phosphorylation as a potential bio-marker for myeloproliferative neoplasms associated with mutant JAK2 kinases. Haematologica 94(4):576–580. doi:10.3324/haematol.2008.002352
Francipane MG, Eterno V, Spina V, Bini M, Scerrino G, Buscemi G, Gulotta G, Todaro M, Dieli F, De Maria R, Stassi G (2009) Suppressor of cytokine signaling 3 sensitizes anaplastic thyroid cancer to standard chemotherapy. Cancer Res 69(15):6141–6148. doi:10.1158/0008-5472.CAN-09-0994
Fujimoto M, Naka T (2003) Regulation of cytokine signaling by SOCS family molecules. Trends Immunol 24(12):659–666. doi:10.1016/j.it.2003.10.008
Garcia R, Yu CL, Hudnall A, Catlett R, Nelson KL, Smithgall T, Fujita DJ, Ethier SP, Jove R (1997) Constitutive activation of Stat3 in fibroblasts transformed by diverse oncoproteins and in breast carcinoma cells. Cell Growth Differ 8(12):1267–1276
Garcia R, Bowman TL, Niu G, Yu H, Minton S, Muro-Cacho CA, Cox CE, Falcone R, Fairclough R, Parsons S, Laudano A, Gazit A, Levitzki A, Kraker A, Jove R (2001) Constitutive activation of Stat3 by the Src and JAK tyrosine kinases participates in growth regulation of human breast carcinoma cells. Oncogene 20(20):2499–2513. doi:10.1038/sj.onc.1204349
He B, You L, Uematsu K, Zang K, Xu Z, Lee AY, Costello JF, McCormick F, Jablons DM (2003) SOCS-3 is frequently silenced by hypermethylation and suppresses cell growth in human lung cancer. Proc Natl Acad Sci USA 100(24):14133–14138. doi:10.1073/pnas.2232790100
Hilton DJ (1999) Negative regulators of cytokine signal transduction. Cell Mol Life Sci 55(12):1568–1577. doi:10.1007/s000180050396
Hirano T, Ishihara K, Hibi M (2000) Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene 19(21):2548–2556. doi:10.1038/sj.onc.1203551
Isobe A, Takeda T, Sakata M, Yamamoto T, Minekawa R, Hayashi M, Auernhammer CJ, Tasaka K, Murata Y (2006) STAT3-mediated constitutive expression of SOCS3 in an undifferentiated rat trophoblast-like cell line. Placenta 27(8):912–918. doi:10.1016/j.placenta.2005.10.003
Isomoto H, Mott JL, Kobayashi S, Werneburg NW, Bronk SF, Haan S, Gores GJ (2007) Sustained IL-6/STAT-3 signaling in cholangiocarcinoma cells due to SOCS-3 epigenetic silencing. Gastroenterology 132(1):384–396. doi:10.1053/j.gastro.2006.10.037
Kim JH, Kim JE, Liu HY, Cao W, Chen J (2008) Regulation of interleukin-6-induced hepatic insulin resistance by mammalian target of rapamycin through the STAT3–SOCS3 pathway. J Biol Chem 283(2):708–715. doi:10.1074/jbc.M708568200
Kinjyo I, Ohishi M, Shouda T, Kobayashi T, Yoshimura A (2007) Positive and negative roles of IL-6, STAT3, and SOCS3 in inflammatory arthritis. Adv Exp Med Biol 602:113–124. doi:10.1007/978-0-387-72009-8
Krishnadasan R, Bifulco C, Kim J, Rodov S, Zieske AW, Vanasse GJ (2006) Overexpression of SOCS3 is associated with decreased survival in a cohort of patients with de novo follicular lymphoma. Br J Haematol 135(1):72–75. doi:10.1111/j.1365-2141.2006.06248.x
Li WC, Ye SL, Sun RX, Liu YK, Tang ZY, Kim Y, Karras JG, Zhang H (2006) Inhibition of growth and metastasis of human hepatocellular carcinoma by antisense oligonucleotide targeting signal transducer and activator of transcription 3. Clin Cancer Res 12(23):7140–7148. doi:10.1158/1078-0432.CCR-06-0484
Martini M, Pallini R, Luongo G, Cenci T, Lucantoni C, Larocca LM (2008) Prognostic relevance of SOCS3 hypermethylation in patients with glioblastoma multiforme. Int J Cancer 123(12):2955–2960. doi:10.1002/ijc.23805
Nakagawa R, Naka T, Tsutsui H, Fujimoto M, Kimura A, Abe T, Seki E, Sato S, Takeuchi O, Takeda K, Akira S, Yamanishi K, Kawase I, Nakanishi K, Kishimoto T (2002) SOCS-1 participates in negative regulation of LPS responses. Immunity 17(5):677–687. doi:10.1016/S1074-7613(02)00449-1
Nakagawa T, Iida S, Osanai T, Uetake H, Aruga T, Toriya Y, Takagi Y, Kawachi H, Sugihara K (2008) Decreased expression of SOCS-3 mRNA in breast cancer with lymph node metastasis. Oncol Rep 19(1):33–39
Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, Heller R, Ellis LM, Karras J, Bromberg J, Pardoll D, Jove R, Yu H (2002) Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene 21(13):2000–2008. doi:10.1038/sj/onc/1205260
Ogata H, Kobayashi T, Chinen T, Takaki H, Sanada T, Minoda Y, Koga K, Takaesu G, Maehara Y, Iida M, Yoshimura A (2006) Deletion of the SOCS3 gene in liver parenchymal cells promotes hepatitis-induced hepato-carcinogenesis. Gastroenterology 131(1):179–193. doi:10.1053/j.gastro.2006.04.025
Quan ML, McCready D (2009) The evolution of lymph node assessment in breast cancer. J Surg Oncol 99(4):194–198. doi:10.1002/jso.21201
Riehle KJ, Campbell JS, McMahan RS, Johnson MM, Beyer RP, Bammler TK, Fausto N (2008) Regulation of liver regeneration and hepatocarcinogenesis by suppressor of cytokine signaling 3. J Exp Med 205(1):91–103. doi:10.1084/jem.20070820
Roberts AW, Robb L, Rakar S, Hartley L, Cluse L, Nicola NA, Metcalf D, Hilton DJ, Alexander WS (2001) Placental defects and embryonic lethality in mice lacking suppressor of cytokine signaling 3. Proc Natl Acad Sci USA 98(16):9324–9329. doi:10.1073/pnas.161271798
Sheen-Chen SM, Huang CC, Tang RP, Chou FF, Eng HL (2008) Prognostic value of signal transducers and activators of transcription 3 in breast cancer. Cancer Epidemiol Biomark Prev 17(9):2286–2290. doi:10.1158/1055-9965.EPI-08-0089
Takahashi Y, Carpino N, Cross JC, Torres M, Parganas E, Ihle JN (2003) SOCS3: an essential regulator of LIF receptor signaling in trophoblast giant cell differentiation. EMBO J 22(3):372–384. doi:10.1093/emboj/cdg057
Takahashi Y, Takahashi M, Carpino N, Jou ST, Chao JR, Tanaka S, Shigeyoshi Y, Parganas E, Ihle JN (2008) Leukemia inhibitory factor regulates trophoblast giant cell differentiation via Janus kinase 1–signal transducer and activator of transcription 3–suppressor of cytokine signaling 3 pathway. Mol Endocrinol 22(7):1673–1681. doi:10.1210/me.2008-0058
Tokita T, Maesawa C, Kimura T, Kotani K, Takahashi K, Akasaka T, Masuda T (2007) Methylation status of the SOCS3 gene in human malignant melanomas. Int J Oncol 30(3):689–694
Weber A, Hengge UR, Bardenheuer W, Tischoff I, Sommerer F, Markwarth A, Dietz A, Wittekind C, Tannapfel A (2005) SOCS-3 is frequently methylated in head and neck squamous cell carcinoma and its precursor lesions and causes growth inhibition. Oncogene 24(44):6699–6708. doi:10.1038/sj.onc.1208818
Weerasinghe P, Garcia GE, Zhu Q, Yuan P, Feng L, Mao L, Jing N (2007) Inhibition of Stat3 activation and tumor growth suppression of non-small cell lung cancer by G-quartet oligonucleotides. Int J Oncol 31(1):129–136
Yang SF, Yeh YT, Wang SN, Hung SC, Chen WT, Huang CH, Chai CY (2008) SOCS-3 is associated with vascular invasion and overall survival in hepatocellular carcinoma. Pathology 40(6):558–563. doi:10.1080/00313020802320432
Yu H, Jove R (2004) The STATs of cancer-new molecular target come of age. Nat Rev Cancer 4(2):97–105. doi:10.1038/nrc1275
Conflict of interest statement
The author(s) indicated no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ying, M., Li, D., Yang, L. et al. Loss of SOCS3 expression is associated with an increased risk of recurrent disease in breast carcinoma. J Cancer Res Clin Oncol 136, 1617–1626 (2010). https://doi.org/10.1007/s00432-010-0819-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-010-0819-6