Abstract
Purpose
Expression of CD44 and its variants has been shown to be relevant to tumor progression in various human malignancies. We evaluated the expression of CD44v6 in the primary lesions of stage I non-small cell lung cancer (NSCLC) and correlated the expression level to its prognosis.
Methods
The expression of CD44v6, measured by immunohistochemistry, was assessed in the tumor specimens from 190 patients with stage I NSCLC. Each slide was assigned a score: the average of the score of tumor cells staining multiplied by the score of staining intensity. And depending on the cut-off score based on receiver operating characteristic (ROC) curve analysis, the CD44v6 expression was categorized into high- and low-level groups, which were then correlated directly with the clinical outcomes.
Results
The high expression of CD44v6 was detected more frequently in the squamous cell carcinoma (38 of 71 patients, 53.5%) than in the other types of carcinoma (p < 0.05). The Kaplan–Meier survival curves showed that high level expression of CD44v6 indicated a better post-operative survival (p = 0.006), especially for stage IB disease (p = 0.049) and squamous cell carcinoma (p = 0.029). The multivariate analysis also confirmed that the expression of CD44v6 was an independent prognostic indicator (p = 0.011).
Conclusions
CD44v6 might be correlated with histogenesis of NSCLC, and its decreased expression may be an adverse prognostic indicator for the patients with stage I NSCLC, especially for those with stage IB diseases. Patients of this subgroup might need adjuvant therapy additionally.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
CD44 is a multifunctional and multistructural transmembrane glycoprotein (Naor et al. 1997; Rudzki and Jothy 1997). It is encoded by a single gene composed of at least 20 exons, ten of which, i.e., exon 6–15 (v1–v10), can be alternatively spliced. Exon 1–5 (s1–s5), and 16–20 (s16–s20) are almost expressed in all types of tissues, producing standard CD44 protein.
CD44 was initially characterized as a lymphocyte-homing receptor and a receptor for hyaluronan (Naor et al. 1997; Rudzki and Jothy 1997). Subsequently, in early 1990s, it was found that the CD44 variants, chiefly CD44v6, regulate tumor invasion, progression, and metastasis of carcinoma in rat experimental models (Gunthert et al. 1991). So far, CD44 expression was shown to be relevant to tumor progression in various human malignancies including liver carcinoma (Coradini et al. 2004), colonic carcinoma (Wielenga et al. 1993; Mulder et al. 1994), breast carcinoma (Kaufmann et al. 1995), lymphoma (Stauder et al. 1995), melanoma (Manten-Horst et al. 1995), gastric carcinoma (Miwa et al. 1996), and lung carcinoma (Hirata et al. 1998; Ramasami et al. 2000). It has been shown that CD44v6 is probably capable to help promoting cancer cells to adhere to the vascular endothelium and base membranes, as well as enhancing the motility of cancer cells (Harn et al. 1996; Dong et al. 2003; Marhaba and Zoller 2004; Kuhn et al. 2007).
Currently, lung cancer is the most common cause of cancer associated death worldwide. The 5-year survival rate of stage I non-small cell lung carcinoma (NSCLC) has been dramatically improved due to the early diagnosis and surgery (approximately 70%) (Bains 1991). To further improve patient’s survival, it is important to identify relevant biomarkers in stage I NSCLC with adverse prognosis and modify the therapeutic strategy for these patients accordingly. In the present study, we assessed the expression of CD44v6 in the primary lesions of stage I NSCLC to elucidate its significance in clinical prognosis.
Patients and methods
Patients
The study was approved by the Research Ethics Committee of the Cancer Center of Sun Yat-Sen University. From January 1990 to March 2002, we enrolled 190 patients with stage I NSCLC who received surgical treatment with curative intent, and the resected specimens were assessed with immunohistochemistry (IHC) analysis. We verified and updated the survival data in the patient records through May 2007 using the database. Patients were selected based on the following eligibility criteria: (a) histopathologically proofed NSCLC; (b) disease stage was T1 ~ 2N0M0 by the 1997 American Joint Committee on Cancer/International Union Against Cancer Staging System; (c) patients were at least 18 years of age, with no evidence of metastatic disease as determined by history, physical examination, and blood chemistry analysis or routine computed tomography; (d) all patients received no adjuvant therapy. Patients were excluded based on the following criteria: history of previously treated cancer other than basal or squamous cell carcinoma of the skin or with preoperative chemotherapy and/or radiotherapy (ASCO 1997).
Immunohistochemistry
Immunoperoxidase stain for CD44 variant 6 (CD44v6; 1:50 dilution; Novocastra Laboratories, Ltd., Newcastle upon Tyne, United Kingdom) was done on 4-μm-thick paraffin sections. The slides were deparaffinized in xylene and then hydrated prior to antigen retrieval by microwaving in sodium citrate buffer (pH 6.0). The slides were then incubated with a peroxidase block, followed by the primary antibody. After a PBS wash, the slides were incubated with the secondary antibody, and 3,3′-diaminobenzidine. The peroxidase block, secondary antibody and 3,3′-diaminobenzidine were from the DakoCytomation EnVision System (Glostrup, Denmark). After a hematoxylin counterstain (Hematoxylin 7211; Richard-Allen Scientific, Kalamazoo, MI), the slides were coverslipped.
IHC scoring
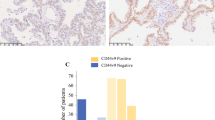
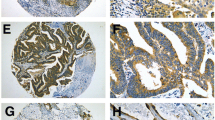
A positive control sample was evaluated with each batch of slides. Each slide was assigned a score: the average of the score of tumor cell staining multiplied by the score of staining intensity (Fig. 1). Tumor cell staining was assigned a score using a semiquantitative six-category grading system: 0, none of tumor cells staining; 1, 1–10% of tumor cells staining; 2, 11–25% of tumor cells staining; 3, 26–50% of tumor cells staining; 4, 51–75% of tumor cells staining; 5, more than 75% of tumor cells staining. Stain intensity was assigned a score using a semiquantitative four-category grading system: 0, non-staining; 1, weak staining; 2, moderate staining; 3, strong staining. Two experienced pathologists independently scored 300 NSCLC samples including the cases used in this study blinded to clinical follow-up data. The complete score agreement between these two pathologists is 86% of the cases, indicating that the scoring method was highly reproducible. The third pathologist intervened and evaluated the cases with different IHC scores. The score was selected when the third pathologist agreed with one of previous pathologists. For the cases with three different score, the three pathologists would review these cases together to reach an agreement.
Selection of cut-off score
We selected the cut-off score for CD44v6 based on receiver operating characteristic (ROC) curve analysis (Hanley 1989; Spira and Ettinger 2004; Zlobec et al. 2007; Zhu et al. 2009). The sensitivity and specificity for the outcome under study was plotted, thus generating an ROC curve. The score having the closest distance to the point with both maximum sensitivity and specificity (i.e., the point [0.0, 1.0] on the curve) was selected as the cut-off score, leading to the greatest number of tumors correctly classified as having or not having the clinical outcome. The area under the ROC curve (AUC) was calculated to estimate the discriminatory power of CD44v6 over the entire range of scores for overall survival. Both generation and analysis of the ROC curve were performed by MedCalc statistical software package 11.0.1 (MedCalc Software bvba, Belgium).
Statistics
The goal of this study was to investigate the possible association between expression of CD44v6 and overall survival of early NSCLC. This is defined as the time between surgery and death or the last follow-up date. Distributions were estimated with the Kaplan–Meier method. The relationship between survival and each variable was determined with the log-rank test. Multivariate analysis of prognostic factors was performed using Cox’s regression model. A significant difference was declared if the p value from a two-tailed test was less than 0.05. All of the statistical analysis was performed using the SPSS 13.0 for Windows software system (SPSS Inc, Chicago, IL).
Results
Table 1 lists the demographic and clinicopathological parameters of the 190 patients. The mean follow-up time is 1,617.5 days. Among the 190 patients, 48 (25.3%) died of tumor and 142 (74.7%) were still alive at last follow-up. The 3- and 5-year survival rates were 78 and 74%, respectively. According to the ROC curve in our study (Fig. 2), threshold value of 13.3333 was the closest to the point with both maximum sensitivity and specificity, and thereby selected as the cut-off score. The AUC of our ROC curve analysis was 0.637 (95% confidence interval: 0.564–0.706). Therefore, the 190 patients with stage I NSCLC were categorized into two groups, namely high expression group (n = 57) and low expression group (n = 133).
The correlation of CD44v6 with histopathological and prognostic indices was shown in Table 2. The high expression of CD44v6 was detected more frequently in the squamous cell carcinoma (38 of 71 patients, 53.5%) than other types of carcinoma (p < 0.05). However, no significant correlations were found between CD44v6 expression and gender, age, tumor size, and pathological stage.
The Kaplan–Meier survival curves showed that patients with high-level expression of CD44v6 had a better post-operative survival than those with low-level expression (p = 0.006, Fig. 3a). In further stratified analysis split by pathological stage, the expression of CD44v6 still had a statistically significant influence on survival of patients with stage IB NSCLC (p = 0.049, Fig. 3c). Although a similar trend was also seen among the patients with stage IA NSCLC, the result was not significant (p = 0.054, Fig. 3b). The stratified analysis split by histological types showed that high CD44v6 expression was a favorable prognostic indicator for the patients with squamous cell carcinoma (p = 0.029, Fig. 4a), but not for those with adenocarcinoma (p = 0.244, Fig. 4b), and bronchioalveolar carcinoma (p = 0.185, Fig. 4c). Patients with large-cell carcinoma, adenosquamous carcinoma, and other unspecified NSCLC were not included in this analysis due to the small sample sizes of these tumors.
We then performed analysis using the Cox proportional hazards model to identify factors involved in overall survival of stage IB NSCLC patients (Table 3). The univariate analysis revealed that tumor diameter (p = 0.006), pathological stage (p = 0.006), and CD44v6 (p = 0.009) were significant prognostic indicators for overall survival, and thereby selected as the parameters to be included in the same Cox regression model. Further multivariate analysis showed that only CD44v6 was confirmed to be an independent prognostic factor (p = 0.011).
Discussion
The present study demonstrated that the staining patterns of CD44v6 variant in neoplastic pulmonary tissue varied with morphological subtypes. High expression of CD44v6 was more frequently seen in squamous cell carcinoma than in any other histological types of NSCLC, which was consistent with the previous studies (Fasano et al. 1997; Hirata et al. 1998). Squamous cell carcinoma of the lung is thought to arise from metaplastic squamous epithelium that progresses through dysplasia, carcinoma in situ, and finally into invasive carcinoma, and the squamous metaplasia is believed to develop through the replacement of the ciliated epithelium by bronchial basal cells that show strong positivity for CD44v6 protein expression. In other words, squamous cell carcinoma actually recapitulates the staining pattern of normal bronchial basal cells. On the contrary, adenocarcinomas of the lung usually show down-regulated or absent CD44 and, in particular, CD44v6 expression (Wimmel et al. 1997; Ramasami et al. 2000). Similarly, high level of CD44v6 expression only existed in 9 out of 60 cases (15%) in our study. Moreover, CD44v6 staining of type 2 pneumocytes was also seen in normal adult lungs (Fasano et al. 1997); thus it is rational to predict that bronchioloalveolar carcinomas might show CD44v6 expression which mirrors that of normal type 2 pneumocytes. However, our data displayed the high expression rate of CD44v6 in bronchioloalveolar carcinomas was only 15.4% (6 out of 39 cases). The inability to identify the trend mentioned earlier may be the result of a different immunohistochemistry scoring system applied. Actually, total negative staining of CD44v6 was merely shown in nine specimens, which means that up to 76.9% of bronchioloalveolar carcinomas (30 out of 39 cases) presented CD44v6 expression to some extent. The staining patterns obtained in our research indicated that CD44v6 may play an important role in lung carcinoma histogenesis and in determining the fate of neoplastic differentiation. Therefore, further analysis of CD44 gene and gene product at different stages in the progression of NSCLC might provide more clues in this regard.
The exact role that CD44v6 expression plays in the prognosis of NSCLC remains to be defined. The present findings are controversial in the literatures. Both Tran et al. (1997) and Miyoshi et al. (1997) have investigated the expression of CD44v6 in primary tumors and lymph node metastasis and found that CD44v6 could have contributed to local lymph node involvement in NSCLC. But there was no significant correlation between the CD44 variant isoform protein expression and tumor stage, recurrence, and survival rates. According to Hirata and his colleagues there was no association observed between CD44v6 expressions and lymphatic or vascular vessel invasion; however, it showed that CD44v6 expression was of prognostic value in predicting clinical progression of patients with stage I NSCLC (Hirata et al. 1998). Another research which concerned pulmonary adenocarcinoma alone reported that the level of CD44v6 expression in primary lesions was not associated with lymph node metastasis or tumor stage and low level of CD44v6 expression predicted a poor post-operative survival, independent of stage (Ramasami et al. 2000). Suzuki and Yamashiro (2002) also obtained a similar result that decreased expressions of CD44v3 and CD44v6 were related to the invasion in the lung adenocarcinoma.
Our data indicate that CD44v6 is an independent prognostic indicator in the multivariate analysis by the Cox’s regression model. And the high expression of CD44v6 predicted a better post-operative survival for the patients with stage I NSCLC, especially for those with stage IB NSCLC. These findings are inconsistent with those of Hirata et al.’s (1998) study, which might be attributed partially to the different immunohistochemistry scoring systems utilized. Compared to most of the previous studies which only considered staining percentage, our scoring system took both staining percentage and intensity into account. Meanwhile, we selected the cut-off score based on receiver operating characteristic (ROC) curve analysis where the score closest to the point with both maximum sensitivity and specificity was chosen. This method allowed the greatest number of tumors to be correctly classified as carrying or not carrying the clinical outcome (Hanley 1989; Spira and Ettinger 2004; Zlobec et al. 2007; Zhu et al. 2009). In addition, it should be noted that 58% of patients received chemotherapy before or after surgery in Hirata’s study, while all the patients in our study received no adjuvant therapy. Finally, because the CD44 molecule has been associated with diverse physiological functions such as cell–cell adhesion (St et al. 1990), cell–matrix interaction (Carter and Wayner 1988), and lymphocyte homing and circulation (Stamenkovic et al. 1989), it is reasonable to predict that tumor invasion and metastasis could be enhanced by either cell–matrix interaction in tumors that show CD44 overexpression or cell–cell interaction in tumors that show CD44 downregulation. Obviously, the critical issue lies in the mechanism which takes the advantage in the natural process of different tumor types in different stages. Contrary to the reports of Ramasami et al. (2000) and Suzuki and Yamashiro (2002), we found that decreased CD44v6 expression was an adverse prognostic indicator for squamous cell carcinoma rather than adenocarcinoma or bronchioalveolar carcinoma. Except for the distinct scoring systems used, this may be attributed to the different distributions of pathological stages studied amongst these researches. Both of the previous studies involved lung carcinomas of stage I–III (even stage IV in Suzuki and Yamashiro’s study) while our study only focused on stage I diseases. In addition, 17 cases of possible bronchial origin and 29 cases of dominant bronchioloalveolar (BAC-like) pattern were also included in Ramasami et al.’s series, which might influence the clinical outcome. However, our findings should also be interpreted with caution, particularly in view of the small number of patients in the high-expression group after stratification by histology (9 cases in adenocarcinomas and 6 cases in bronchioalveolar carcinomas, respectively). Therefore, it is necessary to enhance the statistical power in the future to verify the trend.
In conclusion, the present study elucidates that CD44v6 might be correlated with the histogenesis of NSCLC, and its decreased expression may be an adverse prognostic indicator for the patients with stage I NSCLC, especially for those with stage IB diseases. This finding suggests that patients of this subgroup may benefit from adjuvant therapy; however, further studies with randomization and longer follow-up are needed for the establishment of a safe and effective management plan.
References
ASCO (1997) Clinical practice guidelines for the treatment of unresectable non-small-cell lung cancer. Adopted on May 16, by the American Society of Clinical Oncology. J Clin Oncol 15(8):2996–3018
Bains MS (1991) Surgical treatment of lung cancer. Chest 100(3):826–837
Carter WG, Wayner EA (1988) Characterization of the class III collagen receptor, a phosphorylated, transmembrane glycoprotein expressed in nucleated human cells. J Biol Chem 263(9):4193–4201
Coradini D, Zorzet S, Rossin R et al (2004) Inhibition of hepatocellular carcinomas in vitro and hepatic metastases in vivo in mice by the histone deacetylase inhibitor HA-But. Clin Cancer Res 10(14):4822–4830
Dong WG, Sun XM, Yu BP, Luo HS, Yu JP (2003) Role of VEGF and CD44v6 in differentiating benign from malignant ascites. World J Gastroenterol 9(11):2596–2600
Fasano M, Sabatini MT, Wieczorek R, Sidhu G, Goswami S, Jagirdar J (1997) CD44 and its v6 spliced variant in lung tumors: a role in histogenesis. Cancer 80(1):34–41
Gunthert U, Hofmann M, Rudy W et al (1991) A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell 65(1):13–24
Hanley JA (1989) Receiver operating characteristic (ROC) methodology: the state of the art. Crit Rev Diagn Imaging 29(3):307–335
Harn HJ, Ho LI, Shyu RY et al (1996) Soluble CD44 isoforms in serum as potential markers of metastatic gastric carcinoma. J Clin Gastroenterol 22(2):107–110
Hirata T, Fukuse T, Naiki H, Hitomi S, Wada H (1998) Expression of CD44 variant exon 6 in stage I non-small cell lung carcinoma as a prognostic factor. Cancer Res 58(6):1108–1110
Kaufmann M, Heider KH, Sinn HP, von MG, Ponta H, Herrlich P (1995) CD44 variant exon epitopes in primary breast cancer and length of survival. Lancet 345(8950):615–619
Kuhn S, Koch M, Nubel T et al (2007) A complex of EpCAM, claudin-7, CD44 variant isoforms, and tetraspanins promotes colorectal cancer progression. Mol Cancer Res 5(6):553–567
Manten-Horst E, Danen EH, Smit L et al (1995) Expression of CD44 splice variants in human cutaneous melanoma and melanoma cell lines is related to tumor progression and metastatic potential. Int J Cancer 64(3):182–188
Marhaba R, Zoller M (2004) CD44 in cancer progression: adhesion, migration and growth regulation. J Mol Histol 35(3):211–231
Miwa T, Watanabe A, Yamada Y et al (1996) Progression in gastric carcinoma relative to the ratio of CD44 epithelial variant transcript to CD44 hematopoietic variant transcript. Cancer 77(1):25–29
Miyoshi T, Kondo K, Hino N, Uyama T, Monden Y (1997) The expression of the CD44 variant exon 6 is associated with lymph node metastasis in non-small cell lung cancer. Clin Cancer Res 3(8):1289–1297
Mulder JW, Kruyt PM, Sewnath M et al (1994) Colorectal cancer prognosis and expression of exon-v6-containing CD44 proteins. Lancet 344(8935):1470–1472
Naor D, Sionov RV, Ish-Shalom D (1997) CD44: structure, function, and association with the malignant process. Adv Cancer Res 71:241–319
Ramasami S, Kerr KM, Chapman AD, King G, Cockburn JS, Jeffrey RR (2000) Expression of CD44v6 but not E-cadherin or beta-catenin influences prognosis in primary pulmonary adenocarcinoma. J Pathol 192(4):427–432
Rudzki Z, Jothy S (1997) CD44 and the adhesion of neoplastic cells. Mol Pathol 50(2):57–71
Spira A, Ettinger DS (2004) Multidisciplinary management of lung cancer. N Engl J Med 350(4):379–392
St JT, Meyer J, Idzerda R, Gallatin WM (1990) Expression of CD44 confers a new adhesive phenotype on transfected cells. Cell 60(1):45–52
Stamenkovic I, Amiot M, Pesando JM, Seed B (1989) A lymphocyte molecule implicated in lymph node homing is a member of the cartilage link protein family. Cell 56(6):1057–1062
Stauder R, Eisterer W, Thaler J, Gunthert U (1995) CD44 variant isoforms in non-Hodgkin’s lymphoma: a new independent prognostic factor. Blood 85(10):2885–2899
Suzuki H, Yamashiro K (2002) Reduced expression of CD44 v3 and v6 is related to invasion in lung adenocarcinoma. Lung Cancer 38(2):137–141
Tran TA, Kallakury BV, Sheehan CE, Ross JS (1997) Expression of CD44 standard form and variant isoforms in non-small cell lung carcinomas. Hum Pathol 28(7):809–814
Wielenga VJ, Heider KH, Offerhaus GJ et al (1993) Expression of CD44 variant proteins in human colorectal cancer is related to tumor progression. Cancer Res 53(20):4754–4756
Wimmel A, Schilli M, Kaiser U et al (1997) Preferential histiotypic expression of CD44-isoforms in human lung cancer. Lung Cancer 16(2–3):151–172
Zhu ZH, Sun BY, Ma Y et al (2009) Three immunomarker support vector machines-based prognostic classifiers for stage IB non-small-cell lung cancer. J Clin Oncol 27(7):1091–1099
Zlobec I, Steele R, Terracciano L, Jass JR, Lugli A (2007) Selecting immunohistochemical cut-off scores for novel biomarkers of progression and survival in colorectal cancer. J Clin Pathol 60(10):1112–1116
Acknowledgments
This work was supported by the grant from Sun Yat-sen University Clinical Research 5010 plan ChiCTR-TRC-00000142.
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
D. Situ, H. Long, P. Lin and Z. Zhu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Situ, D., Long, H., Lin, P. et al. Expression and prognostic relevance of CD44v6 in stage I non-small cell lung carcinoma. J Cancer Res Clin Oncol 136, 1213–1219 (2010). https://doi.org/10.1007/s00432-010-0771-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-010-0771-5