Abstract
Purpose
To investigate the clinicopathologic significance and predictive value of Bmi-1 expression in patients with colon cancer.
Methods
Bmi-1 expression was assessed by immunohistochemistry, PCR, and western blotting in specimens from 203 patients and by immunohistochemistry in 66 specimens of lymph node metastasis (LNM).
Results
Positive staining of Bmi-1 occurred in 7.9% (16/203), 66.5% (135/203), and 86.4% (57/66) of specimens from normal tissue, colon cancer, and LNM, respectively. Staining was significantly correlated with clinical stage, depth of invasion, nodal involvement, distant metastasis, and Ki67 level. Bmi-1 was upregulated at the transcriptional and translational levels. Patients with Bmi-1-positive localized tumors had a much lower 5-year disease-free survival (relative risk 2.919, P < 0.0001) and overall survival (relative risk 5.056, P < 0.0001). Bmi-1 immunoreactivity emerged as an independent prognostic factor in the multivariate analysis.
Conclusions
We have shown that expression of Bmi-1 was elevated in colon cancer and might serve as an independent prognostic marker.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colon cancer is one of the most common causes of cancer death throughout the world. Molecular investigations have provided evidence that multiple alterations in genes of the striking signaling pathway, such as adenomatous polyposis coli protein (APC), β-catenin, and c-myc, have been shown to be involved in colonic carcinogenesis (Petrova et al. 2008; Sansom et al. 2007). In spite of intensive study, however, the molecular mechanisms underlying the development and progression of colon cancer remain poorly understood. It is of great clinical importance to further investigate the molecular mechanism of this cancer and find valuable early diagnostic markers with high specificity and sensitivity as well as novel therapeutic targets.
The family of polycomb group (PcG) proteins consists of chromatin-modifying proteins that play important roles in the maintenance of embryonic and adult stem cells and that are involved in the development of cancer (Valk-Lingbeek et al. 2004). The B cell-specific Moloney murine leukemia virus insertion site 1 gene (Bmi-1), the first PcG gene to be found, was originally identified as an oncogene that cooperated with c-Myc in the initiation of mouse pre-B cell lymphomas (Haupt et al. 1991). Recently, the role of Bmi-1 in the self-renewal of stem cells and as a link between the maintenance of cellular homeostasis and tumorigenesis has been highlighted (Liu et al. 2006). A body of evidence has suggested that Bmi-1 was a promising novel target gene involved in the carcinogenesis of various malignancies, including hepatocellular carcinoma, oral cancer, breast cancer, and nasopharyngeal cancer (Berezovska et al. 2006; Kim et al. 2004a, b; Leung et al. 2004; Liu et al. 2008; Nowak et al. 2006; Song et al. 2006; Wang et al. 2008). In colon cancer, it has also been reported that expression of Bmi-1 was dysregulated and correlated with the expression of p16INK4a and p14ARF (Kim et al. 2004a, b). Its overexpression was correlated with the malignant grades of human digestive precancerous tissues, which suggests that advanced Bmi-1 dysregulation might predict malignant progression (Tateishi et al. 2006). However, until now, the role of Bmi-1 in the pathogenesis and its value for the prognosis of colon cancer remain unclear.
In this study, we used immunohistochemistry to explore the expression of Bmi-1 both in primary colon cancer specimens paired with adjacent normal mucosa tissue and archived specimens of lymph node metastasis (LNM) and investigated whether Bmi-1 could be used as an independent biomarker to predict metastasis/recurrence and prognosis in patients with colon cancer.
Materials and methods
Tissue samples
All patient-derived specimens were collected and archived under protocols approved by our institutional review boards. Formalin-fixed, paraffin-embedded samples for immunohistochemistry were obtained from the tumors and adjacent normal tissues of 203 patients with primary colon cancer who had undergone surgery between January 2001 and December 2003, from the Shanghai Jiaotong University affiliated with the Shanghai First People’s Hospital Medical Center. The diagnoses were confirmed by at least two pathologists, and staging was made on the basis of pathological findings according to the American Joint Committee on Cancer (AJCC). There were 86 men and 117 women with a mean age of 65 ± 15 years (range 22–95 years). Sixty-six specimens of primary colon cancer paired with normal colonic mucosa and LNM were available in our archive. Using the tumor (T) node (N) metastasis (M) system, we classified 24 of the 203 patient-derived colon cancer specimens as stage I, 81 as stage II, 80 as stage III, and 18 as stage IV. Among the 18 patients at AJCC stage IV, 10 who had undergone complete metastasis resection (R0) were included for analysis of progression-free survival. Disease-free survival (DFS) and overall survival (OS) rates were defined as the interval from the initial surgery to clinically or radiologically proven metastasis/local recurrence and death, respectively. The final date for conducting the analysis of follow-up data was 29 June 2008, and the median duration of the follow-up period was 61 months (range 9–89 months).
Extent of differentiation
The degree of differentiation was evaluated by a pathologist according to the pathological differences between cancer tissues and normal colon tissues. For adenocarcinoma, grades 1, 2, and 3 correspond to well-differentiated, moderately differentiated, and poorly differentiated tissues, respectively. In the Cox analysis, grades 1 and 2 (well and moderate) were combined for comparison with grade 3 (poor).
TMA construction
After screening hematoxylin and eosin (H&E)-stained slides for optimal tumor tissue and adjacent tissue at a distance of 2 cm from the tumor, we constructed tissue microarray (TMA) slides using the specimens from the 203 patients and the 66 specimens of primary colon cancer paired with normal colonic mucosa and LNM in our archive (in collaboration with Shanghai Biochip, Shanghai, China). Two cores were taken from each specimen of formalin-fixed, paraffin-embedded colon cancer tissue and normal colonic mucosa as well as at least one specimen of LNM using punch cores that measured 2.0 mm in greatest dimension from the non-necrotic area of the tissue.
Immunohistochemical analysis
Immunohistochemistry was performed as described previously (Zhuang et al. 2008). Briefly, immunostaining was performed using an Envision kit (Dako Cytomation, Glostrup, Denmark). Antigen retrieval was performed in preheated (95–98°C) citrate buffer (pH 6.0) for 30 min. Sections were incubated overnight at 4°C with the primary antibody against Bmi-1 (1:50, Abcam, UK) and the proliferation index Ki67 (1:50, Dako), and then incubated with the secondary antibody (Envision System, Dako) for 30 min at room temperature. After rinsing three times in PBS for 10 min each, the sections were incubated with 3,3-diaminobenzidine (DAB) liquid for 1 min, counterstained with Mayer hematoxylin, dehydrated, and then mounted.
Evaluation of immunohistochemical staining
Immunoreactivity was evaluated independently on the basis of the intensity and extent of staining by three researchers who were blinded to patient outcome. The staining intensity for Bmi-1 was scored as 0 (negative), 1 (weak), 2 (moderate), and 3 (strong). The staining extent was scored as 0 (0%), 1 (1–25%), 2 (26–50%), 3 (51–75%), and 4 (76–100%) according to the percentage of positively stained cells. The sum of the intensity and extent scores was used as the final staining score. The specimens were divided into three groups according to their overall scores, which were as follows: 0–1 = negative; 2–4 = weakly positive; 5–7 = strongly positive. For the analysis of the Bmi-1 expression, weakly positive and strongly positive groups were combined for comparison with the negative group. The Ki67 proliferation index was scored according to the percentage of cells with positive nuclear staining and was divided into two groups: negative (less than 10% of cells with positive nuclei) and positive (more than 10% of cells with positive nuclei). In cases of discrepant assessments, slides were reinvestigated by both pathologists under a multi-head microscope, and an agreement was obtained.
RNA extraction and RT-PCR
Eight specimens of frozen tumor tissue and the corresponding normal tissues were obtained from 203 patients and used for semi-quantitative RT-PCR and western blot analysis. RNA isolation was done according to the manufacturer’s instructions (TRIzol, Invitrogen, USA). Reverse transcription was done using 1 μg of RNA according to the recommendations of the manufacturer (Promega, USA). One microliter of the cDNA was used as a template for the specific PCR reactions using the sequence 5′CTCCACCTCTTCTTGTTT3′ as the sense primer and 5′TCATTTACCCAGTAGTCG3′ as the antisense primer for Bmi-1 (168-bp fragment). For β-actin, the sequence of the sense primer was 5′GCGCGGCTACAGCTTCAC3′ and that of the antisense primer was 5′GGGGCCGGACTCGTCATA3′ (490-bp fragment). For amplification, a DNA Engine PTC100 (MJ Research, Watertown, MA) thermocycler was used. Cycling conditions were as follows: initial denaturation (1 min at 95°C) followed by 25 cycles of denaturation (1 min at 94°C), annealing (1 min at 94°C), and elongation (45 s at 72°C). After the last cycle, a final extension (10 min at 72°C) was added, and the amplified samples were then maintained at 4°C. Ten microliters of the products was run on a 2% agarose gel, stained by ethidium bromide, and analyzed under UV light.
Western blot analysis
The protein was extracted from frozen colon cancer tissue using the Whole Protein Extraction Kit (Fermentas, USA), and the concentration was determined using the BCA protein assay kit (PIERCE, Rockford, IL). Equal amounts of protein were separated by electrophoresis on a 9.0% SDS-polyacrylamide gel and transferred onto polyvinylidene difluoride (PVDF) membranes. The membranes were blocked with 5% non-fat milk solution for 1 h, and incubated with a primary monoclonal antibody to Bmi-1 (1:200, Abcam, UK) overnight at 4°C. After washing with TBST, the membranes were incubated with a secondary antibody against mouse immunoglobulin G. The membranes were washed, and protein was detected by enhanced chemiluminescence (Amersham Pharmacia Biotech) according to the manufacturer’s instructions. The membranes were then stripped and probed with an anti-actin rabbit monoclonal antibody (Cell Signaling Technology, USA) to confirm equal loading of the samples. Elevated expression in the cancerous tissue compared with the non-cancerous tissue was confirmed by immunostaining.
Real-time RT-PCR
Eight specimens of frozen tumor tissue and corresponding normal tissue from the 203 patients were used for real-time RT-PCR analysis. RNA was isolated according to the manufacturer’s instructions (TRIzol, Invitrogen, USA). One microgram of total RNA from each sample was subjected to first-strand cDNA synthesis according to the recommendations of the manufacturer (Promega, USA). Twenty nanograms cDNA was used as a template for the specific PCR reactions. The primers for Bmi-1 were the same as those used for semi-quantitative RT-PCR. For β-actin, the sequence of the forward primer was 5′CTCCTTAATGTCACGCACGAT3′, and that for the reverse primer was 5′CATGTACGTTGCTATCCAGGC3′ (251-bp fragment). Quantitative PCRs were performed on a Mastercycler® ep realplex (Eppendorf) with an IQTM SYBR Green Supermix Kit (BIO-RAD) according to the manufacturer’s protocol. Cycling conditions were as follows: initial denaturation (10 min at 95°C) followed by 42 cycles of denaturation (10 s at 95°C), annealing (20 s at 58°C), and elongation (1 min at 72°C). The results were calculated using the formulas below:
where Ct a and Ct b represent the cycle thresholds for Bmi-1 and β-actin, respectively, in the cancer group; and Ct c and Ct d represent the cycle thresholds for Bmi-1 and β-actin, respectively, in the normal group. ΔΔCt (the comparative Ct) represents the difference in cycle thresholds between the cancer group and normal group. The difference in gene expression between the cancer group and the normal group was calculated using 2−(ΔΔCT(a − b) − (c − d)).
Statistical analysis
All statistical analyses were carried out using the SPSS 13.0 statistical software package (SPSS Inc., Chicago, IL). The χ 2 test for proportionality was used to analyze the relationship between the expression of Bmi-1, the Ki67 proliferation index, and the clinicopathological variables. The survival rates were calculated by the Kaplan–Meier method, and the differences between the survival curves were examined by the log-rank test. Univariate Cox proportional hazards regressions were applied to estimate the individual hazard ratio (HR) for the DFS and OS. The significant variables in the univariate analyses (P < 0.05) were then put into the multivariate analysis. The HR with 95% confidence interval was measured to estimate the hazard risk of individual factors. A P value of less than 0.05 was considered to be statistically significant.
Results
Bmi-1 expression in normal colonic mucosa, cancerous tissue, and LNM
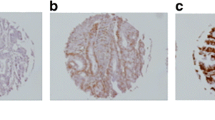
In the colon cancer tissue specimens, staining was observed mainly in the nuclei of the cancer cells (Fig. 1). Among the 203 specimens of normal mucosa, 16 (7.9%) showed weakly positive staining; whereas among the 203 specimens of primary colon cancer in situ, 135 (66.5%) showed positive staining with 56 (27.6%) showing weakly positive and 79 (38.9%) showing strongly positive staining (Table 1). Our investigation of the expression of Bmi-1 protein in primary colon tumors and LNM samples from our archive indicated that Bmi-1 protein expression was elevated in the LNM samples compared with primary tumor samples in 31 of 66 pairs; in 29 pairs, there was no difference in Bmi-1 expression levels; and in 6 pairs, Bmi-1 expression levels were decreased in the LNM samples. Overexpression of Bmi-1 was observed in 57 of the 66 LNM samples, with 44 (66.7%) showing strongly positive staining (Fig. 1d). Statistical analysis indicated that the LNM samples exhibited significantly elevated expression of Bmi-1 relative to that observed in the archived primary colon cancer specimens.
Analysis of Bmi-1 protein expression by immunohistochemistry in colon cancer, normal colonic mucosa, and LNM specimens. Bmi-1 expression was localized within the nuclei. a Elevated Bmi-1 expression in the tumor cells of colon cancer tissue (T) compared with those of adjacent normal mucosa (N); b Bmi-1 staining in well-differentiated colon cancer; c Bmi-1 staining in poorly differentiated colon cancer; d Bmi-1 overexpression in LNM (original magnification ×100)
To confirm the expression levels of Bmi-1 seen by immunostaining in the specimens from our TMA, eight specimens were selected from the 203 patients for semi-quantitative RT-PCR and western blot analysis. All eight of these human colon cancer samples showed higher expression levels of both Bmi-1 mRNA and protein compared to that in the adjacent non-cancerous tissue (Fig. 2). In addition, real-time RT-PCR amplification showed a median difference in Bmi-1 expression of 113.37 (IQR 47.97, 181.02) as shown in Table 2.
Correlation of Bmi-1 expression and clinicopathologic parameters in colon cancer
The relationship between the expression of Bmi-1 (immunohistochemical staining) and clinicopathologic features is summarized in Table 3. Expression (staining) of Bmi-1 was highly correlated with the AJCC stage (P < 0.001), T classification (P < 0.001), nodal involvement (P < 0.001), and the presence of distant metastasis (P = 0.038). Factors not significantly associated with staining included age, sex, location of the tumor, extent of tumor differentiation, and lymphovascular invasion. Moreover, we found that positive immunostaining for Bmi-1 was more frequently detected in specimens that stained positively for Ki67 (P = 0.019).
Survival analysis
The 5-year OS rate of the 203 patients with primary colon cancer was 68%, with 65 deaths observed in the follow-up period. The 5-year DFS rate was 62%, with 74 events observed during follow-up. Fifty-four patients (27%) developed distant metastases, and local tumor recurrence was diagnosed in 20 patients (9%). The proportion of samples with metastasis or local recurrence from primary colon cancer after curative surgery differed significantly between the Bmi-1-positive and Bmi-1-negative groups. More patients with Bmi-1-positive tumors subsequently developed metastases or local recurrence than did those with Bmi-1-negative tumors (P = 0.001) [Bmi-1-positive, 52 (77.8%) of 118 patients, mean follow-up 49 months (range 44–53); Bmi-1-negative, 14 (21.2%) of 67, 65 (61–69)]. On univariate analysis, patients whose localized colon tumors were Bmi-1-positive had a significantly lower 5-year DFS than those with Bmi-1-negative tumors [51.2 vs. 82.2%, hazard ratio 2.919 (95% CI 1.656–5.143)] (Fig. 3a; Table 4). The 5-year OS was also significantly lower in patients with Bmi-1-positive tumors than in those with Bmi-1-negative tumors [54.6 vs. 92.6%, HR 5.056 (95% CI 2.512–10.174)] (Fig. 3b; Table 5). In addition, the AJCC stage (P < 0.001), T classification (P < 0.01), presence of nodal involvement (P < 0.001), metastatic disease (P < 0.001), lymphovascular invasion (P < 0.001), and level of tumor differentiation (P < 0.001) were associated with OS and DFS.
Multivariate analysis was performed using the Cox proportional hazards model for all of the significant variables in the univariate analysis; however, because T stage, lymph node metastasis, and distant metastasis were collinear with AJCC stage, we excluded T stage, lymph node metastasis, and distant metastasis from the final model, as discussed in Dawood et al. 2008. The results from the multivariate analysis showed that Bmi-1 expression (P = 0.018), AJCC stage (P < 0.001), and tumor differentiation (P = 0.002) were independent prognostic factors for DFS (Table 4). Moreover, on Cox proportional hazard analyses of OS, Bmi-1 (P = 0.001), AJCC stage (P < 0.001), and tumor differentiation (P < 0.001) also emerged as significant independent prognostic factors (Table 5).
Discussion
The human Bmi-1 gene is located on the short arm of chromosome 10p13, a region involved in chromosomal translocations in infant leukemias and amplified in occasional non-Hodgkin’s lymphomas (NHLs) and solid tumors (Beà et al. 2001). The Bmi-1 gene product has been identified as a cell cycle regulatory factor and is noted as a negative regulator of cyclin-dependent kinases p16INK4a and p14ARF (Douglas et al. 2008; Molofsky et al. 2005). Abnormalities in Bmi-1 may influence the critical process of cell cycle regulation and the aggressive biological behavior of some malignant tumors through p16INK4a and p14ARF. In colon cancer, Bmi-1 also appeared to be correlated with expression of p16INK4a and p14ARF in an immunostaining study (Kim et al. 2004a, b). However, the significance of Bmi-1 in the development and prognosis of colon cancer has remained unexplored until now.
In the fresh colon tissues examined in the current study, immunostaining, western blotting, and RT-PCR showed that the elevated expression of Bmi-1 occurred both at the transcriptional and post-transcriptional levels. Recent studies have identified Bmi-1 as a potential marker of cancerous stem cells, especially in the initiation of tumors, including hematopoietic cancer, melanoma, ovarian cancer, lung tumorigenesis, pediatric, and brain tumors (Dovey et al. 2008; Hemmati et al. 2003; Hosen et al. 2007; Mihic-Probst et al. 2007; Zhang et al. 2008). This may explain the strong relationship between Bmi-1 and Ki67 in our study, suggesting the potential self-renewal and proliferation capacities of Bmi-1, which may be characteristic of a cancer-initiating gene.
It has also been reported that Bmi-1 was more intensely stained in the invading fronts than in the central portions of primary invasive breast cancers and correlated with axillary lymph node metastases (Kim et al. 2004a, b). Previous studies in lung cancer and B cell non-Hodgkin lymphoma (Vrzalikova et al. 2008) also reported elevated Bmi-1 levels in advanced clinical grades relative to early stages. These findings suggested that Bmi-1 might be involved in tumor progression and metastatic events.
In our study, we found that elevated expression of Bmi-1 correlated with several clinicopathologic factors including AJCC stage (P < 0.0001), T classification (P < 0.0001), nodal involvement (P < 0.0001), and the presence of distant metastasis (P = 0.038). In contrast, Kim et al. (2004a, b) found no correlation between expression of Bmi-1 and clinicopathological parameters, such as age, sex, site of tumor, size, differentiation, or depth of invasion in colorectal cancer. This discrepancy may be explained by the larger number of samples and use of high throughput screening by microarrays to avoid immunostaining bias in our work. It is also possible that there are distinctly different underlying biological behaviors between colon cancer and rectum cancer and that Bmi-1 may play different roles in the pathogenesis of colon cancer and rectum cancer.
In recent years, our understanding of the metastatic process has evolved greatly. However, mechanisms involved in colon cancer metastasis are not fully understood because metastasis is a multistep process and requires altered expression of a spectrum of genes (Ma et al. 2008). In the present study, we examined the expression of Bmi-1 in 66 paired samples of primary colon cancer and matched LNM. The expression of Bmi-1 was upregulated in 31 of the LNM specimens and decreased in 6. Interestingly, Raaphorst et al. (2003) reported increasing levels of Bmi-1 expression during progression from ductal carcinoma in situ to invasive cancer. In addition, a high Bmi-1/low p16 (ink4a) expression pattern was a significant predictor of metastasis in melanoma (Mihic-Probst et al. 2007). Finally, our results provide the first evidence that the expression of Bmi-1 may be a potential molecular marker. Bmi-1 expression was associated with an increased risk of metastasis/local recurrence and was strongly linked to poor survival outcomes, with hazard ratios of 2.919 for DFS and 5.056 for OS in the univariate analysis. On multivariate analysis, Bmi-1 expression appeared to be an independent prognostic factor for OS and DFS in colon cancer.
An unexpected decrease in DFS at 83 months was observed among the patients whose tumor specimens were negative for Bmi-1 expression. Our records show that none of these patients had recurrent tumors at 80 months; the disease-free survival was dramatically dropped at 83 months, and one patient was detected with recurrent tumor and distant metastasis at the 83rd month. It will be interesting to examine such metastatic tissues in future studies to determine whether they are Bmi-1 positive.
Interestingly, the population of Bmi-1-positive patients who did not have metastases at the time of surgery and were disease-free at 60 months appeared to remain disease-free for the remainder of the study. This is in contrast to the continued decrease in OS seen among the Bmi-1-positive population that included the patients who had distant metastases at the time of surgery.
Our results demonstrating that the elevated level of expression of the Bmi-1 protein has a significant correlation with poor prognosis may be helpful in designing future studies to understand the molecular development of colon cancer. They also suggest that level of Bmi-1 may be a valuable predictive marker for colon cancer patients after surgery. This notion is consistent with some previous studies in hepatocellular carcinoma, which showed that Bmi-1 could be a candidate biomarker for long-term survival (Wang et al. 2008). Thus, patients with colon cancer whose expression of Bmi-1 is elevated may need more powerful adjunctive therapy and intensive follow-up. Whether Bmi-1 has value clinically as a marker for predicting outcome or for deciding among potential therapeutic approaches (e.g., surgery vs. chemotherapy or a combination of treatments) in patients with colon cancer should be pursued with additional, properly designed studies.
Conclusion
In this study, we showed that expression of Bmi-1 was elevated in colon cancer. High levels of Bmi-1 protein might serve as an independent prognostic marker for disease-free and overall survival for patients undergoing curative colectomy. A more detailed investigation of the Bmi-1 gene, the elevated expression of which is correlated with metastasis and the establishment of tumors at distant sites, may yield important diagnostic and therapeutic benefits in future.
References
Beà S, Tort F, Pinyol M et al (2001) BMI-1 gene amplification and overexpression in hematological malignancies occur mainly in mantle cell lymphomas. Cancer Res 61:2409–2412
Berezovska O, Glinskii A, Yang Z et al (2006) Essential role for activation of the Polycomb group [PcG] protein chromatin silencing pathway in metastatic prostate cancer. Cell Cycle 5:1886–1901
Dawood S, Broglio K, Gonzalez-Angulo AM et al (2008) Prognostic value of body mass index in locally advanced breast cancer. Clin Cancer Res 14:1718–1725. doi:10.1158/1078-0432.CCR-07-1479
Dimri G, Martinez J, Jacobs J et al (2002) The Bmi-1 oncogene induces telomerase activity and immortalizes human mammary epithelial cells. Cancer Res 62:4736–4745
Douglas D, Hsu J, Hung L et al (2008) BMI-1 promotes Ewing sarcoma tumorigenicity independent of CDKN2A repression. Cancer Res 68:6507–6515. doi:10.1158/0008-5472.CAN-07-6152
Dovey J, Zacharek S, Kim C et al (2008) Bmi1 is critical for lung tumorigenesis and bronchioalveolar stem cell expansion. Proc Natl Acad Sci USA 105:11857–11862. doi:10.1073/pnas.0803574105
Haupt Y, Alexander W, Barri G et al (1991) Novel zinc finger gene implicated as myc collaborator by retrovirally accelerated lymphomagenesis in E mu-myc transgenic mice. Cell 65:753–763. doi:10.1016/0092-8674(91)90383-A
Hemmati H, Nakano I, Lazareff J et al (2003) Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci USA 100:15178–15183. doi:10.1073/pnas.2036535100
Hosen N, Yamane T, Muijtjens M et al (2007) Bmi-1-green fluorescent protein-knock-in mice reveal the dynamic regulation of bmi-1 expression in normal and leukemic hematopoietic cells. Stem Cells 25:1635–1644. doi:10.1634/stemcells.2006-0229
Kang M, Kim R, Kim S et al (2007) Elevated Bmi-1 expression is associated with dysplastic cell transformation during oral carcinogenesis and is required for cancer cell replication and survival. Br J Cancer 96:126–133. doi:10.1038/sj.bjc.6603529
Kim J, Yoon S, Jeong S et al (2004a) Overexpression of Bmi-1 oncoprotein correlates with axillary lymph node metastases in invasive ductal breast cancer. Breast 13:383–388. doi:10.1016/j.breast.2004.02.010
Kim J, Yoon S, Kim C (2004b) The Bmi-1 oncoprotein is overexpressed in human colorectal cancer and correlates with the reduced p16INK4a/p14ARF proteins. Cancer Lett 203:217–224. doi:10.1016/j.canlet.2003.07.009
Leung C, Lingbeek M, Shakhova O et al (2004) Bmi1 is essential for cerebellar development and is overexpressed in human medulloblastomas. Nature 428:337–341. doi:10.1038/nature02385
Liu S, Dontu G, Mantle I et al (2006) Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res 66:6063–6071. doi:10.1158/0008-5472.CAN-06-0054
Liu JH, Song LB, Zhang X et al (2008) Bmi-1 expression predicts prognosis for patients with gastric carcinoma. J Surg Oncol 97:267–272
Ma C, Rong Y, Radiloff D et al (2008) Extracellular matrix protein betaig-h3/TGFBI promotes metastasis of colon cancer by enhancing cell extravasation. Genes Dev 22:308–321. doi:10.1101/gad.1632008
Mihic-Probst D, Kuster A, Kilgus S et al (2007) Consistent expression of the stem cell renewal factor BMI-1 in primary and metastatic melanoma. Int J Cancer 121:1764–1770. doi:10.1002/ijc.22891
Molofsky A, He S, Bydon M et al (2005) Bmi-1 promotes neural stem cell self-renewal and neural development but not mouse growth and survival by repressing the p16Ink4a and p19Arf senescence pathways. Genes Dev 19:1432–1437. doi:10.1101/gad.1299505
Nowak K, Kerl K, Fehr D et al (2006) BMI-1 is a target gene of E2F-1 and is strongly expressed in primary neuroblastomas. Nucleic Acids Res 34:1745–1754. doi:10.1093/nar/gkl119
Petrova T, Nykänen A, Norrmén C et al (2008) Transcription factor PROX1 induces colon cancer progression by promoting the transition from benign to highly dysplastic phenotype. Cancer Cell 13:407–419. doi:10.1016/j.ccr.2008.02.020
Raaphorst F, Meijer C, Fieret E et al (2003) Poorly differentiated breast carcinoma is associated with increased expression of the human polycomb group EZH2 gene. Neoplasia 5:481–488
Sansom O, Meniel V, Muncan V et al (2007) Myc deletion rescues Apc deficiency in the small intestine. Nature 446:676–679. doi:10.1038/nature05674
Song L, Zeng M, Liao W et al (2006) Bmi-1 is a novel molecular marker of nasopharyngeal carcinoma progression and immortalizes primary human nasopharyngeal epithelial cells. Cancer Res 66:6225–6232. doi:10.1158/0008-5472.CAN-06-0094
Tateishi K, Ohta M, Kanai F et al (2006) Dysregulated expression of stem cell factor Bmi1 in precancerous lesions of the gastrointestinal tract. Clin Cancer Res 12:6960–6966. doi:10.1158/1078-0432.CCR-06-0449
Valk-Lingbeek M, Bruggeman S, van Lohuizen M (2004) Stem cells and cancer: the polycomb connection. Cell 118:409–418. doi:10.1016/j.cell.2004.08.005
Vrzalikova K, Skarda J, Ehrmann J et al (2008) Prognostic value of Bmi-1 oncoprotein expression in NSCLC patients: a tissue microarray study. J Cancer Res Clin Oncol 134:1037–1042. doi:10.1007/s00432-008-0361-y
Wang H, Pan K, Zhang H et al (2008) Increased polycomb-group oncogene Bmi-1 expression correlates with poor prognosis in hepatocellular carcinoma. J Cancer Res Clin Oncol 134:535–541. doi:10.1007/s00432-007-0316-8
Zhang S, Balch C, Chan M et al (2008) Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res 68:4311–4320. doi:10.1158/0008-5472.CAN-08-0364
Zhuang P, Zhang J, Zhu X et al (2008) Two pathologic types of hepatocellular carcinoma with lymph node metastasis with distinct prognosis on the basis of CK19 expression in tumor. Cancer 112:2740–2748. doi:10.1002/cncr.23488
Acknowledgments
We are grateful for funding support from: Key Basic Research Project of the Science and Technology Commission of Shanghai Municipality (Grant Number: 05JC1402); Outstanding Medical Academic Leader of Shanghai Municipality (Grant Number: LJ06024).
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
D. Li and H. Tang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, Dw., Tang, Hm., Fan, Jw. et al. Expression level of Bmi-1 oncoprotein is associated with progression and prognosis in colon cancer. J Cancer Res Clin Oncol 136, 997–1006 (2010). https://doi.org/10.1007/s00432-009-0745-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-009-0745-7