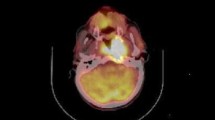
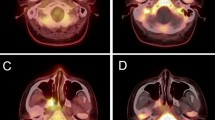
Abstract
Purpose
The purpose of this study was to evaluate the prognostic value of metabolic tumor volume (MTV) and metabolic index (MI) from fluorine-18-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) in patients with nasopharyngeal carcinoma (NPC).
Methods
From October 2002 to July 2004, 41 patients with NPC who underwent 18F-FDG PET-CT scan before and after radiotherapy were reviewed retrospectively. All patients received intensity-modulated radiotherapy using 6MV X-rays. We examined the association of MTV and the results of long-term follow-up of the patients.
Results
Patients having tumors with an MTV below 30 cm3 had significantly better 5-year overall survival (OS) (84.6:46.7%, P = 0.006) and disease-free survival (DFS) (73.1:40.0%, P = 0.014) than patients with an MTV of 30 cm3 or greater. And the patients with MI below 130 had significantly higher 5-year OS (88.0:43.8%, P = 0.002) and DFS (76.0:37.5%, P = 0.005) than other patients. In the Cox multivariate analysis, MI and metabolic response (MR) were predictive of DFS, and we did not find a significant relationship between standard uptake value (SUV) and OS or DFS.
Conclusions
The present study shows that tumor volume parameters, especially the combination of MTV and SUV in the “metabolic index”, are valuable for predicting long-term survival. High MI may be useful for identifying patients requiring more aggressive treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The mainstay treatment for nasopharyngeal carcinoma (NPC) has been radiotherapy as NPC is sensitive to radiotherapy. However, there is a significant rate of local failures and distant metastases (Yeh et al. 2005). Although traditional prognostic factors may provide some useful clinical information, they cannot predict treatment outcome reliably. Therefore, substantial research efforts have focused on the identification of novel prognostic factors to further stratify risk groups with the goal of developing individualized treatment strategies for these patients.
Fluorine-18-fluorodeoxyglucose positron emission tomography with computed tomography (18F-FDG PET/CT) is now widely used with promising results in the initial diagnosis, staging workup, and early detection of recurrence in many kinds of cancer (Tsai et al. 2002; Kao et al. 2002). Furthermore, some studies have shown that tumor FDG uptake may have prognostic significance, in that patients with high FDG uptake generally have less favorable outcomes (Xu et al. 2008; Lin et al. 2007; Allal et al. 2004). Though many studies about the usefulness of FDG uptake have been made, the prognostic value of metabolic tumor volume (MTV) is still under investigation.
In the present study, we used 18F-FDG PET/CT scan MTV and metabolic index (MI) to determine whether 18F-FDG uptake could be used as a prognostic marker of overall survival (OS) and disease-free survival (DFS) in patients with NPC who received definitive radiotherapy.
Patients and methods
Patients
We retrospectively analyzed the medical records of 41 patients with stage I–IV locally NPC who underwent 18F-FDG PET/CT before and after radiotherapy and were referred for definitive radiotherapy to the Department of Radiation Oncology, Shandong Tumor Hospital and Institute, Jinan, China, between October 2002 and July 2004. Eligible patients were those with biopsy-proven carcinoma, including those with differentiated non-keratinizing carcinomas and undifferentiated carcinomas, who had received definitive radiotherapy. Patients in a poor condition (Karnofsky index < 70%) were excluded. All patients were initially evaluated with a complete medical history and physical examination, complete blood count, baseline serum biochemistry, fiberoptic nasopharyngoscopy with nasopharyngeal biopsy, and pre-treatment whole-body 18F-FDG PET/CT scan. Other routine imaging modalities included chest radiography, CT scan or MRI of the head and neck, abdominal ultrasonography, and whole-body bone scan. Tumors were staged according to the American Joint Committee on Cancer (AJCC) staging system.
PET imaging
All patients were fasted for at least 8 h prior to 18F-FDG PET/CT scanning, and their blood glucose level was measured. All patients were rested for at least 1 h before PET/CT scan. 18F-FDG (5.55–7.40 MBq/kg), of radiopharmaceutical purity >95%, was injected intravenously. After 1 h, images were acquired in 2D mode on a Discovery LS PET/CT, GE. The maximal standard uptake value (SUVmax) in each region of interest (ROI) was determined using the whole-body attenuation corrected image and the formula, tissue concentration of 18F-FDG measured by PET/the injected dose/body weight. All the 41 patients underwent the pre- and post-treatment whole-body 18F-FDG PET/CT scan as part of routine follow-up of 2–3 months after treatment completion (Greven et al. 2001).
MTV and MI calculation
First, the PET area was delineated on the FDG-PET images as any place with SUV of 2.5 in the tumor. This means that the PET area was delineated by a circle encompassing regions equal or greater than SUV 2.5. Of note, the cavity in tumors, when present, is excluded as part of the MTV. The metabolic tumor volume of each slice was then determined by multiplying the area within the thresholded margin by CT interval. The final metabolic tumor volume was calculated by adding all metabolic tumor volumes of each slice. Maximum and mean SUV within the MTV were calculated automatically. And MI was calculated by multiplying the metabolic tumor volume by the mean SUV. The formulas of calculating MTV and MI are as follows:
-
S i : the area with abnormal metabolism of each slice;
-
d: the interval of CT scan;
-
MTV i : the metabolic tumor volume of each slice;
-
SUVmean i : the mean SUV of each slice;
-
n: the number of slices with abnormal metabolism.
Treatment
All patients received definitive intensity-modulated radiotherapy (IMRT), or combined with concomitant and adjuvant platinum-based chemotherapy. During treatment planning and radiotherapy, each patient was immobilized in the supine position, using a custom-made thermoplastic mask encompassing the entire head and neck. The CT simulation was performed with administration of intravenous contrast in all patients, and images were acquired at intervals of 3–5 mm from the skull base to the level of the carina using a Philips Brilliance CT simulator (Philips Medical Systems) and transferred to Varian Eclipse 3D Treatment Planning System (Varian Medical Systems). The target volume was defined according to International Commission on Radiation Units (ICRU) publications 50 and 62. The adjacent critical organs were delineated on the same CT slices. In the planning procedure, 5–9 coplanar or non-coplanar fields were usually selected for adequate coverage of the target volume. Radiotherapy was administered as 1.8–2.0 Gy daily fractions using 6 MV photon beams (CLINAC 2100C, Varian), 5 days per week, for a total dose of 70–72 Gy for gross target volume (GTV), 60–66 Gy for clinical target volume (CTV) of high risk, and elective nodal irradiation involved radiation doses of 50–60 Gy. For concomitant chemotherapy, Fluorouracil (500 mg/m2 day, days 1–5) and Cisplatin (12–15 mg/m2 day, days 1–5) were given every 4 weeks on day 1 and day 29. For adjuvant chemotherapy, Fluorouracil (600 mg/m2 per day, days 1–5) and cisplatin (80 mg/m2, day 1) were given every 3 weeks.
Study design and statistical analysis
Recurrence was histologically confirmed when patients developed clinically symptomatic recurrent disease. To evaluate the prognostic value of PET/CT, OS and DFS were chosen as endpoints, and were measured from the date of radiotherapy initiation to the date of death or recurrence. We used SPSS statistical software, version 13.0, for statistical analysis. The differences of MTV, MI and MR among pathologic subtypes were tested using one-way analysis of variance (ANOVA) or Kruskal–Wallis test. The survival function was estimated using the Kaplan–Meier method. The difference in survival rates between groups was tested for significance using the logrank test. Receiver operator curves (ROC) were determined to assess the area under curve (AUC) and the optimal cutoff value for predicting survival. Multivariate analysis was performed to identify the prognostic factors influencing OS and DFS using Cox proportional hazards regression model. All statistical tests were conducted at a two-sided level of significance of 0.05.
Results
-
1.
Patient characteristics are shown in Table 1. Median follow-up for surviving patients was 62 months (range 13–75 months). Twenty-nine patients were alive at last follow-up and 12 had died. All 41 patients had abnormal FDG uptake before treatment. The median of pre-treatment SUVmax was 7.3, ranged from 3.2 to 20.7. Of the 41 patients, 12 (29.3%) had local recurrence during the observation period, 4 (9.8%) showed distant metastases, and the rest displayed no recurrence or metastases. All of the 41 patients’ treatment response was evaluated by 18F-FDG PET/CT scan. The post-treatment PET/CT scan did not show any abnormal FDG uptake (SUVmax < 2.5, metabolic complete response, MCR) in 26 patients. Persistent abnormal FDG uptake (SUVmax ≥ 2.5, metabolic partial response, MPR) was found in 15 patients.
Table 1 Patients characteristics -
2.
The differences of MTV (F = 2.097, P = 0.137), MI (F = 1.933, P = 0.159) and MR (X 2 = 1.533, P = 0.465) among pathologic subtypes (differentiated non-keratinizing carcinoma, vesicular nucleus cell carcinoma, and other undifferentiated carcinomas) are all not significantly in this study.
-
3.
Five-year overall survival rate (OS) and disease-free survival (DFS) rate of all patients were 70.7 and 61.0%, respectively. As shown in Table 2, patients who showed with MPR had significantly lower 5-year OS (X 2 = 11.005, P = 0.001) and DFS (X 2 = 9.084, P = 0.003) than patients with MCR.
Table 2 Logrank test for 5-year OS and DFS -
4.
The ability of MTV and MI to predict prognosis were depicted by ROC curve. Areas under the curve (AUC) are 0.759 and 0.779, respectively. Figure 1 shows the ROC curve of MI. And the best cutoff values are 30 and 130 cm3, respectively. Patients having tumors with an MTV below 30 cm3 had significantly better 5-year OS (84.6:46.7%, P = 0.006) and DFS (73.1:40.0%, P = 0.014) than patients with an MTV of 30 cm3 or greater. And the patients with MI below 130 had significantly higher 5-year OS (88.0:43.8%, P = 0.002) and DFS (76.0:37.5%, P = 0.005) than other patients, as shown in Figs. 2 and 3.
Fig. 1 Fig. 2 -
5.
The mean MI at which there was no evidence of recurrence or metastasis in 5 years was 90.09 (range 8.16–306.28), compared with the mean MI of the recurrent or metastatic patients, which was 234.89 (range 4.42–927.18) (Mann–Whitney U = 116.00, P = 0.025), as shown in Fig. 4.
-
6.
A Cox proportional hazards multivariate model of DFS outcome was constructed to evaluate the pre-treatment tumor stage, tumor size, nodal status, SUVmax, MTV, MI and post-treatment metabolic response as predictors of disease progression and survival. The results indicated that only metabolic response and MI were the significant predictors of DFS in our patient population, as shown in Table 3.
Table 3 Results of Cox multivariate analysis
Discussion
Treatment outcomes in NPC remains heterogeneous; therefore, identification of novel prognostic factors that potentially predict outcome is of great interest.
It has been reported that prognostic factors can be identified by immunohistochemical staining of tumor tissue. However, biopsy samples do not represent the genetic information or protein expression of the entire tumor (Lee et al. 2005; Krishna et al. 2006; Nakao et al. 2006; Wang et al. 2006). Functional imaging, such as 18F-FDG PET or PET/CT, may provide metabolic information on the entire tumor. Additionally, accumulating data suggested that FDG PET may serve as a non-invasive method, which can indirectly measure the expression of various biologic markers of tumor aggressiveness (Allal et al. 2004). Therefore, the SUV which represent the FDG uptake may become one of the potential prognostic factors. Patients with high concentrations of tumor cells or highly metabolic tumor cells would be expected to have poorer prognosis (Huang 2000). At present, besides providing useful diagnostic information regarding pre-treatment staging and post-treatment follow-up (Spiro et al. 2008; Wong et al. 2002; van Tinteren et al. 2002), intensity of FDG uptake is emerging as a valuable predictive factor regarding treatment outcome.
Recently, the degree of tumor uptake of FDG on PET as assessed by the SUV was shown to be an independent prognostic factor in NPC and other tumors (Lee et al. 2008; Chan et al. 2009; Sasaki et al. 2005; Eschmann et al. 2006). However, there have been conflicting results and some studies have not found a significant association between SUV and prognosis (Lee et al. 2007). Thus, more effective ways are needed to identify patients at high risk who may be candidates for more aggressive initial treatment.
Currently, stage is the most prognostic factor in predicting the outcomes of patients with malignant tumors (van Rens et al. 2000). However, stage may simply be a surrogate for or correlate with the underlying tumor burden, which may be a more direct predictor of disease progression and survival (Lee et al. 2007). Until recently, it has been difficult to quantify tumor burden directly and systematically. Therefore, a more objective measuring tool is necessary to accurately assess tumor volume.
Fortunately, it has been reported that tumor burden reflected by the volume of tumor tissue demonstrating increased FDG uptake on PET, or metabolic tumor volume (MTV), is a novel potential prognostic factor (Lee et al. 2007). PET scans tend to be whole-body studies, allowing a comprehensive assessment of tumor burden, with the exception of the brain, and it provides information that is unavailable by MRI or CT imaging. A recent study by La (La et al. 2009) evaluated the usefulness of tumor volume measurement with PET in patients with head and neck cancer. Therefore, instead of measuring tumor volume based on MRI or CT imaging, we used FDG-PET/CT imaging to determine MTV, which may be a more direct and reliable method of quantifying tumor burden because it incorporates functional criteria. In our long-term follow-up analysis with 41 NPC patients, we discovered that 30 cm3 was the most discriminative cutoff of MTV. With furthermore analysis, patients having tumors with lower MTV had higher 5-year OS and DFS than patients with higher MTV, although there is no statistical significance in multivariate analysis. Similar results have been reported in lung cancer and lymphoma where MTV was shown to be highly prognostic for disease progression and death, independent of other established prognostics factors (Lee et al. 2007; Grow et al. 2005).
Interestingly, both SUV and MTV are predictive of survival in patients with NPC. However, SUV can only represent metabolic extent, and MTV represents the size of high metabolic tumor cells only. Thus, it is reasonable to suspect that it may be an even more effective predictor when SUV and MTV are combined. This, indeed, was found to be the case. A principal find of this study is that MI is strongly correlated with DFS and OS in patients with NPC treated by radiotherapy, and thus it is a better predictor of long-term survival than MTV and SUV alone. We also confirmed that patients who presented with local recurrence or distant metastasis within 5 years had a significantly higher MI than the remaining patients. It suggests the intimate correlation between the primary MI and tumor recurrence or metastasis. In our opinion, high MI represents its high activity, thus these tumors maybe more aggressive in metastasizing or because of relative growth conditions. Roedl et al. (2009) observed the similar results using “diameter-SUV index” to predict prognosis.
Quite a number of malignant tumors can result in clinical complete response via appropriate treatment, but it does not mean that the treatments destroyed all tumor cells. It was suggested that FDG uptake value could be a predictor of pathologic response. It has been suggested in previous studies that a metabolic decrease in the FDG uptake of the tumor (decrease in the SUV) between pre- and post-radiotherapy scans is associated with a histopathologic response of the tumor (Song et al. 2005; Swisher et al. 2004; Wieder et al. 2005; Lordick et al. 2007; Cunningham et al. 2006). The metabolic response to therapy as determined by FDG PET has been shown to be predictive of survival outcome after treatment completion for several tumors, including lymphoma, non-small-cell lung cancer and anal cancer (Nahmias et al. 2007; Schwarz et al. 2008; Cheson et al. 2007; Juweid et al. 2007). In this study, we have found that metabolic response is an effective prognostic factor in NPC patients. In our opinion, tumor MCR of post-treatment represented its high sensitivity to radiotherapy, as a result of high response and favorable prognosis. Patients with no evidence of residual PET activity during the course of their therapy may be candidates for dose alterations that could potentially translate into reduced acute and late toxicity.
In multivariable analysis, MI and MR were the most significant independent factor for DFS and OS. Of note, stage (AJCC), which is the most important conventional measure of tumor burden, was not prognostic in our study population (it does not include the new progress in NPC staging, e.g. China 2008 Staging). This is likely because of the heterogeneity of stages (for example, there were only 2 patients of stage I) in this cohort of patients. However, it remains need further investigations to achieve a certain conclusion.
The main limitations of our study are the relatively low number of patients in our cohort, the heterogeneity of the patients and treatments, and the retrospective design. However, despite these limitations, the present study is able to demonstrate that tumor metabolic parameters, especially the tumor metabolic response and the combination of tumor volume and SUV in the “metabolic index” are accurate predictors of treatment response and tumor-free survival in patients with NPC. Our findings suggested that patients with MPR or high MI may be considered at increased risk of failure and may benefit from more aggressive treatment, for instance, higher radiation dose or combined more aggressive chemotherapy, and consequently improve treatment efficiency.
References
Allal AS, Slosman DO, Kebdani T et al (2004) Prediction of outcome in head-and-neck cancer patients using the standardized uptake value of 2-18F-fluoro-2-deoxy-d-glucose. Int J Radiat Oncol Biol Phys 59:1295–1300
Chan SC, Chang JT, Wang HM et al (2009) Prediction for distant failure in patients with stage M0 nasopharyngeal carcinoma: the role of standardized uptake value. Oral Oncol 45:52–58
Cheson BD, Pfistner B, Juweid ME et al (2007) Revised response criteria for malignant lymphoma. J Clin Oncol 25:579–586
Cunningham D, Allum WH, Stenning SP et al (2006) Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 80:11–20
Eschmann SM, Friedel G, Paulsen F et al (2006) Is standardized (18)F-FDG uptake value an outcome predictor in patients with stage III non-small cell lung cancer? Eur J Nucl Med Mol Imaging 33:263–269
Greven KM, Williams DW 3rd, McGuirt WF Sr et al (2001) Serial positron emission tomography scans following radiation therapy of patients with head and neck cancer. Head Neck 23:942–946
Grow A, Quon A, Graves EE et al (2005) Metabolic tumor volume as an independent prognostic factor in lymphoma. J Clin Oncol (ASCO Ann Meeting Proc Suppl) 23:6594
Huang SC (2000) Anatomy of SUV. Standardized uptake value. Nucl Med Biol 27:643–646
Juweid ME, Stroobants S, Hoekstra OS et al (2007) Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol 25:571–578
Kao CH, Shiau YC, Shen YY et al (2002) Detection of recurrent or persistent nasopharyngeal carcinomas after radiotherapy with technetium-99m methoxyisobutylisonitrile single photon emission computed tomography and computed tomography: comparison with 18-fluoro-2-deoxyglucose positron emission tomography. Cancer 94:1981–1986
Krishna SM, James S, Balaram P (2006) Expression of VEGF as prognosticator in primary nasopharyngeal cancer and its relation to EBV status. Virus Res 115:85–90
La TH, Filion EJ, Turnbull BB et al (2009) Metabolic tumor volume predicts for recurrence and death in head-and-neck cancer. Int J Radiat Oncol Biol Phys 74:1335–1341
Lee SW, Cho KJ, Park JH et al (2005) Expressions of Ku70 and DNA-PKcs as prognostic indicators of local control in nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 62:1451–1457
Lee P, Weerasuriya DK, Lavori PW et al (2007) Metabolic tumor burden predicts for disease progression and death in lung cancer. Int J Radiat Oncol Biol Phys 69:328–333
Lee SW, Nam SY, Im KC et al (2008) Prediction of prognosis using standardized uptake value of 2-18F-fluoro-2-deoxy-d-glucose positron emission tomography for nasopharyngeal carcinomas. Radiother Oncol 87:211–216
Lin C, Itti E, Haioun C et al (2007) Early 18F-FDG PET for prediction of prognosis in patients with diffuse large B-cell lymphoma: SUV-based assessment versus visual analysis. J Nucl Med 48:1626–1632
Lordick F, Ott K, Krause BJ et al (2007) PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol 8:797–805
Nahmias C, Hanna WT, Wahl LM et al (2007) Time course of early response to chemotherapy in non-small cell lung cancer patients with 18F-FDG PET/CT. J Nucl Med 48:744–751
Nakao K, Mochiki M, Nibu K et al (2006) Analysis of prognostic factors of nasopharyngeal carcinoma: impact of in situ hybridization for Epstein–Barr virus encoded small RNA 1. Otolaryngol Head Neck Surg 134:639–645
Roedl JB, Halpern EF, Colen RR et al (2009) Metabolic tumor width parameters as determined on PET/CT predict disease-free survival and treatment response in squamous cell carcinoma of the esophagus. Mol Imaging Biol 11:54–60
Sasaki R, Komaki R, Macapinlac H et al (2005) [18F]Fluorodeoxy-glucose uptake by positron emission tomography predicts outcome of non-small-cell lung cancer. J Clin Oncol 23:1136–1143
Schwarz JK, Siegel BA, Dehdashti F et al (2008) Tumor response and survival predicted by post-therapy FDG-PET/CT in anal cancer. Int J Radiat Oncol Biol Phys 71:180–186
Song SY, Kim JH, Ryu JS et al (2005) FDG-PET in the prediction of pathologic response after neoadjuvant chemoradiotherapy in locally advanced, resectable esophageal cancer. Int J Radiat Oncol Biol Phys 63:1053–1059
Spiro SG, Buscombe J, Cook G et al (2008) Ensuring the right PET scan for the right patient. Lung Cancer 59:48–56
Swisher SG, Maish M, Erasmus JJ et al (2004) Utility of PET, CT, and EUS to identify pathologic responders in esophageal cancer. Ann Thorac Surg 78:1152–1160
Tsai MH, Shiau YC, Kao CH et al (2002) Detection of recurrent nasopharyngeal carcinomas with positron emission tomography using 18-fluoro-2-deoxyglucose in patients with indeterminate magnetic resonance imaging findings after radiotherapy. J Cancer Res Clin Oncol 128:279–282
van Rens MT, de la Riviere AB, Elbers HR et al (2000) Prognostic assessment of 2,361 patients who underwent pulmonary resection for non-small cell lung cancer, stage I, II, and IIIA. Chest 117:374–379
van Tinteren H, Hoekstra OS, Smit EF et al (2002) Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non-small-cell lung cancer: the PLUS multicentre randomised trial. Lancet 359:1388–1393
Wang LF, Chai CY, Kuo WR et al (2006) Correlation between proliferating cell nuclear antigen and p53 protein expression and 5-year survival rate in nasopharyngeal carcinoma. Am J Otolaryngol 27:101–105
Wieder HA, Beer AJ, Lordick F et al (2005) Comparison of changes in tumor metabolic activity and tumor size during chemotherapy of adenocarcinomas of the esophagogastric junction. J Nucl Med 46:2029–2034
Wong RJ, Lin DT, Schoder H et al (2002) Diagnostic and prognostic value of [(18)F]fluorodeoxyglucose positron emission tomography for recurrent head and neck squamous cell carcinoma. J Clin Oncol 20:4199–4208
Xu X, Yu J, Sun X et al (2008) The prognostic value of 18F-fluorodeoxyglucose uptake by using serial positron emission tomography and computed tomography in patients with stage III non-small cell lung cancer. Am J Clin Oncol 31:470–475
Yeh SA, Tang Y, Lui CC et al (2005) Treatment outcomes and late complications of 849 patients with nasopharyngeal carcinoma treated with radiotherapy alone. Int J Radiat Oncol Biol Phys 62:672–679
Acknowledgments
The authors thank Dr. Huiqing Li and Jinsong Zheng for their assistance with this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xie, P., Yue, JB., Zhao, Hx. et al. Prognostic value of 18F-FDG PET-CT metabolic index for nasopharyngeal carcinoma. J Cancer Res Clin Oncol 136, 883–889 (2010). https://doi.org/10.1007/s00432-009-0729-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-009-0729-7