Abstract
Objective
Abrogation of the function of TP53 gene is supposed to lead to a more aggressive breast cancer phenotype that produces a less favorable clinical outcome. The p21 gene on chromosome 6p21.2 can be stimulated by an activated TP53 gene. A product of transcription, the p21 protein, an inhibitor of cyclin-dependent kinases, has its function in gene repair and angiogenesis during cell division, and can regulate apoptosis. The purpose of this analysis was to examine for an association between the genotypes measured on two single nucleotide polymorphisms (SNPs) located within the TP53 and p21 genes.
Methods
In a clinical epidemiological case–control study, 814 individuals were recruited. 550 samples (275 cases/275 control) of peripheral blood obtained from women (aged 22–87 years) with breast cancer and from healthy women (aged 23–87 years) were genotyped for frequencies of the following gene variances: R72P/rs1042522 (gene TP53) and S31R/ss4388499 (gene p21).
Results
For the variance in gene TP53 no significant differences between the control group and women with breast cancer could be estimated. For the variance in gene p21 a statistically significant association between the SNP measured within p21 and breast cancer status was observed. The odds ratio for the increased risk for those carrying the CA genotype as opposed to the CC genotype is 1.74 (95% confidence ratio = 1.00–3.05).
Conclusion
Despite this finding p21 does not appear to act as an exclusive prognostic marker for breast cancer disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
By searching genetically determined reasons for cancer, genes have been classified according to their triggering impact. These genes regulate cellular proliferation and differentiation. A mutation can transform a regulating gene into an oncogene. A pivotal part of cancer research is focused on tumor suppressor genes, e.g. TP53 gene on chromosome 17q13 and p21 gene on chromosome 6p21.2. The human tumor suppressor gene TP53 encodes a transcription factor at the centre of a network which inhibits cell growth and stimulates apoptosis in response to cellular stresses such as DNA damage. The TP53 protein is one of the most important regulator proteins and prevents cell division in case of irreparable damages to DNA. Increase in the concentration of TP53 protein results in a transcriptional activation of p21 gene and arrest of cell cycle (Deppert et al. 1990; Kastan et al. 1991; Kuerbitz et al. 1992; Shohat et al. 1987; Steinmeyer et al. 1990). Depending on the phase of cell cycle, varying concentrations of TP53 protein can be determined reflecting the specific function within, for example growth regulation, cell proliferation or lymphocyte stimulation (Mercer et al. 1984; Milner and McCormick 1980; Reich and Levine 1984). Mutation of a gene can impair its protective function and thus promote tumorigenesis.
Studies of the TP53 gene in women with breast cancer show a mutation rate of 20–40% (Osin and Lakhani 1999). Altered TP53 proteins cannot be degraded or are not able to interact correctly with the DNA. Because of the prolonged half-life of the altered protein its concentration increases. Calabretta et al. (1986) have reported this observation as the trigger for exponential cell growth. Analysis of different human and murine tumor tissues (colorectal tumors, lung cancer, bone and lymphoid tumors, breast cancer, sarcomas, Li-Fraumeni syndrome) shows varying TP53 genes (Baker et al. 1989; Doll and Peto 1981; Lavigueur et al. 1989; Malkin et al. 1990; Takahashi et al. 1989; Vogelstein et al. 1988).
Besides the TP53 protein a series of other proteins control cell cycle (cyclins, cyclin-dependent kinases, cyclin-dependent kinase inhibitors) (Soehnge et al. 1997). One of these is the p21 protein, which is called cyclin-dependent kinase inhibitor 1. This protein delays and stops the cell cycle in the G1-phase (Kaul et al. 2003) and has its function during gene repair, within angiogenesis (Weiss et al. 2003). p21 regulates apoptosis by blocking phosphorylation of the cyclin-dependent kinase (Asada et al. 1999; Li et al. 2002). Polymorphism of the p21 gene results in altered transcripts and suppressed apoptosis. The repair mechanisms of the cell are then weakened.
Some publications report of correlations between breast cancer and polymorphisms of p21 gene (el Deiry et al. 1993; Field and Spandidos 1990; Gulbis and Galand 1993). Our study examined a variance of the p21 gene: S31R/ss4388499, which is known to be correlated to an increased risk for breast cancer (Lukas et al. 1997). We focused not only on the single SNPs R72P/rs1042522 of gene TP53 and S31R/ss4388499 of gene p21 but examined both SNPs in parallel in the largest German population-based case–control study to evaluate whether there is an association with breast cancer risk. In addition, we asked whether there is a difference between the group of women with breast cancer and the control group of women without known malignant disease concerning the variances S31R of gene p21 and R72P of gene TP53. Further questions were: Are there differences between the duration of disease and frequency of these variances? Are there differences between tumor classification (TNM system) and frequency of these variances? We also evaluated whether there would be an association between the histological grading of tumors and the genotype.
Our cohort for the present study including 275 German women with breast cancer and 275 age-matched women without known malignant disease is a well-specified and characterized cohort that is described also in the publications of Kammerer et al. (2004) and Hoyal et al. (2005).
Materials and methods
Study population
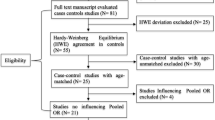
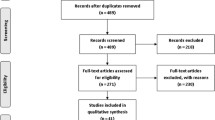
Our case–control study contains 814 questionnaires for which 427 women with breast cancer and 387 women without known malignant disease were recruited. For example, because of missing information within anamnesis, missing matched pair partner or known hereditary history of breast cancer disease, only 275 samples of each group were analyzed. The two groups were matched for age, genetic origin, status of menopause, and substitution with hormones or antagonists. All women involved reported both parents to be of German ancestry. Concerning breast cancer, classification of tumor and histology was evaluated. The study population has been described previously (Hoyal et al. 2005; Kammerer et al. 2004). All women involved in our studies signed a written informed consent. The study was conducted according to protocols approved by the Institutional Ethics Committees.
Primer design
The SpectroDESIGNER 2.0 software supplied by Sequenom (San Diego, CA, USA) was used to design PCR primers, which flank the SNP and result in a product of 80–120 bp length.
The PCR primer were chosen from a distance of 10 bp to the SNP and should fulfill the following criteria: G + C of about 40–70%, mixture of all four bases, one base repeated not more than four times, melting temperature at 60°C. The extension primers were chosen to flank the SNP directly and to result in useful truncations, and a melting temperature of 53°C. All primers were tested whether they bind only once to the genome and that they will not interact among one another. The PCR primer producing the complementary strand to that produced by the extension primer was supplied with an universal sequence, which later on allowed the assembly of a biotin molecule (Table 1).
DNA quantification
The blood samples were frozen at −80°C within 12 h of collection and sent on dry ice to Sequenom, San Diego, CA, USA. DNA from 5 ml blood of each sample was extracted using a desalting method. DNA pools were generated by combining equimolar amounts of each sample as described elsewhere (Bansal et al. 2002; Buetow et al. 2001).
Concentrations of genomic DNA were determined using PicoGreen reagent and protocol from Invitrogen (Carlsbad, CA, USA). Fluorescence was quantitated with the Fluoroskan Ascent Fluorescence 96/384 Plate Reader (Thermo Labsystems, Helsinki, Finland), at an excitation wavelength of 485 nm, and emission wavelength of 538 nm. DNA samples were adjusted to a concentration of 5 ng/μl and 5 μl was used for PCR.
PCR protocol
Standardized conditions were used for PCR. 25 ng genomic DNA were added to 200 μmol of each dNTP, 23 pmol of genespecific primer 1, 1 pmol of genespecific primer 2 with the universal sequence and 10 pmol of biotinylated universal sequence primer (5′-bio-AGA ACC CAT GCG GCA GCA AGG-3′). The reaction was started with 1 U of Taq hotstart polymerase (Qiagen, Chatsworth, CA, USA). The final volume was 50 μl. The PCR primer 2 created a complementary DNA strand to that created by the MassEXTEND primer. The temperature profile used was as follows: denaturing at 95°C for 15 min, then 45 cycles at 95°C for 20 s, 56°C for 30 s and 72°C for 20 s, completed by 72°C for 3 min.
Preparation of the single-stranded DNA for the MassEXTEND reaction
Streptavidin-coated paramagnetic beads (Dynal, Oslo, Norway) were used to immobilize the PCR products through their biotin residues. Then the double strand was denatured by adding 0.1 M NaOH at room temperature. After removal of NaOH and neutralizing by adding 10 mM Tris–HCl solution the beads with single-stranded PCR products were used for the MassEXTEND reaction.
Primer extension protocol
MassEXTEND reactions were carried out with a gene-specific extension primer and a SNP-specific nucleotide termination mixture. This termination mixture enables the extension of the primer or the truncation of the strand depending on the nucleotide at SNP location. 15 μl of reaction volume contained 1 U ThermoSequenase (Amersham Pharmacia Biotech, Piscataway, NJ, USA), 50 μmol of appropriate termination mixture and 10 pmol of test-specific MassEXTEND primers. The extension reaction was carried out as follows: initial denaturation step at 80°C for 30 s, followed by 3 cycles of 45°C for 15 s and 72°C for 1 min. Extension products were solubilized with ammonium hydroxide and 15 nl of each sample applied to a SpectroCHIP by a piezoelectric pipetting machine.
MALDI-TOF mass spectrometry
On a 384-well chip the products together with the matrix (3-hydroxy-picolinic acid) form homogenous crystals. Next the chip was analyzed with a MassARRAY mass spectrometer (Bruker-Sequenom, San Diego, CA, USA). With a 337 nm laser pulse the extension products were unhinged from the crystals, ionized and accelerated within the electric field of the mass spectrometer. The velocity is reciprocally proportional to the quotient of mass and electric charge. The bigger the mass or the longer the product, the longer was the flying time (MALDI-TOF, matrix-assisted laser desorption ionization-time-of-flight mass spectrometry). The mass spectra were analyzed with SpectroTYPER.
Statistical analysis
The χ2 test was used to determine associations between presence of the TP53 and p21 polymorphisms and various clinico-pathological features of the breast tumors, as well as for independence of the alleles (Hardy–Weinberg equilibrium). The associations were also tested with Fisher’s exact test, which is more robust than the χ2 test when class sizes are small. Analysis was carried out using the SPSS software package (Chicago, IL, USA).
Results
The purpose of the study was to test for an association between the genotypes measured on two SNPs located within the TP53 and p21 genes. To verify that systematic genotyping errors were not introduced, the genotype frequencies of each SNP were compared to their expected proportions under Hardy–Weinberg equilibrium in the control subjects. The results of this analysis are shown in Table 2. Neither SNP showed statistically significant deviations. This table also provides the observed relative allele frequencies for each SNP.
To test the association between the genotypes at each SNP and breast cancer affected status, a simple χ2 test of independence was carried out. The genotype frequencies in the cohorts and controls, as well as the results of the hypothesis test are shown in Table 3. Applying a nominal significance level of α = 0.05, a statistical significance could be evaluated for the examined p21 wild type CC-allele and breast cancer (P = 0.03391). A more frequent number of p21 CA-genotypes was recognized compared to the control. The significance could be confirmed by Fisher’s exact test (P = 0.04366). The SNP within the p21 gene is statistically significant, with the cases showing an excess of the CA-genotype as compared to the controls.
In addition to testing for association within each gene separately, they were also combined to evaluate possible interaction effects. The results of this test are also shown in Table 3. This result is not statistically significant. This was also compared using Fisher’s exact test, as well as modeling the interaction effects in a general linear model. Each of these supported the results shown in Table 3.
The total group was stratified according to different parameters: age, age of diagnosis and duration of malignancy, size of tumor (TNM system), lymph node metastases or metastases at all, histological grading of tumors. Concerning the parameter age the observed higher numbers of patients in the groups of age 50–59 and 60–69 showed the typical age relation of breast cancer disease. No statistical significance was found within the evaluated parameters. As example, Tables 4 and 5 are given for duration of malignancy and histological grading.
Histological grading of tumors correlated with genotype did not result in highly significant differences (Table 5). Statistical analysis showed P values between P = 0.21721 and P = 0.87035. For groups G1 and G2, the distribution of CG/CC:CA variances was comparably low (G1 3.125% and G2 7.103%). In group G3, the combination of variances CG/CC:CA was higher 13.333%.
Discussion
In recent times, a plethora of studies has been published which have attempted to enhance our understanding of the determinants of breast cancer disease by analyzing tumor-associated variances of genes thought to be of biological relevance in the carcinogenic process. In the present study, we have evaluated two SNPs located within the TP53 and p21 genes: R72P/rs1042522 (gene TP53) and S31R/ss4388499 (gene p21). Concerning these SNPs a case–control study was performed to epidemiologically distinguish between women without known malignant disease and women with breast cancer. As the origin of patients has an impact on analysis of genes, women with breast cancer were therefore matched for origin and also for age (±3 years) to women without known malignant disease. Women of control group were 23–87 years old (mean 58.36 years) and women with breast cancer were 22–87 years old (mean 59.26 years).
No significant correlation was observed between the combination of p21:TP53 genes with the variances CA:CC/CG and breast cancer compared to control. Also for this combination of genes there was no indication that an increased frequency would be observed by analyzing age subgroups (≤39, 40–49, 50–59, 60–69, 70–79, and ≥80 years). Further the time of diagnosis, duration of disease, did not influence the results.
Several studies have shown a correlation between mutations of gene TP53 and the TNM classification (Castells et al. 1999; Crook et al. 1997). Silva et al. (1999) reported a significant correlation between breast cancer with or without changes of TP53 protein in plasma compared to lymph node contribution, the index of proliferation, and growth of tumors. In our study, we examined possible correlations between the size of tumor and variances in the genes TP53 and p21, but we detected none. Nor did we detect a correlation between variances in these genes and lymph node metastases. In our study, we did not examine concentrations of proteins in plasma, but polymorphisms of alleles, that contribute to changes in proteins.
The most widely studied is the Ex4 + 119C > G polymorphism in codon 72 of exon 4 (TP53-01; rs1042522), which encodes an arginine-proline substitution. Meanwhile 37 SNPs in the TP53 gene have been identified (Packer et al. 2006). However, a recent pooled analysis of more than 8,700 women with breast cancer and 10,000 controls indicated no overall association of the codon 72 polymorphism with breast cancer risk, and no evidence for modification by age at onset (The Breast Cancer Association Consortium 2006). So our study is in accordance with the results of this American study. TP53 variants can also be the consequence of spontaneous mutations within the tumor cell, leading to alterations of tumor progression and chemoresistance (McCubrey et al. 2008). This recent results may elucidate all fruitless efforts to charge TP53 with responsibility for breast cancer disease.
We found no correlation between variances in gene TP53 or p21 concerning lymph node metastases or other forms of metastases. The distribution of histological results represents a normal distribution as it is depicted in the regional analysis TZM (Manual des Tumorzentrums München) (Sauer 2005) of the area of Munich, Bavaria, Germany. To explain the analysis grading of tumor as to be of significance for the G3 tumors (Table 5) the case number is too low. There is no literature about the correlation between histological grading and analysis of genes TP53 and p21.
Dunning et al. (1999) have reported a correlation for the variance R72P and breast cancer. In our study, we could not confirm these results. Our spectroscopic analysis with the described protocol could not show such a correlation. To exclude systemic errors, we compared the analyzed gene types of women with breast cancer to gene types of control by Hardy–Weinberg equation. No significant deviation could be seen within this cohort with a frequency of 6% for the proline allele in gene TP53 R72P polymorphism for the control group.
A frequency of 16% could be shown for the arginine allele in p21 S31R polymorphism. Chedid et al. (1994) report a frequency of 14% for the p21 gene polymorphism in codon 31. Our data also show a significant difference between cohort and control concerning this variance (\( \chi_{1}^{2} = 4.499 \); P = 0.03391). The odds ratio for the higher risk of women with CA gene type compared to CC gene type is 1.74 (95% confidence ratio = 1.00–3.05). Recent studies indicate an impact of the S31R variance and prognosis for other carcinomas (Bahl et al. 2000; Hachiya et al. 1999; Keshava et al. 2002; Yair et al. 2000). Such a correlation between breast cancer and SNP S31R is not yet fully understood (Powell et al. 2002; Själander et al. 1996). Our results indicate for a link within as it is also described by Powell et al. (2002) and Keshava et al. (2002) for Australians and Americans with European, African or South American origin. The serine variant (codon 31 polymorphism) is involved in the increased risk and development of breast cancer.
Small case numbers are better analyzed by Fisher exact test. Even with this test, we were able to demonstrate that the variance in the gene p21 shows a higher risk for breast cancer (P = 0.04366). The group size in the latter publications was in the same range as ours, Powell et al. (2002)––n = 351, Keshava et al. (2002)––n = 160, our study––n = 275. In our cohort we had slightly more polymorphism (16%) than it is reported for the study by Keshava et al. (2002): 12%, but as many as it was reported by Powell et al. (2002): 16%. This study did not analyze a control group but took results from six earlier studies as reference with n = 1,525. Especially for subgroups with African and South American origin a significant correlation between p21 polymorphism and breast cancer was reported. Our study confirmed the strong correlation between p21 polymorphism and breast cancer in our cohort.
The polymorphism S31R/ss4388499 in gene p21 was significantly more frequent in the breast cancer group. Despite this finding p21 does not appear to be a probable candidate biomarker for screening breast cancer disease.
References
Asada M, Yamada T, Ichijo H, Delia D, Miyazono K, Fukumuro K, Mizutani S (1999) Apoptosis inhibitory activity of cytoplasmic p21(Cip1/WAF1) in monocytic differentiation 2. EMBO J 18:1223–1234
Bahl R, Arora S, Nath N, Mathur M, Shukla NK, Ralhan R (2000) Novel polymorphism in p21(waf1/cip1) cyclin dependent kinase inhibitor gene: association with human esophageal cancer. Oncogene 19:323–328
Baker SJ, Fearon ER, Nigro JM, Hamilton SR, Preisinger AC, Jessup JM, van Tuinen P, Ledbetter DH, Baker DF, Nakamura Y, White R, Vogelstein B (1989) Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science 244:217–221
Bansal A, van den Boom D, Kammerer S, Honisch C, Adam G, Cantor CR, Kleyn P, Braun A (2002) Association testing by DNA pooling: an effective initial screen. Proc Natl Acad Sci USA 99:16871–16874
Buetow KH, Edmonson M, MacDonald R, Clifford R, Yip P, Kelley J, Little DP, Strausberg R, Koester H, Cantor CR, Braun A (2001) High-throughput development and characterization of a genome wide collection of gene-based single nucleotide polymorphism markers by chip-based matrix assisted laser desorption/ionization time-of-flight mass spectrometry. Proc Natl Acad Sci USA 98:581–584
Calabretta B, Kaczmarek L, Selleri L, Torelli G, Ming PM, Ming SC, Mercer WE (1986) Growth-dependent expression of human Mr 53,000 tumor antigen messenger RNA in normal and neoplastic cells. Cancer Res 46:5738–5742
Castells A, Puig P, Móra J, Boadas J, Boix L, Urgell E, Solé M, Capellà G, Lluis F, Fernández-Cruz L, Navarro S, Farré A (1999) K-ras mutations in DNA extracted from the plasma of patients with pancreatic carcinoma: diagnostic utility and prognostic significance. J Clin Oncol 17:578–584
Chedid M, Michieli P, Lengel C, Huppi K, Givol D (1994) A single nucleotide substitution at codon 31 (Ser/Arg) defines a polymorphism in a highly conserved region of the p53-inducible gene WAF1/CIP1. Oncogene 9:3021–3024
Crook T, Crossland S, Crompton MR, Osin P, Gusterson BA (1997) P53 mutations in BRCA1-associated familial breast cancer. Lancet 350:638–639
Deppert W, Buschhausen-Denker G, Patschinsky T, Steinmeyer K (1990) Cell cycle control of p53 in normal (3T3) and chemically transformed (Meth A) mouse cells II. Requirement for cell cycle progression. Oncogene 5:1701–1706
Doll R, Peto R (1981) The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst 66:1191–1308
Dunning AM, Healey CS, Pharoah PD, Teare MD, Ponder BA, Easton DF (1999) A systematic review of genetic polymorphisms and breast cancer risk. Cancer Epidemiol Biomarkers Prev 8:843–854
el Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B (1993) WAF1, a potential mediator of p53 tumor suppression. Cell 75:817–825
Field JK, Spandidos DA (1990) The role of ras and myc oncogenes in human solid tumours and their relevance in diagnosis and prognosis (review) 1. Anticancer Res 10:1–22
Gulbis B, Galand P (1993) Immunodetection of the p21-ras products in human normal and preneoplastic tissues and solid tumors: a review. Hum Pathol 24:1271–1285
Hachiya T, Kuriaki Y, Ueoka Y, Nishida J, Kato K, Wake N (1999) WAF1 genotype and endometrial cancer susceptibility. Gynecol Oncol 72:187–192
Hoyal CR, Kammerer S, Roth RB, Reneland R, Marnellos G, Kiechle M, Schwarz-Boeger U, Griffiths LR, Ebner F, Rehbock J, Nelson MR, Braun A (2005) Genetic polymorphisms in DPF3 associated with risk of breast cancer and lymph node metastases. J Carcinog 4:13
Kammerer S, Roth RB, Reneland R, Marnellos G, Hoyal CR, Markward NJ, Ebner F, Kiechle M, Schwarz-Boeger U, Griffiths LR, Ulbrich C, Chrobok K, Forster G, Praetorius GM, Meyer P, Rehbock J, Cantor CR, Nelson MR, Braun A (2004) Large-scale association study identifies ICAM gene region as breast and prostate cancer susceptibility locus. Cancer Res 64:8906–8910
Kastan MB, Onyekwere O, Sidransky D, Vogelstein B, Craig RW (1991) Participation of p53 protein in the cellular response to DNA damage. Cancer Res 51:6304–6311
Kaul R, Mukherjee S, Ahmed F, Bhat MK, Chhipa R, Galande S, Chattopadhyay S (2003) Direct interaction with and activation of p53 by SMAR1 retards cell-cycle progression at G2/M phase and delays tumor growth in mice. Int J Cancer 103:606–615
Keshava C, Frye BL, Wolff MS, McCanlies EC, Weston A (2002) Waf-1 (p21) and p53 polymorphisms in breast cancer. Cancer Epidemiol Biomarkers Prev 11:127–130
Kuerbitz SJ, Plunkett BS, Walsh WV, Kastan MB (1992) Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc Natl Acad Sci USA 89:7491–7495
Lavigueur A, Maltby V, Mock D, Rossant J, Pawson T, Bernstein A (1989) High incidence of lung, bone, and lymphoid tumors in transgenic mice overexpressing mutant alleles of the p53 oncogene. Mol Cell Biol 9:3982–3991
Li Y, Dowbenko D, Lasky LA (2002) AKT/PKB phosphorylation of p21Cip/WAF1 enhances protein stability of p21Cip/WAF1 and promotes cell survival 1. J Biol Chem 277:11352–11361
Lukas J, Groshen S, Saffari B, Niu N, Reles A, Wen WH, Felix J, Jones LA, Hall FL, Press MF (1997) WAF1/Cip1 gene polymorphism and expression in carcinomas of the breast, ovary, and endometrium. Am J Pathol 150:167–175
Malkin D, Li FP, Strong LC, Fraumeni JF Jr, Nelson CE, Kim DH, Kassel J, Gryka MA, Bishoff FZ, Tainsky MA et al (1990) Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science 250:1233–1238
McCubrey JA, Abrams SL, Ligresti G, Misaghian N, Wong EW, Steelman LS, Bäsecke J, Troppmair J, Libra M, Nicoletti F, Molton S, McMahon M, Evangelisti C, Martelli AM (2008) Involvement of p53 and Raf/MEK/ERK pathways in hematopoietic drug resistance. Leukemia 22:2080–2090
Mercer WE, Avignolo C, Baserga R (1984) Role of the p53 protein in cell proliferation as studied by microinjection of monoclonal antibodies. Mol Cell Biol 4:276–281
Milner J, McCormick F (1980) Lymphocyte stimulation: concanavalin A induces the expression of a 53 K protein. Cell Biol Int Rep 4:663–667
Osin PP, Lakhani SR (1999) The pathology of familial breast cancer: Immunohistochemistry and molecular analysis. Breast Cancer Res 1:36–40
Packer BR, Yeager M, Burdett L, Welch R, Beerman M, Qi L, Sicotte H, Staats B, Acharya M, Crenshaw A, Eckert A, Puri V, Gerhard DS, Chanock SJ (2006) SNP500Cancer: a public resource for sequence validation, assay development, and frequency analysis for genetic variation in candidate genes. Nucleic Acids Res 34:617–621
Powell BL, van Staveren IL, Roosken P, Grieu F, Berns EM, Iacopetta B (2002) Associations between common polymorphisms in TP53 and p21WAF1/Cip1 and phenotypic features of breast cancer. Carcinogenesis 23:311–315
Reich NC, Levine AJ (1984) Growth regulation of a cellular tumour antigen, p53, in nontransformed cells. Nature 308:199–201
Sauer H (ed) Manual Mammakarzinom 2005, Tumorzentrum München, Zuckschwert Verlag München, Wien, New York
Shohat O, Greenberg M, Reisman D, Oren M, Rotter V (1987) Inhibition of cell growth mediated by plasmids encoding p53 anti-sense. Oncogene 1:277–283
Silva JM, Dominguez G, Garcia JM, Gonzalez R, Villanueva MJ, Navarro F, Provencio M, San Martin S, Espana P, Bonilla F (1999) Presence of tumor DNA in plasma of breast cancer patients: clinicopathological correlations. Cancer Res 59:3251–3256
Själander A, Birgander R, Hallmans G, Cajander S, Lenner P, Athlin L, Beckman G, Beckman L (1996) p53 polymorphisms and haplotypes in breast cancer. Carcinogenesis 17:1313–1316
Soehnge H, Ouhtit A, Ananthaswamy HN (1997) Mechanisms of induction of skin cancer by UV radiation. Front Biosci 2:d538–d551
Steinmeyer K, Maacke H, Deppert W (1990) Cell cycle control by p53 in normal (3T3) and chemically transformed (Meth A) mouse cells. I. Regulation of p53 expression. Oncogene 5:1691–1699
Takahashi T, Nau MM, Chiba I, Birrer MJ, Rosenberg RK, Vinocour M, Levitt M, Pass H, Gazdar AF, Mima JD (1989) p53: a frequent target for genetic abnormalities in lung cancer. Science 246:491–494
The Breast Cancer Association Consortium (2006) Commonly studies single-nucleotide polymorphisms and breast cancer: results from the breast cancer association consortium. J Natl Cancer Inst 98:1382–1396
Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL (1988) Genetic alterations during colorectal-tumor development. N Engl J Med 319:525–532
Weiss RH, Marshall D, Howard L, Corbacho AM, Cheung AT, Sawai ET (2003) Suppression of breast cancer growth and angiogenesis by an antisense oligodeoxynucleotide to p21(Waf1/Cip1). Cancer Lett 189:39–48
Yair D, Ben Baruch G, Chetrit A, Friedman T, Hirsh Yechezkel G, Gotlieb WH, Fishman A, Beller U, Bar-Am A, Fishman E (2000) p53 and WAF1 polymorphisms in Jewish-Israeli women with epithelial ovarian cancer and its association with BRCA mutations. BJOG 107:849–854
Acknowledgments
The authors wish to thank the staff of the I Frauenklinik, Ludwig-Maximilians-University Munich, for their excellent contributions and Dr.rer.nat. Dr.med. Andreas Braun and Dr.rer.nat. Stephan Kammerer, Sequenom Inc. San Diego, CA, USA, for all their outstanding help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ebner, F., Schremmer-Danninger, E. & Rehbock, J. The role of TP53 and p21 gene polymorphisms in breast cancer biology in a well specified and characterized German cohort. J Cancer Res Clin Oncol 136, 1369–1375 (2010). https://doi.org/10.1007/s00432-010-0788-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-010-0788-9